UV-Induced Aging in Thermochromic Pigment-Integrated Food-Grade Polymers: A Performance Assessment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Thermochromic Polyproplyene Blends
2.3. Injection Molding
2.4. UV Exposure Protocol
2.5. ATR-FTIR Analysis (Pre-Exposure)
2.6. Visual Inspection of the Surface Area
2.7. Color Stability Analysis (CIE L*a*b*)
2.8. Tensile Property (Post-UV Exposure)
2.9. Impact Strength Testing (Charpy Method)
2.10. Mass Variation Measurement
2.11. Statistical Analyses
3. Results
3.1. ATR-FTIR Spectral Characterization (Pre-Exposure)
3.2. Visual Property Results
3.3. Color Stability
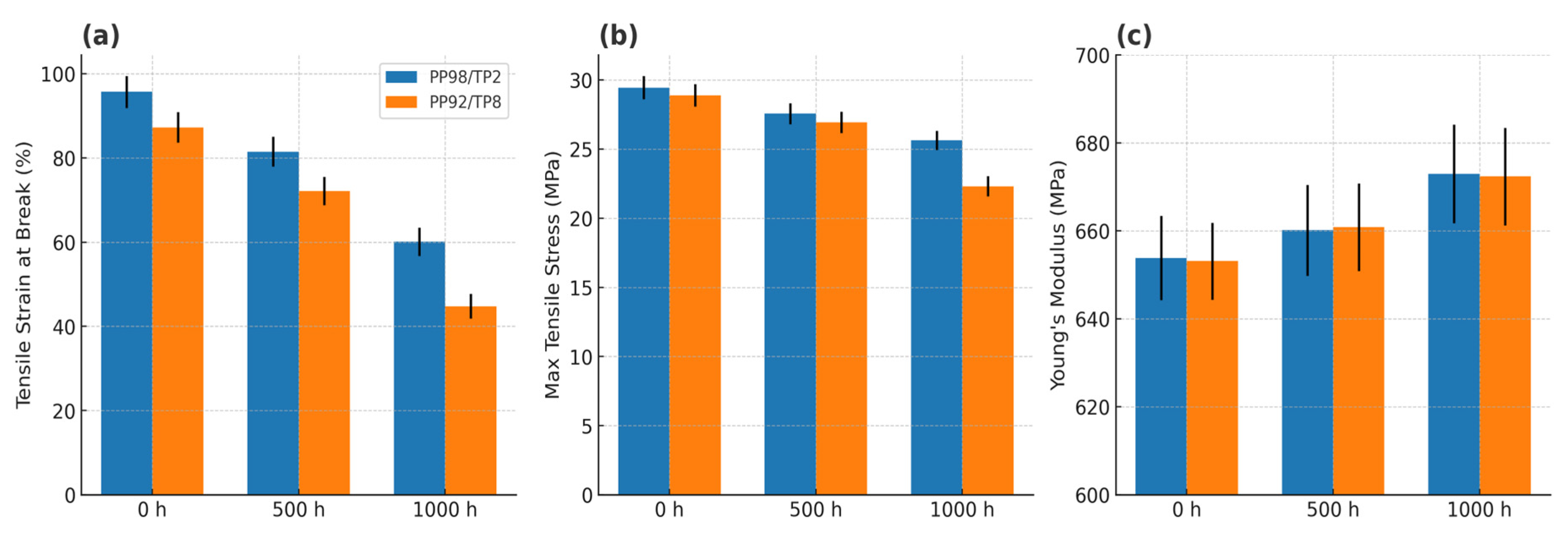
3.4. Tensile Properties After UV Exposure
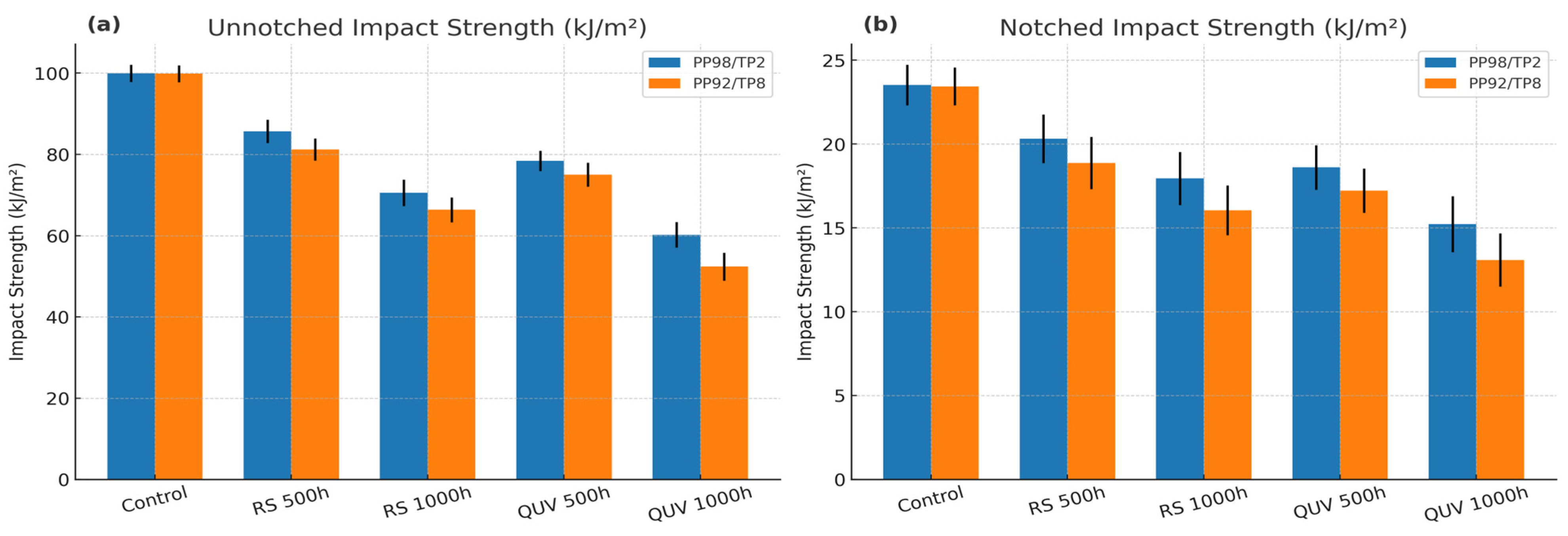
3.5. Impact Strength After UV Exposure
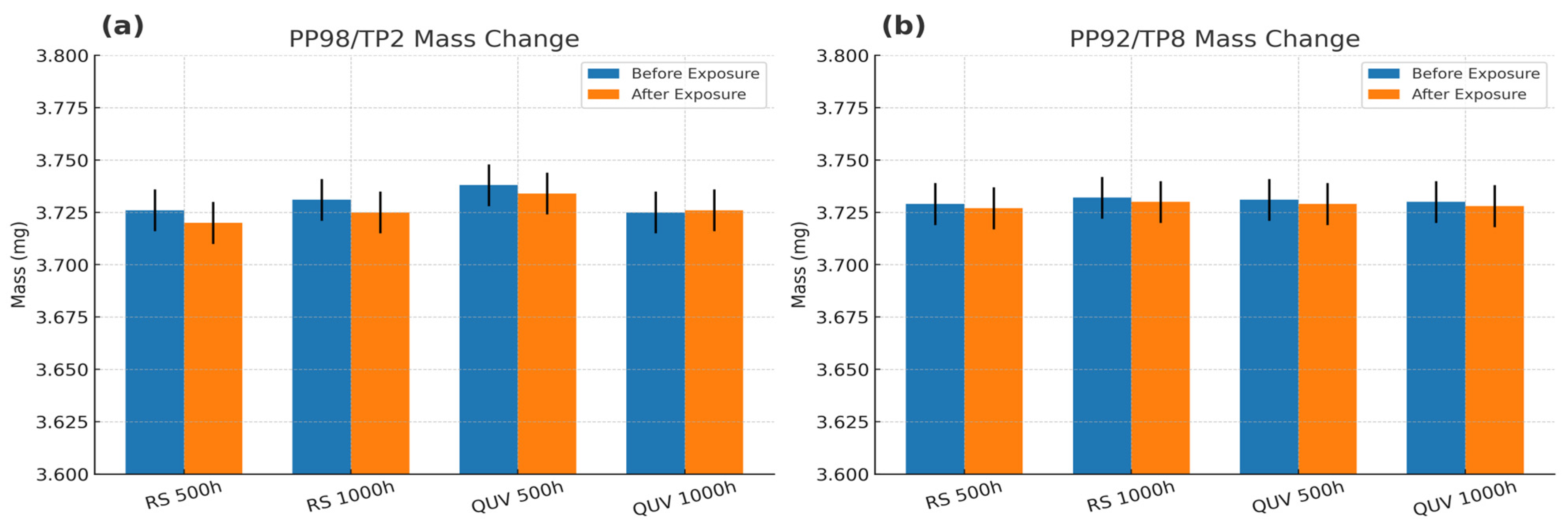
3.6. Mass Change After UV Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, V.; Srivastava, S.K. The Safety–Quality Dominant View of Food Chain Integrity: Implications for Consumer-centric Food Chain Governance. Int. J. Manag. Rev. 2022, 24, 3–24. [Google Scholar] [CrossRef]
- Saha, N.C. Food Packaging: Concepts and Its Significance. In Food Packaging: Materials, Techniques and Environmental Issues; Saha, N.C., Ghosh, A.K., Garg, M., Sadhu, S.D., Eds.; Springer Nature: Singapore, 2022; pp. 1–45. ISBN 978-981-16-4233-3. [Google Scholar]
- Ibrahim, I.D.; Hamam, Y.; Sadiku, E.R.; Ndambuki, J.M.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Snyman, J. Need for Sustainable Packaging: An Overview. Polymers 2022, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; He, J.; Li, W.; Deng, J.; Lin, Q. A Review on Takeaway Packaging Waste: Types, Ecological Impact, and Disposal Route. Environ. Pollut. 2023, 337, 122518. [Google Scholar] [CrossRef]
- Fasake, V.; Shelake, P.S.; Srivastava, A.; Dashora, K. Characteristics of Different Plastic Materials, Properties and Their Role in Food Packaging. Curr. Nutr. Food Sci. 2021, 17, 944–954. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. Chapter 4—Plastic Waste in a Circular Economy. In Environmental Materials and Waste, 2nd ed.; Vara Prasad, M.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 99–134. ISBN 978-0-443-22069-2. [Google Scholar]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability Governance and Contested Plastic Food Packaging—An Integrative Review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Behera, A. Chromogenic Materials. In Advanced Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 157–191. ISBN 978-3-030-80358-2. [Google Scholar]
- Hakami, A.; Srinivasan, S.S.; Biswas, P.K.; Krishnegowda, A.; Wallen, S.L.; Stefanakos, E.K. Review on Thermochromic Materials: Development, Characterization, and Applications. J. Coat. Technol. Res. 2022, 19, 377–402. [Google Scholar] [CrossRef]
- Breheny, C.; Donlon, K.; Harrington, A.; Colbert, D.M.; Bezerra, G.S.N.; Geever, L.M. Thermochromic Polymers in Food Packaging: A Comprehensive Systematic Review and Patent Landscape Analysis. Coatings 2024, 14, 1252. [Google Scholar] [CrossRef]
- Kavuncuoglu, H. Packaging Aspects of Food Analogues. In Food Analogues: Emerging Methods and Challenges; Can, Ö.P., Göksel Saraç, M., Aslan Türker, D., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 437–463. ISBN 978-3-031-69872-9. [Google Scholar]
- Gao, T.; Sun, D.-W.; Tian, Y.; Zhu, Z. Gold–Silver Core-Shell Nanorods Based Time-Temperature Indicator for Quality Monitoring of Pasteurized Milk in the Cold Chain. J. Food Eng. 2021, 306, 110624. [Google Scholar] [CrossRef]
- Albrecht, A.; Ibald, R.; Raab, V.; Reichstein, W.; Haarer, D.; Kreyenschmidt, J. Implementation of Time Temperature Indicators to Improve Temperature Monitoring and Support Dynamic Shelf Life in Meat Supply Chains. J. Package Technol. Res. 2020, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Salim, S.A.; Hasanvand, S.; Assadpour, E.; Azizi-Lalabadi, M.; Prieto, M.A.; Jafari, S.M. Application of Smart Packaging for Seafood: A Comprehensive Review. Comp. Rev. Food Sci. Food Safe 2023, 22, 1438–1461. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.M.; Chang, Y.S. Cold Chain Monitoring Tools. In Cold Chain Management; Springer Series in Advanced Manufacturing; Springer International Publishing: Cham, Switzerland, 2023; pp. 77–91. ISBN 978-3-031-09565-8. [Google Scholar]
- Supian, A.B.M.; Asyraf, M.R.M.; Syamsir, A.; Najeeb, M.I.; Alhayek, A.; Al-Dala’ien, R.N.; Manar, G.; Atiqah, A. Thermochromic Polymer Nanocomposites for the Heat Detection System: Recent Progress on Properties, Applications, and Challenges. Polymers 2024, 16, 1545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. [Google Scholar] [CrossRef]
- Jamnicki Hanzer, S.; Kulčar, R.; Vukoje, M.; Marošević Dolovski, A. Assessment of Thermochromic Packaging Prints’ Resistance to UV Radiation and Various Chemical Agents. Polymers 2023, 15, 1208. [Google Scholar] [CrossRef]
- Thamrin, E.S.; Warsiki, E.; Bindar, Y.; Kartika, I.A. Thermochromic Ink as a Smart Indicator on Cold Product Packaging—Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012021. [Google Scholar] [CrossRef]
- Nagare, S.M.; Hakami, A.; Biswas, P.K.; Stefanakos, E.K.; Srinivasan, S.S. A Review of Thermochromic Materials for Coating Applications: Production, Protection, and Degradation of Organic Thermochromic Materials. J. Coat. Technol. Res. 2025, 22, 91–115. [Google Scholar] [CrossRef]
- Sadoh, A.; Hossain, S.; Ravindra, N.M. Thermochromic Polymeric Films for Applications in Active Intelligent Packaging—An Overview. Micromachines 2021, 12, 1193. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Duan, H.; Wu, C.; Zhang, S. Long-Term Photo Oxidation Aging Investigation of Temperature-Regulating Bitumen Based on Thermochromic Principle. Fuel 2021, 286, 119403. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Y.; He, M.; Huang, L.; Wang, X.; Yu, J. Thermochromic Paint with Three-Stage Colour Transition Effect Based on Thermochromic Microcapsules and Its Crosslinking Modification. Color. Technol. 2025. [Google Scholar] [CrossRef]
- Vanzetto, A.B.; Neves, G.; Alves, T.P.; Moura, J.H.; de Bortoli, B.F.; Santana, L.; Polkowski, R.D.d.O. A Systematic Review on Smart Coatings for Automotive Applications; SAE International: Warrendale, PA, USA, 2024. [Google Scholar]
- Aldas, M.; Paladines, A.; Valle, V.; Pazmiño, M.; Quiroz, F. Effect of the Prodegradant-Additive Plastics Incorporated on the Polyethylene Recycling. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- European Parliament and Council Regulation (EC). No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food; European Parliament and Council Regulation (EC): Strasbourg, France, 2004. [Google Scholar]
- European Commission Commission Regulation (EU). No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; European Commission Commission Regulation (EU): Brussels, Belgium, 2011. [Google Scholar]
- Conradie, W.; Dorfling, C.; Chimphango, A.; Booth, A.M.; Sørensen, L.; Akdogan, G. Investigating the Physicochemical Property Changes of Plastic Packaging Exposed to UV Irradiation and Different Aqueous Environments. Microplastics 2022, 1, 456–476. [Google Scholar] [CrossRef]
- Doğan, M. Ultraviolet Light Accelerates the Degradation of Polyethylene Plastics. Microsc. Res. Tech. 2021, 84, 2774–2783. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-Based UV Protection Functional Films for Food Packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D.J. Development of Sustainable UV-Screening Food Packaging Materials: A Review of Recent Advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of Objects, Products, and Packaging Surfaces and Their Characterization in Different Fields of Industry: The Status in 2020. Phys. Status Solidi A 2021, 218, 2000732. [Google Scholar] [CrossRef]
- Finardi, S.; Hoffmann, T.G.; Schmitz, F.R.W.; Bertoli, S.L.; Khayrullin, M.; Neverova, O.; Ponomarev, E.; Goncharov, A.; Kulmakova, N.; Dotsenko, E.; et al. Comprehensive Study of Light-Emitting Diodes (LEDs) and Ultraviolet-LED Lights Application in Food Quality and Safety. J. Pure Appl. Microbiol. 2021, 15, 1125–1135. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-Blocking, and High Barrier Cellulose-Based Bioplastics with Naringin as Active Food Packaging Materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Patil, A.S.; Nille, O.S.; Kolekar, G.B.; Sohn, D.; Gore, A.H. UV-Shielding Biopolymer@Nanocomposites for Sustainable Packaging Applications. In Sustainable Packaging; Muthu, S.S., Ed.; Environmental Footprints and Eco-design of Products and Processes; Springer: Singapore, 2021; pp. 177–202. ISBN 978-981-16-4608-9. [Google Scholar]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Blatchley, E.R. Photochemical Reactors: Theory, Methods, and Ultraviolet Radiation Applications; Wiley: Hoboken, NJ, USA, 2022; ISBN 978-1-119-87135-4. [Google Scholar]
- Korkut, G.O.; Gunes, G. Ultraviolet Light Technology: Applications for Fresh Produce. In Handbook of Research on Food Processing and Preservation Technologies; Apple Academic Press: Ontario, CA, USA, 2021; ISBN 978-1-003-15322-1. [Google Scholar]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, S.A.; Alshawish, N.K.; Abdalla, Y.K.; Ibrahim, M.K. Ultraviolet Radiation: Health Risks and Benefits. Saudi J. Eng. Technol. 2022, 7, 533–541. [Google Scholar] [CrossRef]
- Breheny, C.; Colbert, D.M.; Bezerra, G.; Geever, J.; Geever, L.M. Towards Sustainable Food Packaging: Mechanical Recycling Effects on Thermochromic Polymers Performance. Polymers 2025, 17, 1042. [Google Scholar] [CrossRef]
- Breheny, C.; Geever, L.M.; Colbert, D.M.; Bezerra, G.; Geever, J. Evaluating the Chemical Resistance and Performance of Thermochromic Polymers for Food Packaging. Materials 2025, 18, 2085. [Google Scholar] [CrossRef] [PubMed]
- Crosby, P.H.N.; Netravali, A.N. Green Thermochromic Materials: A Brief Review. Adv. Sustain. Syst. 2022, 6, 2200208. [Google Scholar] [CrossRef]
- Sørensen, L.; Groven, A.S.; Hovsbakken, I.A.; Del Puerto, O.; Krause, D.F.; Sarno, A.; Booth, A.M. UV Degradation of Natural and Synthetic Microfibers Causes Fragmentation and Release of Polymer Degradation Products and Chemical Additives. Sci. Total Environ. 2021, 755, 143170. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and Fragmentation of Plastics in a Changing Environment; from UV-Radiation to Biological Degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Al-Khazrajy, O.S.A.; Abdallh, M.; Alanazi, S.A. Modifications of Polymers through the Addition of Ultraviolet Absorbers to Reduce the Aging Effect of Accelerated and Natural Irradiation. Polymers 2021, 14, 20. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Gao, Y.; Tang, Y. Sustainable Developments in Polyolefin Chemistry: Progress, Challenges, and Outlook. Prog. Polym. Sci. 2023, 143, 101713. [Google Scholar] [CrossRef]
- Pospíšil, P.; Prasad, A.; Rác, M. Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Papac Zjačić, J.; Vujasinović, M.; Kovačić, M.; Lončarić Božić, A.; Kušić, H.; Katančić, Z.; Hrnjak-Murgić, Z. From Macro to Micro Plastics; Influence of Photo-Oxidative Degradation. Kem. U Ind. 2023, 72, 463–471. [Google Scholar] [CrossRef]
- Pfaff, G. (Ed.) Antraquinonoid Pigments—Color Fundamentals; De Gruyter: Berlin, Germany, 2022; ISBN 978-3-11-058807-1. [Google Scholar]
- Anwer, M.B.; Al-Mashhadani, M.H.; Alsayed, R.; Mohammed, A.H.; Alshareef, S.A.; Alhuwaymil, Z.; Alyami, M.S.S.; Yousif, E. Optimizing PVC Photostability and UV Blocking Capability through Nanoparticles Incorporation: A Comprehensive Review. J. Vinyl Addit. Technol. 2025, 31, 259–278. [Google Scholar] [CrossRef]
- ISO 179-1; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. International Organization for Standardization ISO: Geneva, Switzerland, 2023.
- ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization ISO: Geneva, Switzerland, 2012.
- Farooque, R.; Asjad, M.; Rizvi, S.J.A. A Current State of Art Applied to Injection Moulding Manufacturing Process—A Review. Mater. Today Proc. 2021, 43, 441–446. [Google Scholar] [CrossRef]
- ISO 294-1; Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 1: General Principles, and Moulding of Multipurpose and Bar Test Specimens. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Silva, M.R.F.; Alves, M.F.R.P.; Cunha, J.P.G.Q.; Costa, J.L.; Silva, C.A.; Fernandes, M.H.V.; Vilarinho, P.M.; Ferreira, P. Nanostructured Transparent Solutions for UV-Shielding: Recent Developments and Future Challenges. Mater. Today Phys. 2023, 35, 101131. [Google Scholar] [CrossRef]
- ISO 4892-3; Plastics—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps. International Organization for Standardization: Geneva, Switzerland, 2024.
- Q-Lab Corporation. QUV & Q-SUN: Which Is Right for You? Q-Lab Corporation: Westlake, OH, USA, 2006. [Google Scholar]
- Kaur, H.; Rana, B.; Tomar, D.; Kaur, S.; Jena, K.C. Fundamentals of ATR-FTIR Spectroscopy and Its Role for Probing In-Situ Molecular-Level Interactions. In Modern Techniques of Spectroscopy; Singh, D.K., Pradhan, M., Materny, A., Eds.; Progress in Optical Science and Photonics; Springer: Singapore, 2021; Volume 13, pp. 3–37. ISBN 978-981-336-083-9. [Google Scholar]
- Nandiyanto, A.B.D.; Ragadhita, R.; Fiandini, M. Interpretation of Fourier Transform Infrared Spectra (FTIR): A Practical Approach in the Polymer/Plastic Thermal Decomposition. Indones. J. Sci. Technol. 2022, 8, 113–126. [Google Scholar] [CrossRef]
- Siddique, I. Exploring Functional Groups and Molecular Structures: A Comprehensive Analysis Using FTIR Spectroscopy. SSRN J. 2024, 1, 2. [Google Scholar] [CrossRef]
- Fiore, T.; Pellerito, C. Infrared Absorption Spectroscopy. In Spectroscopy for Materials Characterization; Agnello, S., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 129–167. ISBN 978-1-119-69732-9. [Google Scholar]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space. International Organization for Standardization: Geneva, Switzerland, 2019.
- Gdoutos, E.; Konsta-Gdoutos, M. Tensile Testing. In Mechanical Testing of Materials; Gdoutos, E., Konsta-Gdoutos, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–34. ISBN 978-3-031-45990-0. [Google Scholar]
- Zainuddin, H.; Ali, M.B.; Zakaria, K.A.; Paijan, L.H.; Mamat, M.F.; Abu Bakar, M.H. Investigation of Impact Properties under Instrumented Charpy Test. J. Eng. Technol. Sci. 2024, 56, 329–339. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint Analysis of FTIR Spectra of Polymers Containing Vinyl Acetate. Dyna 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Randhawa, K.S. Polymer Composites with Advanced Pigments: Enhancing Properties and Applications. Pigment. Resin Technol. 2025, 54, 620–636. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers II: Polyethylene. Spectroscopy 2021, 36, 24–29. [Google Scholar] [CrossRef]
- Kotnarowska, D. Influence of Ageing with UV Radiation on Physicochemical Properties of Acrylic-Polyurethane Coatings. J. Surf. Eng. Mater. Adv. Technol. 2018, 8, 95–109. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV Degradation Model for Polymers and Polymer Matrix Composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Hedir, A.; Slimani, F.; Moudoud, M.; Lamrous, O.; Touam, T.; Teguar, M.; Haddad, A.; Durmuş, A. Quantifying Time-Dependent Structural and Mechanical Properties of UV-Aged LDPE Power Cables Insulations. Turk. J. Chem. 2022, 46, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M.; Öztaş, A.; Mutlu, R. Assessing The Relationship Between Color Change and Tensile Strength In Thermoplastic Polyolefin Outer Sheaths of Low-Voltage Power Cables. Trak. Üniversitesi Mühendislik Bilim. Derg. 2024, 25, 11–19. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Fang, C.; Chen, J.; Wang, Z. Discoloration Mechanism, Structures and Recent Applications of Thermochromic Materials via Different Methods: A Review. J. Mater. Sci. Technol. 2018, 34, 2225–2234. [Google Scholar] [CrossRef]
- Bulderberga, O.; Aniskevich, A. The Effect of UV Exposure on the Service-Life of Thermochromic Microcapsules Integrated into the Epoxy Matrix. Mater. Sci. 2022, 28, 333–339. [Google Scholar] [CrossRef]
- Pecho, O.E.; Martos, J.; Pinto, K.V.A.; Pinto, K.V.A.; Baldissera, R.A. Effect of Hydrogen Peroxide on Color and Whiteness of Resin-based Composites. J. Esthet. Restor. Dent. 2019, 31, 132–139. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of Blueberry Residue Incorporated Cassava Starch Film as pH Indicator in Different Simulants and Foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Kheur, M.; Sethi, T.; Coward, T.; Kakade, D.; Rajkumar, M. Evaluation of the Effect of Ultraviolet Stabilizers on the Change in Color of Pigmented Silicone Elastomer: An in Vitro Study. J. Indian. Prosthodont. Soc. 2016, 16, 276. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Chang, Y.; Lu, R.; Miyakoshi, T. The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface. Polymers 2019, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Korayem, A.-E.; Mohammadi, H.; Dargazany, R. Experimental Investigation on the Performance Loss of Cross-Linked Polymers During Photo-Oxidative Aging. In Proceedings of the Volume 12: Mechanics of Solids, Structures, and Fluids; American Society of Mechanical Engineers, Virtual, 16 November 2020; p. V012T12A002. [Google Scholar]
- Gewert, B.; Plassmann, M.; Sandblom, O.; MacLeod, M. Identification of Chain Scission Products Released to Water by Plastic Exposed to Ultraviolet Light. Environ. Sci. Technol. Lett. 2018, 5, 272–276. [Google Scholar] [CrossRef]
- Erdmann, M.; Niebergall, U.; Wachtendorf, V.; Böhning, M. Evaluation of UV-Induced Embrittlement of PE-HD by Charpy Impact Test. J. Appl. Polym. Sci. 2020, 137, 49069. [Google Scholar] [CrossRef]
- Scida, D.; Alix, S.; Buet, S.; Hadi Saidane, E.; Courmont, F.; Behlouli, K.; Ayad, R. Hygrothermal/UV Aging Effect on Visual Aspect and Mechanical Properties of Non-Woven Natural-Fiber Composites. J. Renew. Mater. 2019, 7, 865–875. [Google Scholar] [CrossRef]
- Varghese, A.M.; Rangaraj, V.M.; Luckachan, G.; Mittal, V. UV Aging Behavior of Functionalized Mullite Nanofiber-Reinforced Polypropylene. ACS Omega 2020, 5, 27083–27093. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-C.; Kim, J.H.; Orski, S.; Fairbrother, A.; Jacobs, D.; Perry, L.; Hunston, D.; White, C.; Sung, L. Micro and Macroscopic Mechanical Behaviors of High-Density Polyethylene under UV Irradiation and Temperature. Polym. Degrad. Stab. 2020, 174, 109098. [Google Scholar] [CrossRef]
- Kuka, E.; Cirule, D.; Andersone, I.; Vasiljevs, L.O.; Merna, J.; Sarakovskis, A.; Kurnosova, N.; Sansonetti, E.; Vevere, L.; Andersons, B. A Step to Microplastic Formation: Microcracking and Associated Surface Transformations of Recycled LDPE, LLDPE, HDPE, and PP Plastics Exposed to UV Radiation. Polym. Degrad. Stab. 2024, 229, 110967. [Google Scholar] [CrossRef]
- De Freitas, A.D.S.M.; Rodrigues, J.S.; Botaro, V.R.; Lemes, A.P.; Cruz, S.A.; Waldman, W.R. Formation of Craze-like Pattern in Polypropylene UV-Induced Surface Cracking. J. Polym. Res. 2022, 29, 506. [Google Scholar] [CrossRef]
- Arencón, D.; Velasco, J.I. Fracture Toughness of Polypropylene-Based Particulate Composites. Materials 2009, 2, 2046–2094. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Mansoor, B.; Ayoub, G.; Colin, X.; Benzerga, A.A. Effect of UV-Aging on the Mechanical and Fracture Behavior of Low Density Polyethylene. Polym. Degrad. Stab. 2020, 180, 109185. [Google Scholar] [CrossRef]
- Cundiff, K.N.; Madi, Y.; Benzerga, A.A. Photo-Oxidation of Semicrystalline Polymers: Damage Nucleation versus Growth. Polymer 2020, 188, 122090. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of UV Degradation and Stabilization; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-1-927885-57-4. [Google Scholar]
- Wypych, A.; Wypych, G. Databook of UV Stabilizers; Elsevier: Amsterdam, The Netherlands, 2025; ISBN 978-1-77467-055-2. [Google Scholar]
- Davand, R.; Rahimpour, M.R.; Hassanajili, S.; Rashedi, R. Theoretical and Experimental Assessment of UV Resistance of high-density Polyethylene: Screening and Optimization of hindered amine light stabilizers. J. Appl. Polym. Sci. 2021, 138, 51262. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Yin, D. Benzotriazole Ultraviolet Stabilizers in the Environment: A Review of Occurrence, Partitioning and Transformation. Sci. Total Environ. 2024, 954, 176362. [Google Scholar] [CrossRef] [PubMed]
- Al-Borno, A.; Van Der Veen, A. Field and Laboratory Studies on the Effect of UV Degradation and UV Protector Paints on Stockpiles of FBE Coated Pipeline. In Proceedings of the Conference 2022; AMPP: San Antonio, TX, USA, 2022; pp. 1–10. [Google Scholar]
- Liu, C.; Mei, C.; Xu, B.; Chen, W.; Yong, C.; Wang, K.; Wu, Q. Light Stabilizers Added to the Shell of Co-Extruded Wood/High-Density Polyethylene Composites to Improve Mechanical and Anti-UV Ageing Properties. R. Soc. Open Sci. 2018, 5, 180074. [Google Scholar] [CrossRef] [PubMed]
- Sabah, A.; Tasleem, S.; Murtaza, M.; Nazir, M.; Rashid, F. Effect of Polymer Capping on Photonic Multi-Core–Shell Quantum Dots CdSe/CdS/ZnS: Impact of Sunlight and Antibacterial Activity. J. Phys. Chem. C 2020, 124, 9009–9020. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Tao, W.; Yang, Y. Preparation of UV Curable Organic/Inorganic Hybrid Coatings-a Review. Prog. Org. Coat. 2020, 145, 105685. [Google Scholar] [CrossRef]
- Chylińska, M.; Kaczmarek, H.; Moszyński, D.; Królikowski, B.; Kowalonek, J. Surface Studies of UV Irradiated Polypropylene Films Modified with Mineral Fillers Designed as Piezoelectric Materials. Polymers 2020, 12, 562. [Google Scholar] [CrossRef]
- Larché, J.-F.; Bussière, P.-O.; Thérias, S.; Gardette, J.-L. Photooxidation of Polymers: Relating Material Properties to Chemical Changes. Polym. Degrad. Stab. 2012, 97, 25–34. [Google Scholar] [CrossRef]
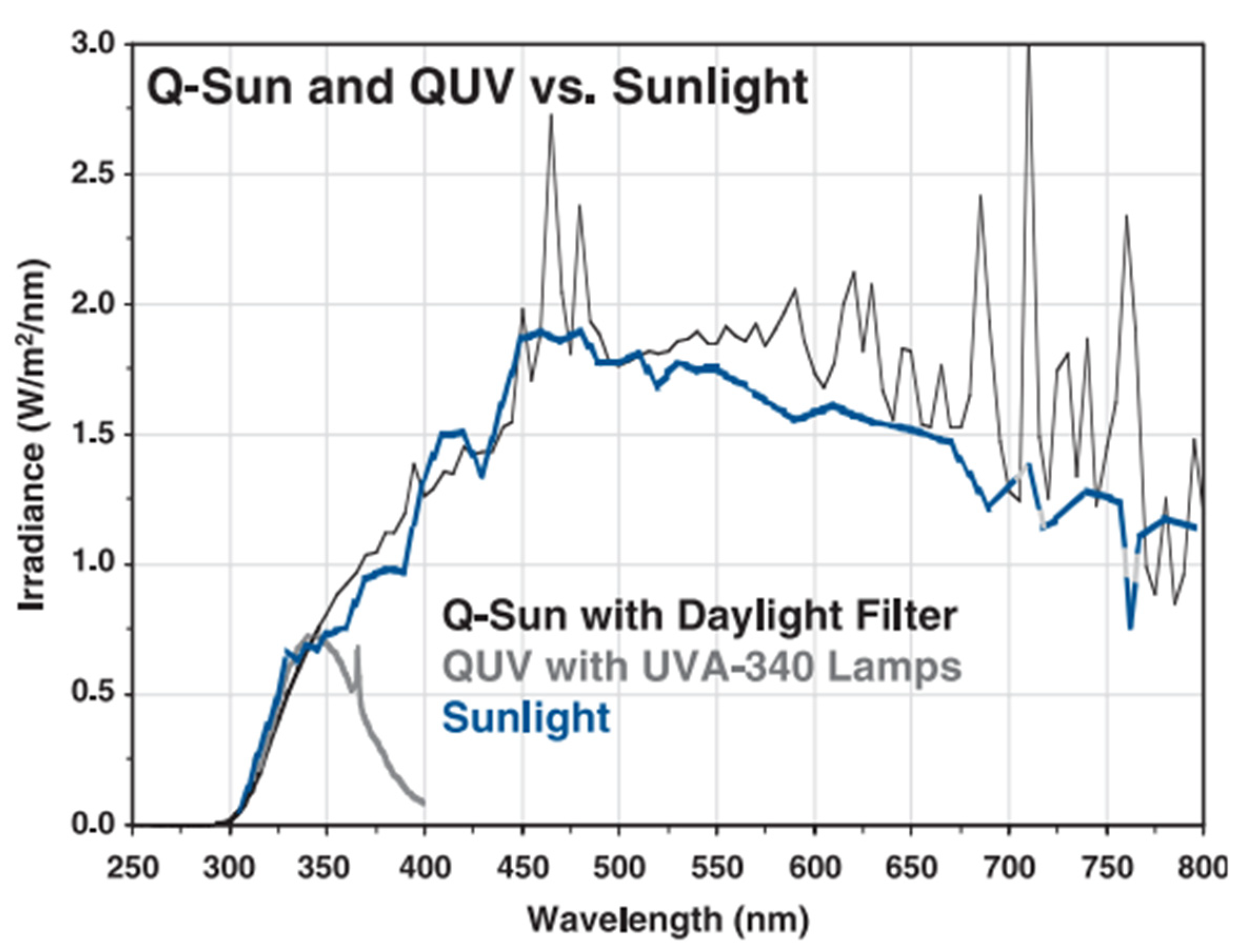
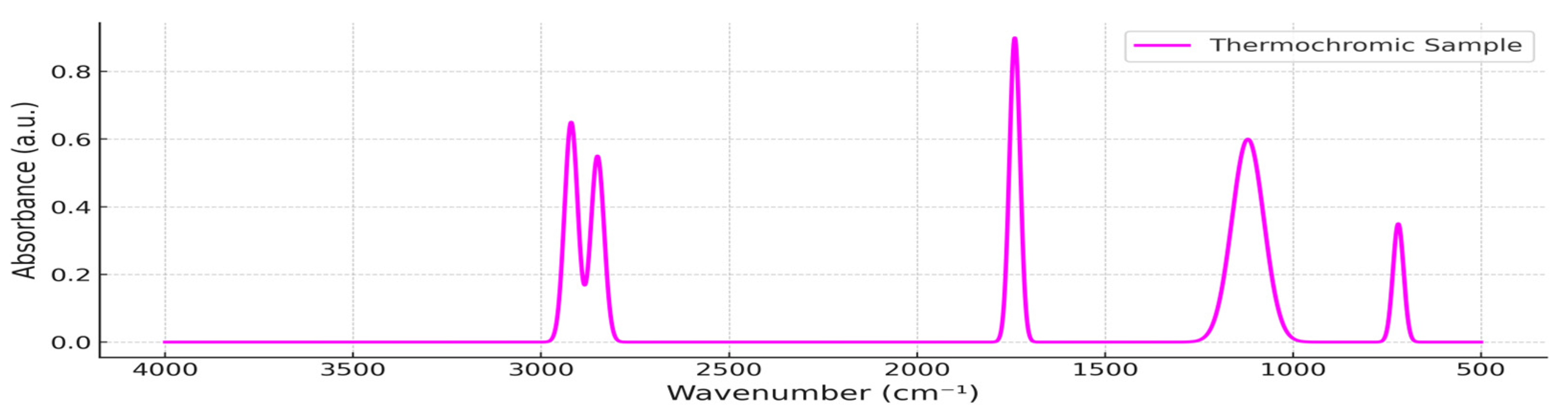
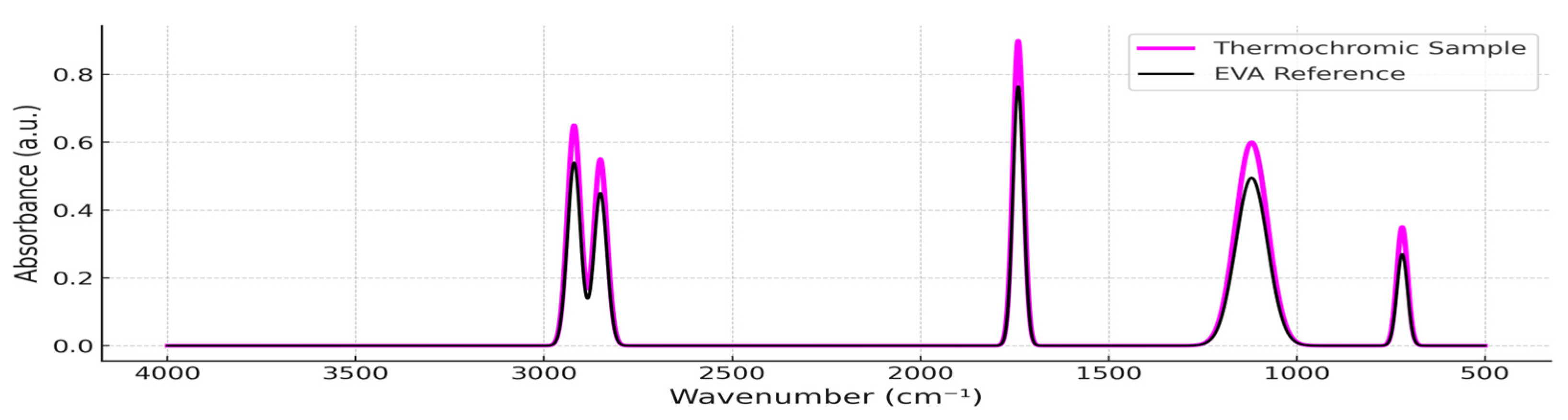
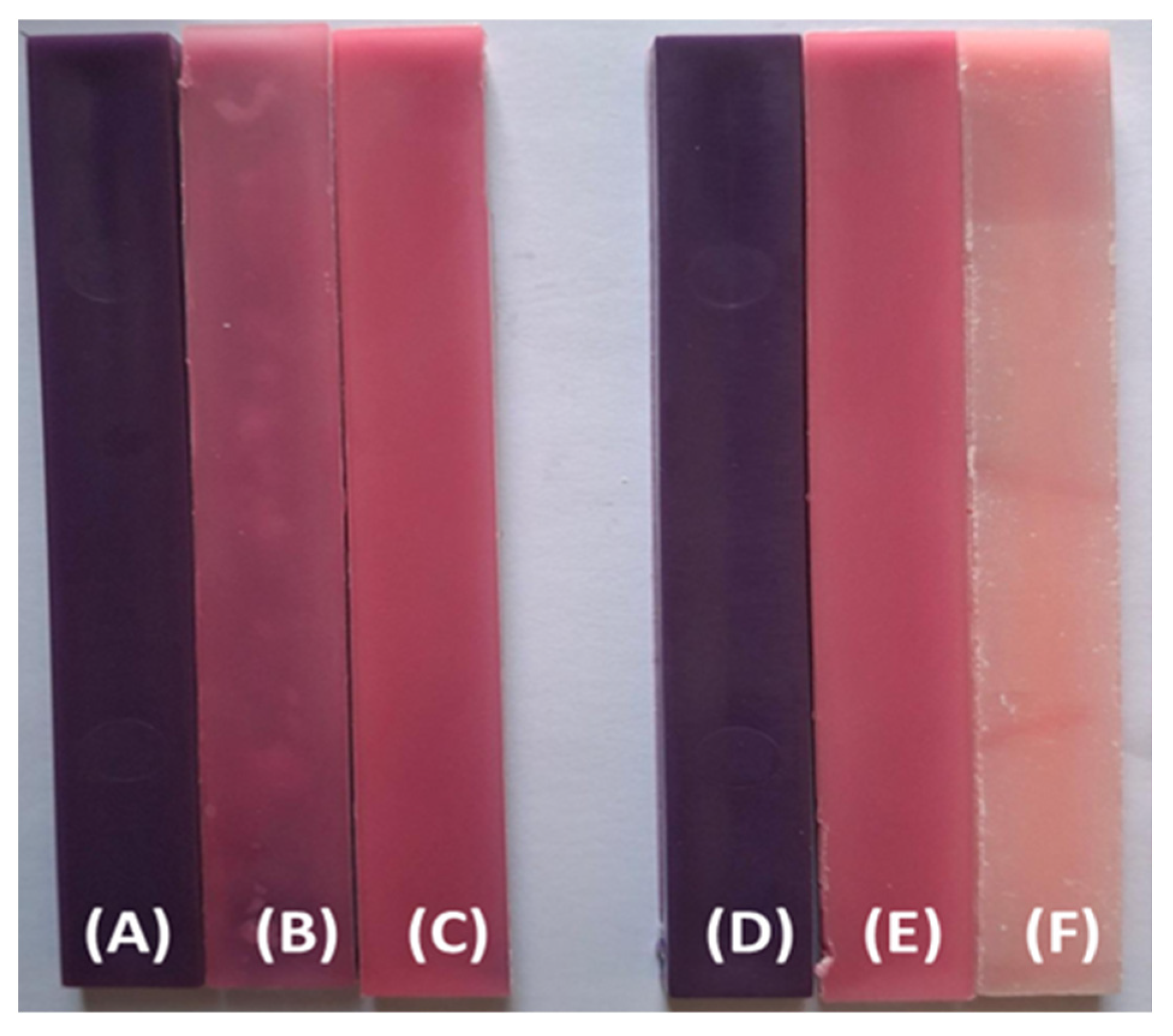
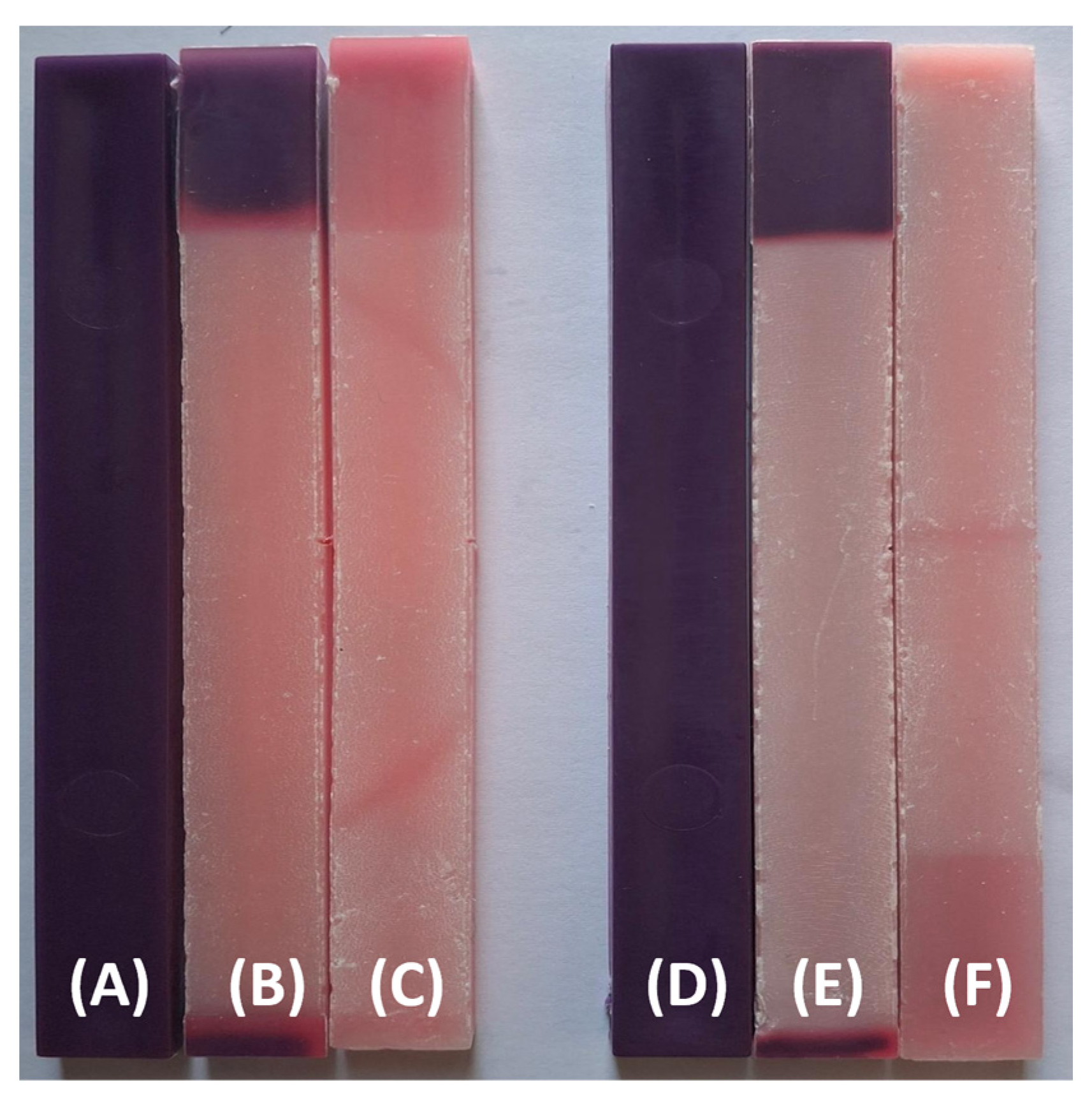

| Sample Name | PP (wt. %) | TP (wt. %) |
|---|---|---|
| PP98/TP2 | 98 | 2 |
| PP92/TP8 | 92 | 8 |
| Device | Wavelength | UV Type | Use Case | Exposure Duration (h) |
|---|---|---|---|---|
| RS 559-934 UV Exposure Unit | 360 nm | UVA | Simulated indoor lighting | 500 h and 1000 h |
| QUV/Solar Eye (SE) | 340 nm | UVA | Simulated outdoor lighting | 500 h and 1000 h |
| Specimen ID | UV Device | Exposure Hours (h) | ΔL* | Δa* | Δb* | (ΔE*ab) | RGB |
|---|---|---|---|---|---|---|---|
| PP98/TP2 | — | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 155, 112, 125 |
| RS 559-934 | 500 | 14.22 (±0.82) | 8.16 (±1.03) | 11.38 (±0.92) | 19.29 (±1.13) d | 213, 142, 142 | |
| RS 559-934 | 1000 | 19.58 (±0.91) | 6.03 (±0.75) | 13.53 (±0.61) | 23.50 (±1.02) b | 227, 158, 152 | |
| QUV/SE | 500 | 19.21 (±1.00) | 10.78 (±1.26) | 14.46 (±0.45) | 26.36 (±1.97) c | 233, 153, 150 | |
| QUV/SE | 1000 | 24.62 (±1.06) | 8.07 (±1.01) | 15.65 (±0.48) | 30.29 (±1.08) a | 246, 170, 162 | |
| PP92/TP8 | — | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 148, 109, 121 |
| RS 559-934 | 500 | 13.14 (±0.53) | 7.51 (±0.86) | 10.81 (±0.15) | 17.82 (±0.91) c | 206, 140, 141 | |
| RS 559-934 | 1000 | 24.78 (±0.45) | 3.27 (±0.62) | 12.98 (±0.49) | 27.57 (±0.62) a | 234, 174, 167 | |
| QUV/SE | 500 | 17.57 (±0.62) | 10.13 (±0.96) | 13.43 (±0.58) | 24.34 (±1.10) d | 224, 149, 148 | |
| QUV/SE | 1000 | 30.34 (±0.43) | 4.09 (±0.66) | 14.96 (±0.30) | 34.07 (±0.21) b | 252, 188, 179 |
| Specimen ID | UV Device | Exposure Hours (h) | Tensile Strain at Break (%) | Maximum Tensile Stress (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| PP98/TP2 | None (control) | 0 | 95.69 (±3.81) a | 29.45 (±0.85) a | 653.82 (±9.57) a |
| RS 559-934 (360 nm) | 500 | 81.49 (±3.52) b | 27.57 (±0.76) b | 660.12 (±10.34) b | |
| RS 559-934 (360 nm) | 1000 | 60.11 (±3.34) c | 25.64 (±0.69) c | 672.97 (±11.21) c | |
| PP92/TP8 | None (control) | 0 | 87.25 (±3.63) a | 28.89 (±0.81) a | 653.10 (±8.72) a |
| RS 559-934 (360 nm) | 500 | 72.13 (±3.38) b | 26.94 (±0.78) b | 660.82 (±9.94) b | |
| RS 559-934 (360 nm) | 1000 | 44.79 (±2.92) c | 22.31 (±0.73) c | 672.33 (±11.06) c |
| Specimen ID | UV Device | Exposure Hours (h) | Unnotched Impact Strength (kJ/m2) | Notched Impact Strength (kJ/m2) |
|---|---|---|---|---|
| PP98/TP2 | None (control) | 0 | 99.94 (±2.12) a | 23.52 (±1.21) a |
| RS 559-934 (360 nm) | 500 | 85.65 (±2.87) b | 20.32 (±1.44) b | |
| RS 559-934 (360 nm) | 1000 | 70.54 (±3.23) c | 17.95 (±1.58) c | |
| QUV/Solar Eye (340 nm) | 500 | 78.41 (±2.51) d | 18.61 (±1.33) d | |
| QUV/Solar Eye (340 nm) | 1000 | 60.22 (±3.13) e | 15.23 (±1.67) e | |
| PP92/TP8 | None (Control) | 0 | 99.86 (±2.07) a | 23.44 (±1.13) a |
| RS 559-934 (360 nm) | 500 | 81.19 (±2.72) b | 18.87 (±1.56) b | |
| RS 559-934 (360 nm) | 1000 | 66.37 (±3.08) c | 16.05 (±1.49) c | |
| QUV/Solar Eye (340 nm) | 500 | 75.04 (±2.95) d | 17.22 (±1.32) d | |
| QUV/Solar Eye (340 nm) | 1000 | 52.37 (±3.43) e | 13.09 (±1.58) e |
| Specimen ID | Exposure Condition | Exposure Duration (h) | Mass Before Exposure (mg) | Mass After Exposure (mg) | Δ Mass% |
|---|---|---|---|---|---|
| PP98/TP2 | Indoor (360 nm, RS unit) | h500 h | 3.726 (±0.01) | 3.720 (±0.01) | −0.16 |
| Indoor (360 nm, RS unit) | 1000 h | 3.731 (±0.01) | 3.725 (±0.01) | −0.16 | |
| Outdoor (340 nm, QUV) | 500 h | 3.738 (±0.01) | 3.734 (±0.01) | −0.11 | |
| Outdoor (340 nm, QUV) | 1000 h | 3.725 (±0.01) | 3.726 (±0.01) | 0.03 | |
| PP92/TP8 | Indoor (360 nm, RS unit) | 500 h | 3.729 (±0.01) | 3.727 (±0.01) | −0.05 |
| Indoor (360 nm, RS unit) | 1000 h | 3.732 (±0.01) | 3.730 (±0.01) | −0.05 | |
| Outdoor (340 nm, QUV) | 500 h | 3.731 (±0.01) | 3.729 (±0.01) | −0.05 | |
| Outdoor (340 nm, QUV) | 1000 h | 3.730 (±0.01) | 3.728 (±0.01) | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breheny, C.; Colbert, D.M.; Bezerra, G.; Geever, J.; Geever, L.M. UV-Induced Aging in Thermochromic Pigment-Integrated Food-Grade Polymers: A Performance Assessment. Appl. Sci. 2025, 15, 6039. https://doi.org/10.3390/app15116039
Breheny C, Colbert DM, Bezerra G, Geever J, Geever LM. UV-Induced Aging in Thermochromic Pigment-Integrated Food-Grade Polymers: A Performance Assessment. Applied Sciences. 2025; 15(11):6039. https://doi.org/10.3390/app15116039
Chicago/Turabian StyleBreheny, Colette, Declan Mary Colbert, Gilberto Bezerra, Joseph Geever, and Luke M. Geever. 2025. "UV-Induced Aging in Thermochromic Pigment-Integrated Food-Grade Polymers: A Performance Assessment" Applied Sciences 15, no. 11: 6039. https://doi.org/10.3390/app15116039
APA StyleBreheny, C., Colbert, D. M., Bezerra, G., Geever, J., & Geever, L. M. (2025). UV-Induced Aging in Thermochromic Pigment-Integrated Food-Grade Polymers: A Performance Assessment. Applied Sciences, 15(11), 6039. https://doi.org/10.3390/app15116039









