The Relationship Between Age and the Propofol Dose for Anesthesia Induction: A Single-Center Retrospective Study Utilizing Neural Network Model Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Ethics
2.2. Clinical Data
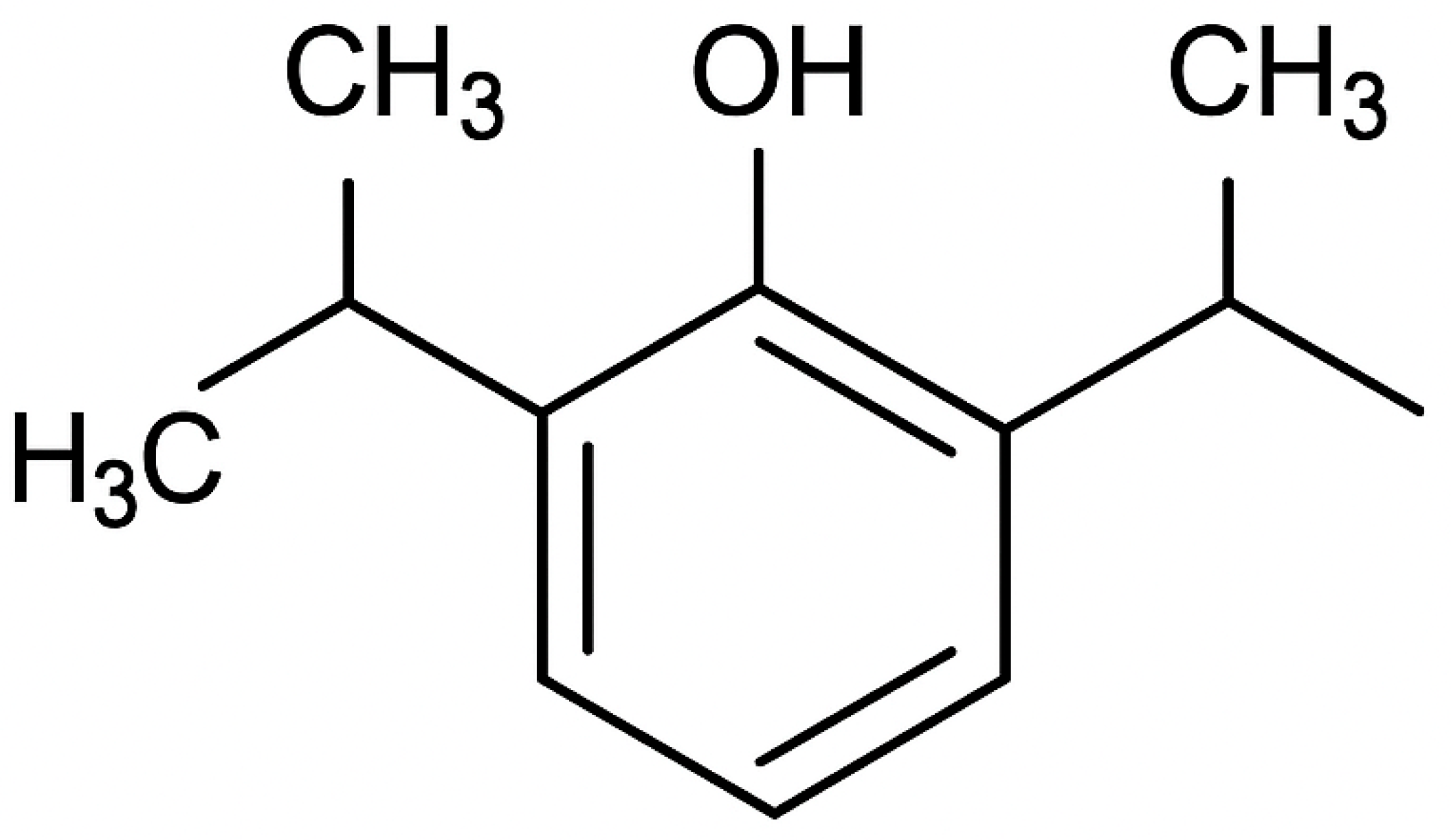
2.3. Propofol Induction Dose and Post-Induction Hemodynamic Fluctuations
2.4. Statistical Analysis and Model Training and Simulation
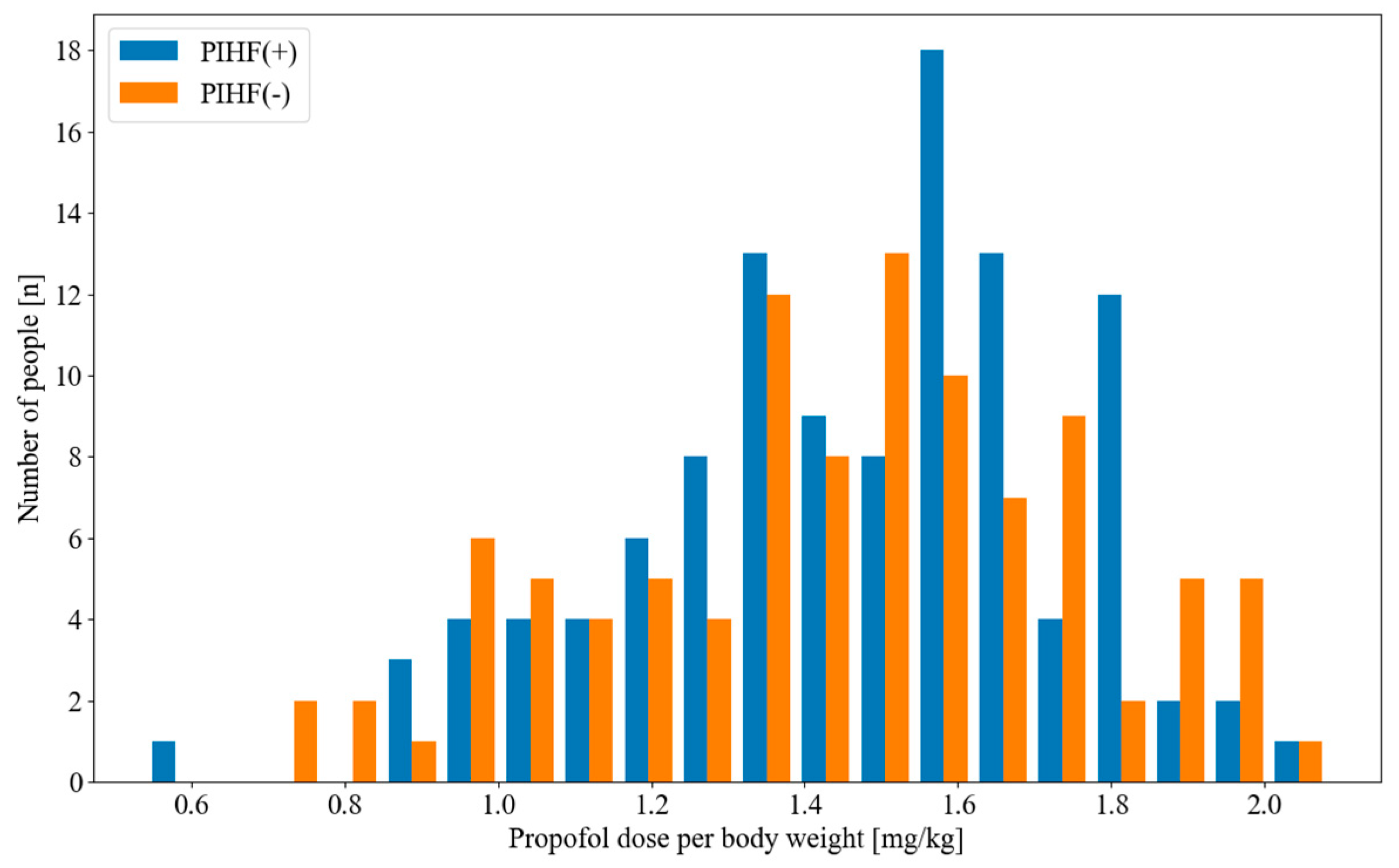
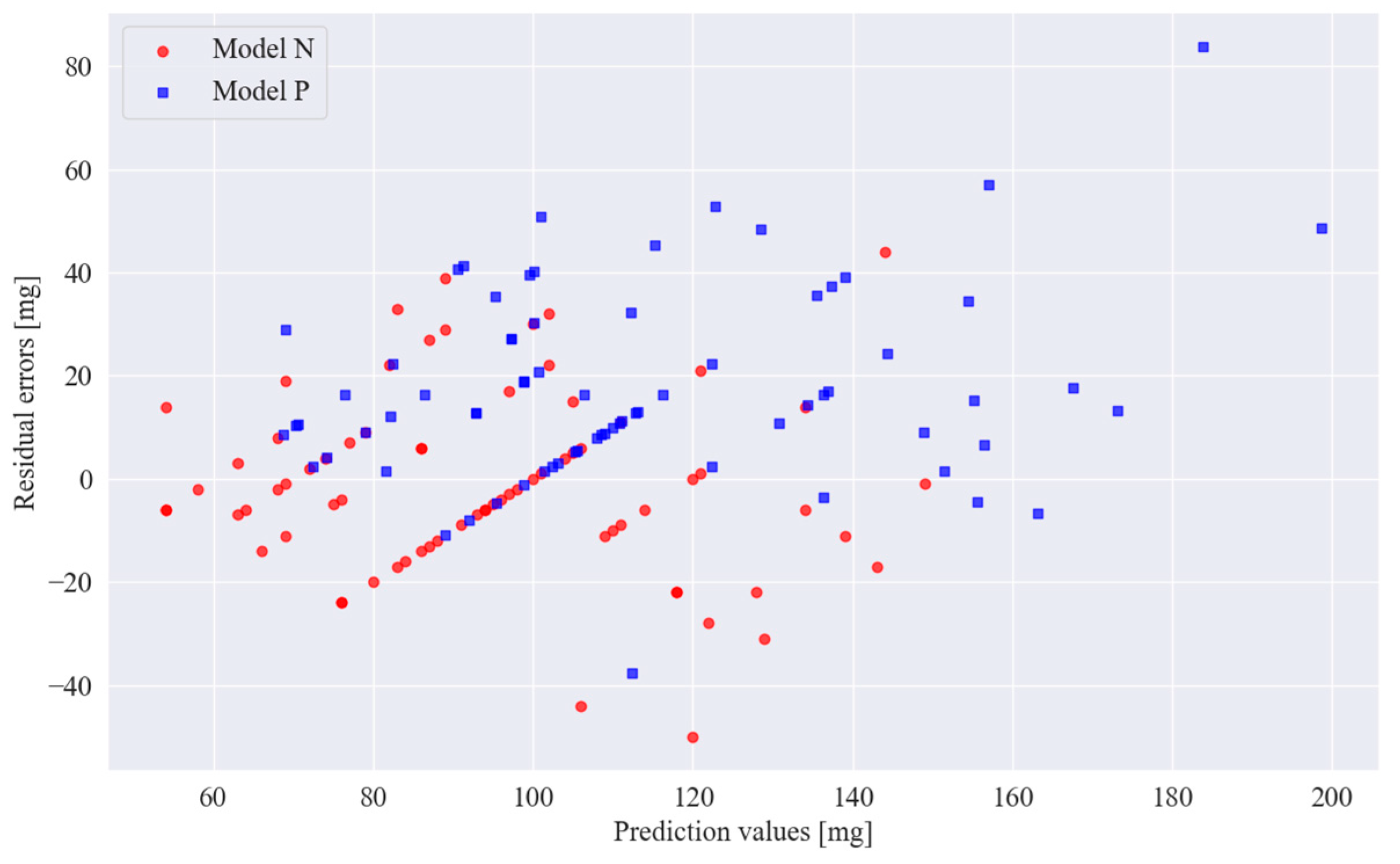
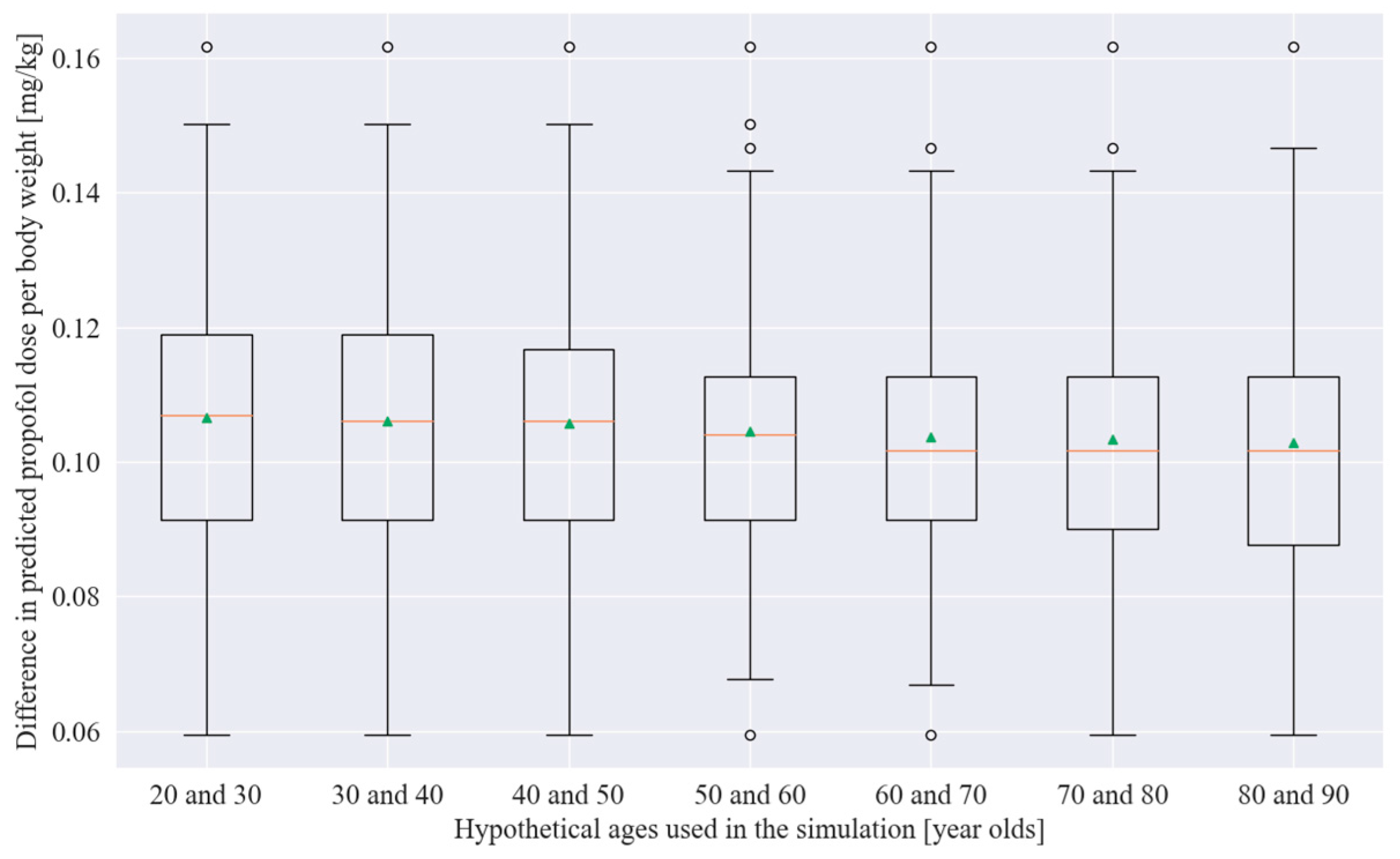
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RMSE | Root mean squared error |
| MAE | Mean absolute error |
| IRB | Institutional review board |
| PIHF | Post-induction hemodynamic fluctuations |
| SBP | Systolic blood pressure |
| MAP | Mean arterial blood pressure |
| ASA-PS | American Society of Anesthesiologists physical status |
| DBP | Diastolic blood pressure |
| HR | Heart rate |
| CIs | Confidence intervals |
| TCI | Target-controlled infusion |
References
- Degtyarenko, K.; de Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcántara, R.; Darsow, M.; Guedj, M.; Ashburner, M. ChEBI: A Database and Ontology for Chemical Entities of Biological Interest. Nucleic Acids Res. 2008, 36, D344–D350. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Altomare, C.; Liso, G.; Sanna, E.; Biggio, G. Propofol in Anesthesia. Mechanism of Action, Structure-Activity Relationships, and Drug Delivery. Curr. Med. Chem. 2000, 7, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.C.; McLeskey, C.H.; Nahrwold, M.L.; Roizen, M.F.; Stanley, T.H.; Thisted, R.A.; Walawander, C.A.; White, P.F.; Apfelbaum, J.L.; Grasela, T.H. Hemodynamic Effects of Propofol: Data from over 25,000 Patients. Anesth. Analg. 1993, 77, S21–S29. [Google Scholar] [PubMed]
- Hojo, T.; Kimura, Y.; Shibuya, M.; Fujisawa, T. Predictors of Hypotension during Anesthesia Induction in Patients with Hypertension on Medication: A Retrospective Observational Study. BMC Anesthesiol. 2022, 22, 343. [Google Scholar] [CrossRef]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.M.; Kalkman, C.J.; Kappelle, L.J.; van Klei, W.A. Intraoperative Hypotension and Perioperative Ischemic Stroke after General Surgery: A Nested Case-Control Study. Anesthesiology 2012, 116, 658–664. [Google Scholar] [CrossRef]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery: Toward an Empirical Definition of Hypotension. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.T.J.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between Intraoperative Hypotension and Hypertension and 30-Day Postoperative Mortality in Noncardiac Surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Shafer, S.L.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Youngs, E.J. The Influence of Age on Propofol Pharmacodynamics. Anesthesiology 1999, 90, 1502–1516. [Google Scholar] [CrossRef]
- Saugel, B.; Bebert, E.-J.; Briesenick, L.; Hoppe, P.; Greiwe, G.; Yang, D.; Ma, C.; Mascha, E.J.; Sessler, D.I.; Rogge, D.E. Mechanisms Contributing to Hypotension after Anesthetic Induction with Sufentanil, Propofol, and Rocuronium: A Prospective Observational Study. J. Clin. Monit. Comput. 2022, 36, 341–347. [Google Scholar] [CrossRef]
- Schonberger, R.B.; Dai, F.; Michel, G.; Vaughn, M.T.; Burg, M.M.; Mathis, M.; Kheterpal, S.; Akhtar, S.; Shah, N.; Bardia, A. Association of Propofol Induction Dose and Severe Pre-Incision Hypotension among Surgical Patients over Age 65. J. Clin. Anesth. 2022, 80, 110846. [Google Scholar] [CrossRef]
- Shafer, S.L. The Pharmacology of Anesthetic Drugs in Elderly Patients. Anesthesiol. Clin. N. Am. 2000, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Kiyohara, C.; Tokunaga, S.; Hoka, S. Prediction of Hemodynamic Fluctuations after Induction of General Anesthesia Using Propofol in Non-Cardiac Surgery: A Retrospective Cohort Study. BMC Anesthesiol. 2018, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Hirata, K.; Fujita, N.; Watabe, A.; Morimoto, Y. Quantification of the Relationship between Aging and Propofol Induction Dose Using Neural Network Model Simulation: A Single-Center Retrospective Observational Study. In Proceedings of the 71st Annual Meeting of the Japanese Society of Anesthesiologists, Kobe, Japan, 6–8 June 2024. [Google Scholar]
- Goldman, L.; Caldera, D.L.; Southwick, F.S.; Nussbaum, S.R.; Murray, B.; O’Malley, T.A.; Goroll, A.H.; Caplan, C.H.; Nolan, J.; Burke, D.S.; et al. Cardiac Risk Factors and Complications in Non-Cardiac Surgery. Medicine 1978, 57, 357–370. [Google Scholar] [CrossRef]
- Reich, D.L.; Hossain, S.; Krol, M.; Baez, B.; Patel, P.; Bernstein, A.; Bodian, C.A. Predictors of Hypotension after Induction of General Anesthesia. Anesth. Analg. 2005, 101, 622–628. [Google Scholar] [CrossRef]
- Südfeld, S.; Brechnitz, S.; Wagner, J.Y.; Reese, P.C.; Pinnschmidt, H.O.; Reuter, D.A.; Saugel, B. Post-Induction Hypotension and Early Intraoperative Hypotension Associated with General Anaesthesia. Br. J. Anaesth. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Jor, O.; Maca, J.; Koutna, J.; Gemrotova, M.; Vymazal, T.; Litschmannova, M.; Sevcik, P.; Reimer, P.; Mikulova, V.; Trlicova, M.; et al. Hypotension after Induction of General Anesthesia: Occurrence, Risk Factors, and Therapy. A Prospective Multicentre Observational Study. J. Anesth. 2018, 32, 673–680. [Google Scholar] [CrossRef]
- Phillips, A.T.; Deiner, S.; Mo Lin, H.; Andreopoulos, E.; Silverstein, J.; Levin, M.A. Propofol Use in the Elderly Population: Prevalence of Overdose and Association With 30-Day Mortality. Clin. Ther. 2015, 37, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Michel, G.; Zhou, B.; Dai, F.; Akhtar, S.; Schonberger, R.B. An Analysis of Anesthesia Induction Dosing in Female Older Adults. Drugs Aging 2020, 37, 435–446. [Google Scholar] [CrossRef]
- Yildirim, S.A.; Dogan, L.; Sarikaya, Z.T.; Ulugol, H.; Gucyetmez, B.; Toraman, F. Hypotension after Anesthesia Induction: Target-Controlled Infusion Versus Manual Anesthesia Induction of Propofol. J. Clin. Med. 2023, 12, 5280. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, T.; Lu, G.; Luo, H.; Lai, Z.; Zhang, L. Efficacy and Safety of Remimazolam Compared with Propofol in Hypertensive Patients Undergoing Breast Cancer Surgery: A Single-Center, Randomized, Controlled Study. BMC Anesthesiol. 2023, 23, 409. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, A.; Sethi, A.; Pathania, J. Diagnostic Accuracy of Left Ventricular Outflow Tract Velocity Time Integral versus Inferior Vena Cava Collapsibility Index in Predicting Post-Induction Hypotension during General Anesthesia: An Observational Study. Acute Crit. Care 2024, 39, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.M.; Wahdan, R.A.; Salah, A.A.A.; Elnakera, A.M. Inferior Vena Cava Collapsibility Index as a Predictor of Hypotension after Induction of General Anesthesia in Hypertensive Patients. BMC Anesthesiol. 2023, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Tarao, K.; Daimon, M.; Son, K.; Nakanishi, K.; Nakao, T.; Suwazono, Y.; Isono, S. Risk Factors Including Preoperative Echocardiographic Parameters for Post-Induction Hypotension in General Anesthesia. J. Cardiol. 2021, 78, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Qiao, N.; Pan, Y.; Simayi, A.; Chen, N. The Appropriate Dose of Propofol for Anesthesia Induction in Morbidly Obese Patients. Ann. Palliat. Med. 2020, 9, 1921–1927. [Google Scholar] [CrossRef]
- Iwakiri, H.; Nishihara, N.; Nagata, O.; Matsukawa, T.; Ozaki, M.; Sessler, D.I. Individual Effect-Site Concentrations of Propofol Are Similar at Loss of Consciousness and at Awakening. Anesth. Analg. 2005, 100, 107–110. [Google Scholar] [CrossRef]



| Variables | |
|---|---|
| Age (years), mean (range) | 63.8 (18–95) |
| Female sex, n (%) | 209 (57.3) |
| Height (cm), mean (95% CI) | 160.4 (159.4–161.4) |
| Weight (kg), mean (95% CI) | 61.7 (60.4–63.1) |
| ASA-PS, n (%) | |
| 1 | 54 (14.8) |
| 2 | 265 (72.6) |
| 3 | 45 (12.3) |
| SBP before induction (mmHg), mean (range) | 139.5 (79–203) |
| DBP before induction (mmHg), mean (range) | 76.8 (45–121) |
| HR before induction (bpm), mean (range) | 73.5 (34–144) |
| Induction propofol dose (mg), mean (range) | 98.5 (20–200) |
| Induction fentanyl dose (μg), mean (range) | 93.4 (0–200) |
| Induction remifentanil dose (μg/kg/min), mean (range) | 0.2 (0.0–0.5) |
| PIHF(+) | PIHF (−) | |
|---|---|---|
| Residual error < 5 mg | 9 | 7 |
| Residual error ≥ 5 mg | 36 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Hirata, K.; Noriaki, F.; Watabe, A.; Morimoto, Y. The Relationship Between Age and the Propofol Dose for Anesthesia Induction: A Single-Center Retrospective Study Utilizing Neural Network Model Simulation. Appl. Sci. 2025, 15, 6052. https://doi.org/10.3390/app15116052
Nishimura K, Hirata K, Noriaki F, Watabe A, Morimoto Y. The Relationship Between Age and the Propofol Dose for Anesthesia Induction: A Single-Center Retrospective Study Utilizing Neural Network Model Simulation. Applied Sciences. 2025; 15(11):6052. https://doi.org/10.3390/app15116052
Chicago/Turabian StyleNishimura, Kazuki, Kenji Hirata, Fujita Noriaki, Akira Watabe, and Yuji Morimoto. 2025. "The Relationship Between Age and the Propofol Dose for Anesthesia Induction: A Single-Center Retrospective Study Utilizing Neural Network Model Simulation" Applied Sciences 15, no. 11: 6052. https://doi.org/10.3390/app15116052
APA StyleNishimura, K., Hirata, K., Noriaki, F., Watabe, A., & Morimoto, Y. (2025). The Relationship Between Age and the Propofol Dose for Anesthesia Induction: A Single-Center Retrospective Study Utilizing Neural Network Model Simulation. Applied Sciences, 15(11), 6052. https://doi.org/10.3390/app15116052






