Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors
Abstract
Featured Application
Abstract
1. Introduction
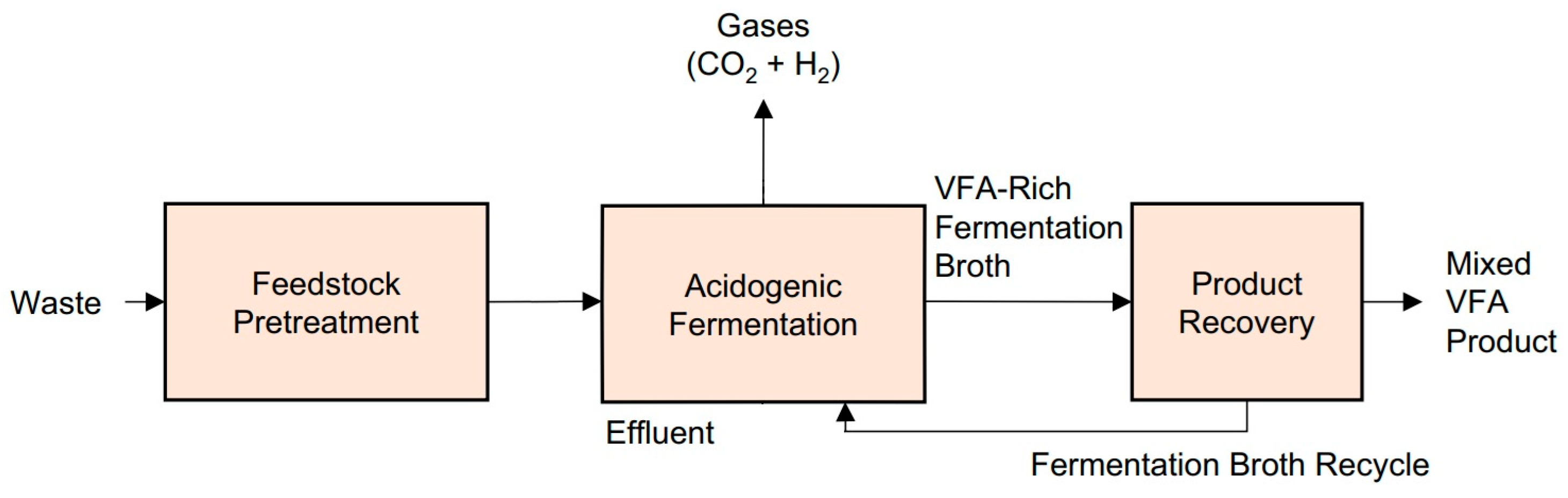
2. Mechanisms of Acidogenic Fermentation
2.1. Biological Processes Involved in Acidogenic Fermentation
2.2. Key Microbial Communities Involved in VFA Production
2.3. Biotechnological Production of Volatile Fatty Acids
2.4. Technical and Operational Challenges
3. VFA as Bioplastic Precursors
4. Waste Streams as a Resource for VFA Production
| Substrate | Operational Setup | Yield | VFA Composition (%) | Reference |
|---|---|---|---|---|
| Citrus residues | Batch fermentation: pH 6, 37.5 °C, S:I ratio 1:1, duration 14 days | 0.79 g-VFA/g-VS | Acetic: 40, Propionic: 5.3, Butyric: 18.3, Others: 36.4 | [114] |
| Potato peels | Batch fermentation: pH 7, 37 °C, duration 5 days | 0.63 g-COD/g-VS | Acetic: 45.8, Propionic: 28.5, Butyric: 24.2, Others: 1.5 | [115] |
| Brewery spent grain | Batch fermentation: pH 6.5, 30 °C, duration 10 days | 0.68 g-VFA/g-VS | Acetic: 50, Propionic: 20, Butyric: 25, Others: 5 | [116] |
| Rice straw hydrolysate | Anaerobic digestion; pH 9.0; 37 °C; 1 day HRT | 0.63 g VFA/g COD | Acetic: 45, Propionic: 30, Butyric: 25 | [106] |
| Food waste | Semi-continuous membrane bioreactor: uncontrolled pH, OLR 2 g-VS/L·d | 0.54 g-VFA/g-VS | Acetic: 20–30, Propionic: 3–10, Butyric: 14–23, Others: 35–65 | [117] |
| Food waste | Leach bed fermentation: pH 7, 22 °C, S:I ratio 25:1, duration 6 days | 0.65 g-COD/g-VS | Acetic: 27.9, Propionic: 12.9, Butyric: 33.7, Others: 25.5 | [118] |
| Food waste | Anaerobic digestion: pH 7.0; 35 °C; 20 days | 0.4–0.6 g VFA/g VS | Acetic: 50–60, Propionic: 15–25, Butyric: 10–20 | [119] |
| Food waste | Batch fermentation: initial pH 10 (alkaline condition), inoculum acclimated to food waste, 35 °C, 15 days | 21.98 g COD/L (highest VFA concentration reported) | Acetic: 38–57, Propionic: 25–30 | [120] |
| Dairy manure | Semi-continuous anaerobic membrane bioreactor: uncontrolled pH, OLR 4.7 g-VS/L·d, 114 days | 0.41 g-VFA/g-VS | Acetic: 53–89, Propionic: 4–15, Butyric: 1–12 | [121] |
| Poultry manure | Batch fermentation: uncontrolled pH, 37 °C, S:I ratio 3:1, duration 35 days | 0.53 g-VFA/g-VS | Acetic: 80–90, Propionic: 10–15 | [122] |
| Chicken manure | Batch reactor: pH 5.5, 37 °C, duration 30 days | 1.20 g-VFA/g-VS | Acetic: 60, Propionic: 15, Butyric: 20, Others: 5 | [123] |
| Waste sludge | Semi-continuous bioreactor: S:I ratio 2:1, 35 °C, duration 12 days | 0.33 g-COD/g-VS | Acetic: 25–43, Propionic: 8–33, Butyric: 11–50, Others: 14–34 | [124] |
| Waste sludge | Batch fermentation: pH 9, S:I ratio 1:1, 55 °C, duration 10 days | 0.52 g-VFA/g-VS | Acetic: 53, Propionic: 10, Butyric: 10, Others: 27 | [125] |
| Waste sludge and apple pulp | Co-fermentation under thermophilic conditions (55 °C), alkaline pH (9), batch operation | 15.50 g COD/L; 255.68 mg VFA/g COD substrate | Acetic acid: 60%, Butyric acid: 30%, Others: 10% | [126] |
| Sewage sludge and organic waste | Acidogenic fermentation: pH 5.5, ambient temperature, HRT of 5 days | VFA production of 4.5 g/L | Acetic acid: 45%, Propionic acid: 25%, Butyric acid: 20%, Others: 10% | [127] |
| Organic fraction of municipal solid waste | Anaerobic digestion: pH 6.0; 37 °C; 15 days | 0.5–0.7 g VFA/g VS | Acetic: 40–50, Propionic: 20–30, Butyric: 15–25 | [5] |
| Organic solid waste leachate | Upflow anaerobic bioreactors: uncontrolled pH, 186 days of operation | Net acidification degree (NAD) of 0.45 | Acetic acid: 50%, Propionic acid: 20%, Butyric acid: 15%, Others: 15% | [128] |
5. Targeted Acidogenic Fermentation for Bioplastics Production
6. Summary and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COD | Chemical oxygen demand |
| HRT | Hydraulic retention time |
| mcl-PHA | Medium-chain-length polyhydroxyalkanoates |
| MES | Microbial electrosynthesis |
| MMC | Mixed microbial cultures |
| OLR | Organic loading rate |
| PHA | Polyhydroxyalkanoates |
| S:I | Substrate/inoculum ratio |
| VFA | Volatile fatty acids |
| VS | Volatile solids |
References
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about bioproduction of volatile fatty acids from wastes and wastewaters: Influence of operating conditions and organic composition of the substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Bruni, C.; Foglia, A.; Eusebi, A.L.; Frison, N.; Akyol, C.; Fatone, F. Targeted bio-based volatile fatty acid production from waste streams through anaerobic fermentation: Link between process parameters and operating scale. ACS Sustain. Chem. Eng. 2021, 9, 9970–9987. [Google Scholar] [CrossRef]
- Palar, S. Comparison of Conventional and Electro-Selective Fermentations of Swine Manure to Produce Short and Medium Chain Volatile Fatty Acids. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2024. Available online: https://krex.k-state.edu/bitstreams/3c7fec1c-3b20-4b2a-8358-503623ca1915/download (accessed on 18 October 2024).
- Moza, A.; Ram, N.R.; Srivastava, N.K.; Nikhil, G.N. Bioprocessing of low-value food waste to high value volatile fatty acids for applications in energy and materials: A review on process-flow. Bioresour. Technol. Rep. 2022, 19, 101123. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A glimpse of the world of volatile fatty acids production and application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- IMARC. Fatty Acid Prices, Trend, Chart, Demand, Market Analysis, News, Historical and Forecast Data Report. 2024. Available online: https://www.imarcgroup.com/fatty-acid-pricing-report (accessed on 18 October 2024).
- PR (Precedence Research). Butyric Acid Market Size, Share, and Trends 2024 to 2034. 2023. Available online: https://www.precedenceresearch.com/butyric-acid-market (accessed on 18 October 2024).
- RN (Research Nester). Valeric Acid Market Size and Share. 2023. Available online: https://www.researchnester.com/reports/valeric-acid-market/5809 (accessed on 18 October 2024).
- MarkWide Research. Volatile Fatty Acids Market—Industry Analysis, Trends, and Forecast (2024–2032). MarkWide Research. 2024. Available online: https://markwideresearch.com/volatile-fatty-acids-market/ (accessed on 18 October 2024).
- Duong, T.H.; Nga, T.T.V. Production of volatile fatty acids from organic-rich waste streams: Current issues, challenges, and opportunities. Curr. Pollut. Rep. 2024, 10, 594–605. [Google Scholar] [CrossRef]
- Ram, N.R.; Nikhil, G.N. Assessment of microbial consortiums and their metabolic patterns during the bioconversion of food waste. Biomass Convers. Biorefin. 2023, 1–14. [Google Scholar] [CrossRef]
- Varghese, V.K.; Poddar, B.J.; Shah, M.P.; Purohit, H.J.; Khardenavis, A.A. A comprehensive review on current status and future perspectives of microbial volatile fatty acids production as platform chemicals. Sci. Total Environ. 2022, 815, 152500. [Google Scholar] [CrossRef]
- Castro-Fernandez, A.; Rodríguez-Hernández, L.; Castro-Barros, C.M.; Lema, J.M.; Taboada-Santos, A. Scale-up and economic assessment of volatile fatty acids production from food waste. Biomass Bioenergy 2024, 182, 107112. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Sánchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile fatty acids production by acidogenic fermentation of wastewater: A bibliometric analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- Wang, Q.; Xin, W.; Shao, Z.; Usman, M.; Li, J.; Shang, P.; Kou, Y.; El-Din, M.G.; Chen, C. Role of pretreatment type and microbial mechanisms on enhancing volatile fatty acids production during anaerobic fermentation of refinery waste activated sludge. Bioresour. Technol. 2023, 381, 129122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, Y.; Wang, H.; Simeonov, I.; Christov, N. A volatile fatty acids adaptive observer-based hierarchical optimal controller design to maximum gas production of two-stage anaerobic digestion process. Comput. Chem. Eng. 2023, 181, 108524. [Google Scholar] [CrossRef]
- Carvalheira, M.; Marreiros, B.C.; Reis, M.A.M. Acids (VFA) and bioplastic (PHA) recovery. Clean Energy Resour. Recovery 2022, 2, 245–254. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, K.S.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFA): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Ren, J.L.; Zhang, M.M.; Wu, Q.F.; Yuan, S.; Liu, W.; Lu, D. Highly efficient utilization of sugar in molasses for butyric acid production by Clostridium tyrobutyricum. Sugar Tech 2023, 25, 580–591. [Google Scholar] [CrossRef]
- Guo, X.; Ye, F.; Nan, C.; Cheng, J.; Feng, J.; Fu, H.; Wang, J. Efficient production of butyl butyrate from mannitol by engineered Clostridium tyrobutyricum. Chem. Eng. J. 2024, 487, 150577. [Google Scholar] [CrossRef]
- Conrado, L.; McCoy, J.; Rabinovich, L.; Davoudimehr, M.; Stamatopoulou, P.; Scarborough, M. Anaerobic conversion of proteinogenic amino acids when methanogenesis is inhibited: Carboxylic acid production from single amino acids. Fermentation 2024, 10, 237. [Google Scholar] [CrossRef]
- Vijande, C.; Bevilacqua, R.; Balboa, S.; Carballa, M. Altering operational conditions during protein fermentation to volatile fatty acids modifies the associated bacterial community. Microb. Biotechnol. 2024, 17, e14505. [Google Scholar] [CrossRef]
- Law, A.W.S.; Rincón, F.R.; van de Vossenberg, J.; Al Saffar, Z.; Welles, L.; Rene, E.R.; Vazquez, C.L. Volatile fatty acid production from food waste: The effect of retention time and lipid content. Bioresour. Technol. 2023, 367, 128298. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of extreme pH conditions on methanogenesis: Methanogen metabolism and community structure. Sci. Total Environ. 2023, 877, 162702. [Google Scholar] [CrossRef]
- Qin, W.; Han, S.; Meng, F.; Chen, K.; Gao, Y.; Li, J.; Lin, L.; Hu, E.; Jiang, J. Impacts of seasonal variation on volatile fatty acids production of food waste anaerobic fermentation. Sci. Total Environ. 2024, 912, 168764. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Gonzalez-Fernandez, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Cetecioglu, Z. Bioaugmentation as a strategy for tailor-made volatile fatty acid production. J. Environ. Manag. 2021, 295, 113093. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yang, Y.H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Biotechnol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest trends in industrial vinegar production and the role of acetic acid bacteria: Classification, metabolism, and applications—A comprehensive review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef]
- Paul, S.K.; Wartu, J.R.; Orukotan, A.A. Production of vinegar from waste fruits using Acetobacter species. Ife J. Sci. 2024, 26, 179–200. [Google Scholar] [CrossRef]
- Nayak, J.; Chakrabortty, S. Production of acetic acid and whey protein from cheese whey in a hybrid reactor under response surface optimized conditions. Fine Chem. Eng. 2023, 4, 58–73. [Google Scholar] [CrossRef]
- Pawar, P.R.; Rao, P.; Prakash, G.; Lali, A.M. Organic waste streams as feedstock for the production of high volume-low value products. Environ. Sci. Pollut. Res. 2021, 28, 11904–11914. [Google Scholar] [CrossRef]
- Tammali, R.; Seenayya, G.; Reddy, G. Fermentation of cellulose to acetic acid by Clostridium lentocellum SG6: Induction of sporulation and effect of buffering agent on acetic acid production. Lett. Appl. Microbiol. 2003, 37, 304–308. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, M.; Chen, X.; Li, D.; Qin, L.; Li, Z.; Yao, J.; Liang, X. Mixed culture of Saccharomyces cerevisiae and Acetobacter pasteurianus for acetic acid production. Biochem. Eng. J. 2013, 79, 41–45. [Google Scholar] [CrossRef]
- Ranaei, V.; Pilevar, Z.; Khaneghah, A.M.; Hosseini, H. Propionic acid: Method of production, current state and perspectives. Food Technol. Biotechnol. 2020, 58, 115. [Google Scholar] [CrossRef]
- Duran-Cruz, V.; Hernández, S.; Ortíz, I. Propionic acid production by Propionibacterium acidipropionici CDBB-B-1981 from enzymatic hydrolysates of Agave bagasse pretreated by steam explosion. Eng. Rep. 2024, 6, e12858. [Google Scholar] [CrossRef]
- Dishisha, T.; Jain, M.; Hatti-Kaul, R. High cell density sequential batch fermentation for enhanced propionic acid production from glucose and glycerol/glucose mixture using Acidipropionibacterium acidipropionici. Microb. Cell Fact. 2024, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.A.; de Oliveira Meira, A.C.F.; da Silva, F.L.; Veríssimo, L.A.A.; Piccoli, R.H.; Paiva, L.V.; Mondragon-Bernal, L.V.; Alves, J.G.L.F. Biosynthesis of propionic acid from whey permeate and corn steep liquor by Propionibacterium freudenreichii subsp ATCC 6207 and partial purification using ion exchange cryogels. Braz. J. Chem. Eng. 2024, 1–13. [Google Scholar] [CrossRef]
- Ammar, E.M.; Martin, J.; Brabo-Catala, L.; Philippidis, G.P. Propionic acid production by Propionibacterium freudenreichii using sweet sorghum bagasse hydrolysate. Appl. Microbiol. Biotechnol. 2020, 104, 9619–9629. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kot, A.M.; Kieliszek, M.; Bonin, S. Use of Propionibacterium freudenreichii T82 strain for effective biosynthesis of propionic acid and trehalose in a medium with apple pomace extract and potato wastewater. Molecules 2021, 26, 3965. [Google Scholar] [CrossRef]
- Llamas, M.; Magdalena, J.A.; Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Insights on the microbial communities developed during the anaerobic fermentation of raw and pretreated microalgae biomass. Chemosphere 2021, 263, 127942. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Duan, C.; Liu, Z.; Wang, Y.; Zhong, Y.; Hu, X.; Song, Z.; Yi, J.; Wang, T. A synergistic fermentation system of probiotics with low-cost and high butyric acid production: Lactiplantibacillus plantarum and Clostridium tyrobutyricum. Food Biosci. 2024, 62, 105152. [Google Scholar] [CrossRef]
- Kelbert, M.; Machado, T.O.; Araújo, P.H.; Sayer, C.; de Oliveira, D.; Maziero, P.; Carciofi, B.A. Perspectives on biotechnological production of butyric acid from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2024, 202, 114717. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, G.; Chen, L.; Yang, N.; Wu, Y.; Fang, W.; Zhang, R.; Wang, X.; Fu, C.; Zhang, P. Volatile fatty acid production in anaerobic fermentation of food waste saccharified residue: Effect of substrate concentration. Waste Manag. 2023, 164, 29–36. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, L.; Chen, T.; Yuan, W.; Geng, Y. Butyric acid fermentation in xylose and glucose by Clostridium tyrobutyricum. Bioresources 2017, 12, 2930–2940. [Google Scholar] [CrossRef]
- Bao, T.; Feng, J.; Jiang, W.; Fu, H.; Wang, J.; Yang, S.T. Recent advances in n-butanol and butyrate production using engineered Clostridium tyrobutyricum. World J. Microbiol. Biotechnol. 2020, 36, 138. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Cetecioglu, Z. Butyric acid dominant volatile fatty acids production: Bio-augmentation of mixed culture fermentation by Clostridium butyricum. J. Environ. Chem. Eng. 2020, 8, 104496. [Google Scholar] [CrossRef]
- Romans-Casas, M.; Feliu-Paradeda, L.; Tedesco, M.; Hamelers, H.V.; Bañeras, L.; Balaguer, M.D.; Puig, S.; Dessì, P. Selective butyric acid production from CO2 and its upgrade to butanol in microbial electrosynthesis cells. Environ. Sci. Ecotechnol. 2024, 17, 100303. [Google Scholar] [CrossRef]
- Ruan, X.; Zhang, S.; Song, W.; Liu, J.; Chen, X.; Liu, L.; Wu, J. Efficient synthesis of tyrosol from L-tyrosine via heterologous Ehrlich pathway in Escherichia coli. Chin. J. Chem. Eng. 2022, 47, 18–30. [Google Scholar] [CrossRef]
- Lang, K.; Zierow, J.; Buehler, K.; Schmid, A. Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites. Microb. Cell Fact. 2014, 13, 2. [Google Scholar] [CrossRef]
- Godoy, P.; Udaondo, Z.; Duque, E.; Ramos, J.L. Biosynthesis of fragrance 2-phenylethanol from sugars by Pseudomonas putida. Biotechnol. Biofuels Bioprod. 2024, 17, 51. [Google Scholar] [CrossRef]
- Nitschel, R.; Ankenbauer, A.; Welsch, I.; Wirth, N.T.; Massner, C.; Ahmad, N.; McColm, S.; Borges, F.; Fotheringham, I.; Takors, R.; et al. Engineering Pseudomonas putida KT2440 for the production of isobutanol. Eng. Life Sci. 2020, 20, 148–159. [Google Scholar] [CrossRef]
- Vemuri, G.N.; Altman, E.; Sangurdekar, D.P.; Khodursky, A.B.; Eiteman, M.A. Overflow metabolism in Escherichia coli during steady-state growth: Transcriptional regulation and effect of the redox ratio. Appl. Environ. Microbiol. 2006, 72, 3653–3661. [Google Scholar] [CrossRef]
- Hossain, M.N.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Production of short chain fatty acids and vitamin B12 during the in-vitro digestion and fermentation of probiotic chocolate. Food Biosci. 2022, 47, 101682. [Google Scholar] [CrossRef]
- Veras, S.T.S.; Cavalcante, W.A.; Gehring, T.A.; Ribeiro, A.R.; Ferreira, T.J.T.; Kato, M.T.; Rojas-Ojeda, P.; Sanz-Martin, J.L.; Leitão, R.C. Anaerobic production of valeric acid from crude glycerol via chain elongation. Int. J. Environ. Sci. Technol. 2020, 17, 1847–1858. [Google Scholar] [CrossRef]
- Gasser, B.G.; Fuchsmann, P.; Fröhlich-Wyder, M.T. Sensory Characteristics of Swiss-Type Cheese Varieties. In Sensory Profiling of Dairy Products; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 195–224. [Google Scholar] [CrossRef]
- Xiong, M.; Deng, J.; Woodruff, A.P.; Zhu, M.; Zhou, J.; Park, S.W.; Li, H.; Fu, Y.; Zhang, K. A bio-catalytic approach to aliphatic ketones. Sci. Rep. 2012, 2, 311. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Ayeni, A.O.; Soni, D.; Chandrashekhar, B. Microbial Consortia: A Mixed Cell Catalyst for Biotransformation of Biomass into Biofuels and Chemicals. In Whole-Cell Biocatalysis; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 269–307. [Google Scholar]
- Zhou, S.P.; Ke, X.; Jin, L.Q.; Xue, Y.P.; Zheng, Y.G. Sustainable management and valorization of biomass wastes using synthetic microbial consortia. Bioresour. Technol. 2024, 395, 130391. [Google Scholar] [CrossRef] [PubMed]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total Environ. 2021, 798, 149292. [Google Scholar] [CrossRef]
- Tian, L.; Pan, L.; Wang, L. Effect of inoculum pretreatment and substrate/inoculum ratio on acidogenic fermentation of chemically enhanced primary treatment sludge. Sustainability 2024, 16, 3347. [Google Scholar] [CrossRef]
- Mulyawati, A.I.; Suraraksa, B.; Chaiprasert, P. Efficacies of anaerobic microbial consortium for starchy lignocellulose hydrolysis and acidogenic fermentation of cassava pulp. BioEnergy Res. 2023, 16, 2314–2330. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Feng, L.; Chen, Y. The mechanisms of pH regulation on promoting volatile fatty acids production from kitchen waste. J. Environ. Sci. 2025, 147, 414–423. [Google Scholar] [CrossRef]
- Atasoy, M.; Scott, W.T.; Regueira, A.; Mauricio-Iglesias, M.; Schaap, P.J.; Smidt, H. Biobased short chain fatty acid production—Exploring microbial community dynamics and metabolic networks through kinetic and microbial modeling approaches. Biotechnol. Adv. 2024, 73, 108363. [Google Scholar] [CrossRef]
- Gyadi, T.; Bharti, A.; Basack, S.; Kumar, P.; Lucchi, E. Influential factors in anaerobic digestion of rice-derived food waste and animal manure: A comprehensive review. Bioresour. Technol. 2024, 413, 131398. [Google Scholar] [CrossRef]
- Tennison-Omovoh, C.A.; Fagbohungbe, M.O.; Bankole, P.O.; Semple, K.T. The effect of different C-N ratios on volatile fatty acid (VFA) production from acidogenic fermentation of sucrose in continuous-stirred tank reactors. Biomass Conv. Bioref. 2023, 13, 9339–9351. [Google Scholar] [CrossRef]
- Duong, T.H.; Grolle, K.; Nga, T.T.V.; Zeeman, G.; Temmink, H.; van Eekert, M. Protein hydrolysis and fermentation under methanogenic and acidifying conditions. Biotechnol. Biofuels 2019, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Peña-Picola, S.; Serra-Toro, A.; Da Silva, C.; Peces, M.; Jordán, M.; Vila, J.; Grifoll, M.; Valentino, F.; Astals, S.; Dosta, J. Acidogenic fermentation of biowaste coupled with nitrogen recovery using selective membranes to produce a VFA-rich liquid with a high C/N ratio. J. Environ. Chem. Eng. 2024, 12, 112352. [Google Scholar] [CrossRef]
- Baksi, S.; Sarkar, U.; Villa, R.; Basu, D.; Sengupta, D. Conversion of biomass to biofuels through sugar platform: A review of enzymatic hydrolysis highlighting the trade-off between product and substrate inhibitions. Sustain. Energy Technol. Assess. 2023, 55, 102963. [Google Scholar] [CrossRef]
- Ram, N.R.; Nikhil, G.N. A critical review on sustainable biogas production with focus on microbial-substrate interactions: Bottlenecks and breakthroughs. Bioresour. Technol. Rep. 2022, 19, 101170. [Google Scholar] [CrossRef]
- Duong, T.H.; van Eekert, M.; Grolle, K.; Tran, T.V.N.; Zeeman, G.; Temmink, H. Effect of carbohydrates on protein hydrolysis in anaerobic digestion. Water Sci. Technol. 2022, 86, 66–79. [Google Scholar] [CrossRef]
- Avelar Molina, G.; Schaller, K.; Kari, J.; Schiano-di-Cola, C.; Peters, G.H.J.; Borch, K.; Westh, P. Interrelationship of substrate crystallinity, enzyme binding strength, and cellulase activity. bioRxiv 2024. biorXiv:20.607150. [Google Scholar] [CrossRef]
- Kohli, V.; Singha, S. Protein digestibility of soybean: How processing affects seed structure, protein and non-protein components. Discov. Food 2024, 4, 7. [Google Scholar] [CrossRef]
- Zheng, X.; Juan, M.; Kou, X.; Gao, X.; Liu, J.; Li, S.; Zheng, B.; Liu, Y.; Xue, Z. Investigation on the emulsification mechanism in aqueous enzymatic extraction of edible oil from Schizochytrium sp. J. Sci. Food Agric. 2023, 103, 2904–2913. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Agbede, O.O.; Agarry, S.E.; Ajani, A.O.; Ogunkunle, O.; Laseinde, O.T.; Rahman, S.M.A.; Fattah, I.M.R. Decoding anaerobic digestion: A holistic analysis of biomass waste technology, process kinetics, and operational variables. Energies 2023, 16, 3378. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic digestion of food waste—A short review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Deng, Z.; Sierra, J.M.; Ferreira, A.L.M.; Cerqueda-Garcia, D.; Spanjers, H.; van Lier, J.B. Effect of operational parameters on the performance of an anaerobic sequencing batch reactor (AnSBR) treating protein-rich wastewater. Environ. Sci. Ecotechnol. 2024, 17, 100296. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Otto, P.; Puchol-Royo, R.; Ortega-Legarreta, A.; Tanner, K.; Tideman, J.; de Vries, S.J.; Pascual, J.; Porcar, M.; Latorre-Pérez, A.; Abendroth, C. Multivariate comparison of taxonomic, chemical and operational data from 80 different full-scale anaerobic digester-related systems. Biotechnol. Biofuels Bioprod. 2024, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P. Techno-economic analysis of cyanobacterial PHB bioplastic production. J. Environ. Chem. Eng. 2022, 10, 107502. [Google Scholar] [CrossRef]
- Bravo-Porras, G.; Fernández-Güelfo, L.A.; Álvarez-Gallego, C.J.; Carbú, M.; Sales, D.; Romero-García, L.I. Influence of the total concentration and the profile of volatile fatty acids on polyhydroxyalkanoates (PHA) production by mixed microbial cultures. Biomass Conv. Bioref. 2024, 14, 239–253. [Google Scholar] [CrossRef]
- Urbina, L.; Rovira-Cal, E.; Nafarrate, I.; Urkiaga, A.; Berganza, J.; Aymerich, E.; Suárez, M.J. Acidogenic fermentation of dairy by-products for the utilization of volatile fatty acids in PHBV production by Haloferax mediterranei. Sustain. Food Technol. 2025, 3, 300–310. [Google Scholar] [CrossRef]
- Kalampokidis, A.; Klontza, E.; Vakalis, S.; Naddeo, V.; Lekkas, D.F. Is it possible to produce polyhydroxyalkanoate (PHA) bioplastics of consistent composition from organic wastes? A review. Circ. Econ. Sustain. 2024, 4, 2775–2797. [Google Scholar] [CrossRef]
- Katagi, V.N.; Bhat, S.G.; Paduvari, R.; Kodavooru, D.; Somashekara, D.M. Waste to value-added products: An innovative approach for sustainable production of microbial biopolymer (PHA)—Emphasis on inexpensive carbon feedstock. Environ. Technol. Rev. 2023, 12, 570–587. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Liu, H.; Zhou, T.; Qin, Z.; Mou, J.; Sun, J.; Huang, S.; Chaces, A.V.; Gao, L.; et al. Bioproduction and applications of short-chain fatty acids from secondary sludge anaerobic fermentation: A critical review. Renew. Sustain. Energy Rev. 2023, 183, 113502. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, X.; Zhang, Y.; Zhu, T.; Peng, L.; Xu, Y.; Chen, X.; Wang, D.; Ni, B.-J.; et al. Towards scaling-up implementation of polyhydroxyalkanoate (PHA) production from activated sludge: Progress and challenges. J. Clean. Prod. 2024, 447, 141542. [Google Scholar] [CrossRef]
- Chen, X.; Bai, C.; Li, Z.; Cao, D.; Zu, Y.; Zhang, Z.; Zhao, G.; Nan, J.; Wang, H.; Liang, B.; et al. Directional bioelectrochemical dechlorination of trichloroethene to valuable ethylene by introduction poly-3-hydroxybutyrate as a slow release carbon source. Chem. Eng. J. 2023, 455, 140737. [Google Scholar] [CrossRef]
- Crisafi, F.; Valentino, F.; Micolucci, F.; Denaro, R. From organic wastes and hydrocarbons pollutants to polyhydroxyalkanoates: Bioconversion by terrestrial and marine bacteria. Sustainability 2022, 14, 8241. [Google Scholar] [CrossRef]
- Ingram, H.R.; Winterburn, J.B. Influence of emulsified plant oil composition on growth and biopolymer production of Cupriavidus necator DSM 545. Food Bioprod. Process. 2022, 132, 23–34. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 263, 130204. [Google Scholar] [CrossRef]
- Montiel-Jarillo, G.; Gea, T.; Artola, A.; Fuentes, J.; Carrera, J.; Suárez-Ojeda, M.E. Towards PHA production from wastes: The bioconversion potential of different activated sludge and food industry wastes into VFA through acidogenic fermentation. Waste Biomass Valor. 2021, 12, 6861–6873. [Google Scholar] [CrossRef]
- Regueira, A.; Bevilacqua, R.; Lema, J.M.; Carballa, M.; Mauricio-Iglesias, M. A metabolic model for targeted volatile fatty acids production by cofermentation of carbohydrates and proteins. Bioresour. Technol. 2020, 298, 122535. [Google Scholar] [CrossRef]
- Penumathsa, B.K.V.; Premier, G.C.; Kyazze, G.; Dinsdale, R.; Guwy, A.J.; Esteves, S.; Rodríguez, J. ADM1 can be applied to continuous bio-hydrogen production using a variable stoichiometry approach. Water Res. 2008, 42, 4379–4385. [Google Scholar] [CrossRef]
- Savun-Hekimoğlu, B. On the use of mathematical models for wastewater treatment: A review and analysis of activated sludge models ASM1 and ASM3. Int. J. Environ. Geoinf. 2021, 8, 1–18. [Google Scholar] [CrossRef]
- González-Cabaleiro, R.; Lema, J.M.; Rodríguez, J. Metabolic energy-based modelling explains product yielding in anaerobic mixed culture fermentations. PLoS ONE 2015, 10, e0126739. [Google Scholar] [CrossRef]
- Regueira, A.; González-Cabaleiro, R.; Ofiţeru, I.D.; Rodríguez, J.; Lema, J.M. Electron bifurcation mechanism and homoacetogenesis explain products yields in mixed culture anaerobic fermentations. Water Res. 2018, 141, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Regueira, A.; Lema, J.M.; Carballa, M.; Mauricio-Iglesias, M. Metabolic modeling for predicting VFA production from protein-rich substrates by mixed-culture fermentation. Biotechnol. Bioeng. 2020, 117, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, W.W.; Huang, L.; Liu, H.Q.; Wang, Y.K.; Geng, Y.K.; Kwan-Sing Lam, P.; Yu, H.Q. Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Regueira, A.; Turunen, R.; Vuoristo, K.S.; Carballa, M.; Lema, J.M.; Uusitalo, J.; Mauricio-Iglesias, M. Model-aided targeted volatile fatty acid production from food waste using a defined co-culture microbial community. Sci. Total Environ. 2023, 857, 159521. [Google Scholar] [CrossRef]
- Kacaribu, A.A.; Darwin, D. Sustainable bioproduct production via anaerobic bioconversion by landfill soil inoculum in various carbohydrate wastes. Acta Technol. Agric. 2024, 27, 61–68. [Google Scholar] [CrossRef]
- Urfi, M.; Babar, Z.B.; Munir, S.; Rizwan, K.; Majeed, I. Production of volatile fatty acids from biomass, their recovery and applications in fuel and other valued products formation. In Nanomaterials in Biomass Conversion; Woodhead Publishing: Cambridge, UK, 2024; pp. 349–367. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- More, P.P.; Gore, S.; Dargode, P.; Sharma, M.D.; Lali, A.M. Volatile Fatty Acids (VFA) Production through altered anaerobic digestion (AD) process for efficient utilization of residual liquid stream of pretreated lignocellulosic biomass. Bioenerg. Res. 2022, 15, 1616–1625. [Google Scholar] [CrossRef]
- Vogel, K.P.; Dien, B.S.; Jung, H.G.; Casler, M.D.; Masterson, S.D.; Mitchell, R.B. Quantifying actual and theoretical ethanol yields for switchgrass strains using NIRS analyses. Bioenerg. Res. 2011, 4, 96–110. [Google Scholar] [CrossRef]
- Sakthivel, U. Bio-Valorization of Physical and Chemical Pretreated Switchgrass on Volatile Fatty Acid Production. Ann. Agric. Crop Sci. 2021, 6, 1085. [Google Scholar]
- Battista, F.; Strazzera, G.; Valentino, F.; Gottardo, M.; Villano, M.; Matos, M.; Silva, F.; Reis, M.A.; Mata-Alvarez, J.; Astals, S.; et al. New insights in food waste, sewage sludge and green waste anaerobic fermentation for short-chain volatile fatty acids production: A review. J. Environ. Chem. Eng. 2022, 10, 108319. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, G.; Brar, S.K. Tailored production of butyric acid from mixed culture fermentation of food waste. Food Bioprod. Process. 2025, 150, 1–11. [Google Scholar] [CrossRef]
- Ma, S.; Huang, X. Volatile fatty acid (VFA) production from sludge and chicken manure co-fermentation: Role of acid/alkali-treatment and microbial characteristics. J. Environ. Chem. Eng. 2024, 12, 112215. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, W.; Xue, T.; Zhou, Y.; Dong, R. Generation of high concentration free volatile fatty acids from continuous anaerobic digestion of chicken manure by suppressing the decomposition of nitrogenous components. Mol. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, H.; Zhang, Q.; Xi, J.; Zhang, H.; Zheng, M.; Xi, B. A review on polyhydroxyalkanoates production from various organic waste streams: Feedstocks, strains, and production strategy. Resour. Conserv. Recycl. 2023, 198, 107166. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Pérez-Barragán, J.; Martínez-Fraile, C.; Muñoz, R.; Quijano, G.; Maya-Yescas, R.; León-Becerril, E.; Castro-Muñoz, R.; García-Depraect, O. Brewery spent grain valorization through fermentation: Targeting biohydrogen, carboxylic acids, and methane production. Process Saf. Environ. Prot. 2024, 191, 206–217. [Google Scholar] [CrossRef]
- Wainaina, S.; Parchami, M.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J. Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Bioresour. Technol. 2019, 274, 329–334. [Google Scholar] [CrossRef]
- Shewa, W.A.; Hussain, A.; Chandra, R.; Lee, J.; Saha, S.; Lee, H.S. Valorization of food waste and economical treatment: Effect of inoculation methods. J. Clean. Prod. 2020, 261, 121170. [Google Scholar] [CrossRef]
- Sukphun, N.; Sittijunda, S.; Reungsang, A. Volatile fatty acid production from food waste with the emphasis on membrane-based recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef] [PubMed]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFA) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, J.; Wang, J.; Fang, H.; Yu, X.; He, J.; Chen, T. Targeted volatile fatty acid production based on lactate platform in mixed culture fermentation: Insights into carbon conversion and microbial metabolic traits. Bioresour. Technol. 2025, 417, 131835. [Google Scholar] [CrossRef]
- Xin, X.; She, Y.; Hong, J. Insights into microbial interaction profiles contributing to volatile fatty acids production via acidogenic fermentation of waste activated sludge assisted by calcium oxide pretreatment. Bioresour. Technol. 2021, 320, 124287. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From sewage sludge and agri-food waste to VFA: Individual acid production potential and up-scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Cerdán Morillo, J.M.A. Potencial de Producción de Ácidos Grasos Volátiles en Lodos de PTAR y Residuos Agroindustriales en Condiciones Termofílicas. Master’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2020. Available online: https://hdl.handle.net/20.500.12996/4445 (accessed on 27 December 2024).
- AquaVall. Producción de Ácidos Grasos Volátiles a Partir de Lodos de Depuradora y Residuos Orgánicos Mediante Fermentación Acidogénica. Apuntes de Innovación AquaVall, 1. 2020. Available online: https://aquavall.es/wp-content/uploads/2020/07/Apuntes-de-Innovacion-AquaVall-01-Produccion-de-acidos-grasos-TROVANT-web.pdf (accessed on 27 December 2024).
- Ortegón Velandia, M.F. Evaluación del Potencial Acidogénico para Producción de Ácidos Grasos Volátiles (AGV) a Partir de Lixiviados de los Residuos Sólidos Orgánicos, Como Plataforma de Biorefinería. Bachelor’s thesis, Universidad Santo Tomás, Manila, Phillipines, 2016. Available online: https://repository.usta.edu.co/handle/11634/2471 (accessed on 27 December 2024).
- Dong, W.; Yang, Y.; Liu, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste: Pathways and microbial perspective. Renew. Sustain. Energy Rev. 2023, 175, 113181. [Google Scholar] [CrossRef]
- Sánchez-Ledesma, L.M.; Rodríguez-Victoria, J.A.; Ramírez-Malule, H. Effect of fermentation time, pH, and their interaction on the production of volatile fatty acids from cassava wastewater. Water 2024, 16, 1514. [Google Scholar] [CrossRef]
- Carvalheira, M.; Duque, A.F. From food waste to volatile fatty acids towards a circular economy. In Fermentation—Processes, Benefits and Risks; IntechOpen: London, UK, 2021; pp. 165–185. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Assessing the relevance of acidic pH on primary intermediate compounds when targeting carboxylate accumulation. Biomass Conv. Bioref. 2022, 12, 4519–4529. [Google Scholar] [CrossRef]
- Tourang, M.; Xiong, X.; Sarkhosh, S.; Chen, S. Polyhydroxybutyrate (PHB) biosynthesis by an engineered Yarrowia lipolytica strain using co-substrate strategy. Fermentation 2023, 9, 1003. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Dartiailh, C.; Blunt, W.; Sharma, P.K.; Liu, S.; Cicek, N.; Levin, D.B. The thermal and mechanical properties of medium chain-length polyhydroxyalkanoates produced by Pseudomonas putida LS46 on various substrates. Front. Bioeng. Biotechnol. 2021, 8, 617489. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Short-chain fatty acids and hydrogen production in one single anaerobic fermentation stage using carbohydrate-rich food waste. J. Clean. Prod. 2021, 284, 124727. [Google Scholar] [CrossRef]
- Ibáñez-López, M.E.; Frison, N.; Bolzonella, D.; García-Morales, J.L. Enhancing anaerobic digestion with an UASB reactor of the winery wastewater for producing volatile fatty acid effluent enriched in caproic acid. Fermentation 2023, 9, 958. [Google Scholar] [CrossRef]
- Lim, J.X.; Vadivelu, V.M. Enhanced volatile fatty acid production in sequencing batch reactor: Microbial population and growth kinetics evaluation. AIP Conf. Proc. 2019, 2124, 020040. [Google Scholar] [CrossRef]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile fatty acids (VFA) production and recovery from chicken manure using a high-solid anaerobic membrane bioreactor (AnMBR). Membranes 2022, 12, 1133. [Google Scholar] [CrossRef]
- Ben, M.; Mato, T.; Lopez, A.; Vila, M.; Kennes, C.; Veiga, M.C. Bioplastic production using wood mill effluents as feedstock. Water Sci. Technol. 2011, 63, 1196–1202. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic anaerobic digestion: An advancement towards enhanced biogas production from lignocellulosic biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, J.Y. Feasibility assessment of thermophilic anaerobic digestion process of food waste. J. Mater. Cycles Waste Manag. 2016, 18, 413–418. [Google Scholar] [CrossRef]
- Sakarika, M.; Regueira, A.; Rabaey, K.; Ganigué, R. Thermophilic caproic acid production from grass juice by sugar-based chain elongation. Sci. Total Environ. 2023, 860, 160501. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Liu, L.; Song, B.; Liu, H.; Shi, H.; Zhu, Y. Mesophilic and thermophilic fermentation of activated sludge for volatile fatty acids production: Focusing on anaerobic degradation of carbohydrate and protein. Environ. Technol. 2024, 45, 5745–5757. [Google Scholar] [CrossRef]
- Nie, E.; He, P.; Zhang, H.; Hao, L.; Shao, L.; Lü, F. How does temperature regulate anaerobic digestion? Renew. Sustain. Energy Rev. 2021, 150, 111453. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; He, Z.; Li, M.; Zong, S.; Wang, Z.; Ge, B.; Zhang, O.; Li, X.; Wang, A. Insights into methanogenesis of mesophilic-psychrophilic varied anaerobic digestion of municipal sludge with antibiotic stress. J. Environ. Manag. 2023, 331, 117278. [Google Scholar] [CrossRef]
- Aboudi, K.; Greses, S.; González-Fernández, C. Hydraulic retention time as an operational tool for the production of short-chain carboxylates via anaerobic fermentation of carbohydrate-rich waste. Molecules 2023, 28, 6635. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, P.; Liu, C.; Zheng, Y.; Chen, X. Feasibility of hydrogen recovery and optimization of gas production from protein-rich food waste by bio-electrochemical system. Int. J. Hydrogen Energy 2022, 47, 31241–31254. [Google Scholar] [CrossRef]
- Li, S.; Yin, Y.; Zhang, R.; Wang, C. The impacts of cellulose on volatile fatty acid production and the microbial community in anaerobic fermentation of sludge at high and medium temperatures. Appl. Biochem. Biotechnol. 2024, 197, 631–648. [Google Scholar] [CrossRef]
- Zigova, J.; Šturdík, E. Advances in biotechnological production of butyric acid. J. Ind. Microbiol. Biotechnol. 2000, 24, 153–160. [Google Scholar] [CrossRef]
- Wu, P.; Ding, P.; Cao, Q.H.; Zhang, C.; Fu, B.; Liu, H.B.; Chen, C.J.; Liu, H. Amino acids as in-situ electron donors drive medium chain fatty acids production from sludge acidogenic fermentation liquid by electro-fermentation enhancement. Chem. Eng. J. 2023, 476, 146537. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Mohan, S.V. Understanding acidogenesis towards green hydrogen and volatile fatty acid production–critical analysis and circular economy perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Tomás-Pejó, E.; Ballesteros, M.; González-Fernandez, C. Volatile fatty acids production from protease pretreated Chlorella biomass via anaerobic digestion. Biotechnol. Prog. 2018, 34, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Tonanzi, B.; Gallipoli, A.; Frugis, A.; Gianico, A.; Lazzazzara, M.; Angelini, S.; Cecchini, C.; Braguglia, C.M. Bio-based production of medium-chain carboxylic acids from food waste and sludge without chemical addition: The pivotal role of mix ratio and pretreatment. J. Clean. Prod. 2024, 436, 140560. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Zhou, X.; Wang, X.; Zhu, J. Effect of different vegetable wastes on the performance of volatile fatty acids production by anaerobic fermentation. Sci. Total Environ. 2020, 748, 142390. [Google Scholar] [CrossRef]
- Jimenez, J.; Charnier, C.; Kouas, M.; Latrille, E.; Torrijos, M.; Harmand, J.; Patureau, D.; Spérandio, M.; Morgenroth, E.; Béline, F.; et al. Modelling hydrolysis: Simultaneous versus sequential biodegradation of the hydrolysable fractions. Waste Manag. 2020, 101, 150–160. [Google Scholar] [CrossRef]
- Alawad, I.; Ibrahim, H. Pretreatment of agricultural lignocellulosic biomass for fermentable sugar: Opportunities, challenges, and future trends. Biomass Convers. Biorefin. 2024, 14, 6155–6183. [Google Scholar] [CrossRef]
- Hu, M.; Bao, W.; Peng, Q.; Hu, W.; Yang, X.; Xiang, Y.; Yang, X.; Li, M.; Xu, P.; He, Q.; et al. Metabolic engineering of Zymomonas mobilis for co-production of D-lactic acid and ethanol using waste feedstocks of molasses and corncob residue hydrolysate. Front. Bioeng. Biotechnol. 2023, 11, 1135484. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Zhang, S.; Vo, H.N.P.; Bui, X.T.; Hoang, B.N. Volatile fatty acids production from waste streams by anaerobic digestion: A critical review of the roles and application of enzymes. Bioresour. Technol. 2022, 359, 127420. [Google Scholar] [CrossRef]
- García, E.A. Safe Chassis: Engineering Biosafety for Industrial Biotechnology. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2022. Available online: https://www.proquest.com/openview/29323d322bea3d94c573927b338c571d/1?cbl=2026366&diss=y&pq-origsite=gscholar (accessed on 10 May 2025).
- Ko, Y.J.; Cha, J.; Jeong, W.Y.; Lee, M.E.; Cho, B.H.; Nisha, B.; Jeong, H.J.; Park, S.E.; Han, S.O. Bio-isopropanol production in Corynebacterium glutamicum: Metabolic redesign of synthetic bypasses and two-stage fermentation with gas stripping. Bioresour. Technol. 2022, 354, 127171. [Google Scholar] [CrossRef]




| Acid | Chemical Formula | Molecular Mass (g/mol) | Density (g/L) at 20 °C | Boiling Point (°C) | pKa | Average Price (USD/kg, 2024) | Market Size 2023 |
|---|---|---|---|---|---|---|---|
| Acetic (HAc) | CH3COOH | 60 | 1050 | 118 | 4.76 | 0.80–1.25 | 12.1 US$ Billion |
| Propionic (HPr) | CH3CH2COOH | 74 | 990 | 141 | 4.88 | 2.00–2.45 | 1.11 US$ Billion |
| Butyric (HBu) | CH3CH2CH2COOH | 88 | 960 | 164 | 4.82 | 2.20–2.75 | 389.2 US$ Million |
| Isobutyric (iHBu) | (CH3)2CHCOOH | 88 | 950 | 154 | 4.84 | 2.50–3.00 | 182.4 US$ Million |
| Valeric (HVa) | CH3(CH2)3COOH | 102 | 940 | 186 | 4.84 | 4.20–4.65 | 13 US$ Billion |
| Isovaleric (iHVa) | (CH3)2CHCH2COOH | 102 | 930 | 177 | 4.77 | 3.50–4.00 | 1.6 US$ Billion |
| Volatile Fatty Acid | Related Microorganisms | Key Notes |
|---|---|---|
| Acetic | Acetobacter aceti Moorella thermoacetica Aurantiochytrium limacinum Clostridium lentocellum Clostridium ljungdahlii Saccharomyces cerevisiae Acetobacter pasteurianus | Efficient production from carbohydrate-rich substrates like whey and agricultural residues. High theoretical yields with agricultural residues. |
| Propionic | Propionibacterium acidipropionici Propionibacterium freudenreichii Peptostreptococcus Anaerotignum propionicum | Utilizes glucose, glycerol, and protein-rich substrates for high yields of propionic acid. |
| Butyric | Clostridium butyricum Clostridium tyrobutyricum Clostridium acetobutylicum Megasphaera Butyrivibrio fibrisolvens | High yields in various substrates; chain elongation in microbial electrosynthesis. Unique metabolic pathways allow high titers of n-butanol and butyrate, demonstrating versatility in bioaugmentation systems. |
| Valeric | Clostridium spp. Propionibacterium freudenreichii Megasphaera Lachnospiraceae bacterium | Production through chain elongation and robust fermentation pathways. |
| Caproic | Clostridium kluyveri Ruminococcaceae bacterium | Produced via reverse β-oxidation with lignocellulosic residues. |
| Isobutyric | Escherichia coli Pseudomonas sp. Corynebacterium glutamicum | Engineered pathways for selective production with minimal by-products. Optimized through metabolic engineering to achieve higher purity and yield. |
| Isovaleric | Propionibacterium freudenreichii Escherichia coli Leuconostoc mesenteroides | Production from branched-chain amino acid pathways; useful in dairy fermentations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, D.; Garrote, L.; Infante, F.; Martín-Marroquín, J.M.; Pérez-Zapatero, E.; Corona, F. Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors. Appl. Sci. 2025, 15, 5923. https://doi.org/10.3390/app15115923
Hidalgo D, Garrote L, Infante F, Martín-Marroquín JM, Pérez-Zapatero E, Corona F. Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors. Applied Sciences. 2025; 15(11):5923. https://doi.org/10.3390/app15115923
Chicago/Turabian StyleHidalgo, Dolores, Lidia Garrote, Francisco Infante, Jesús M. Martín-Marroquín, Enrique Pérez-Zapatero, and Francisco Corona. 2025. "Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors" Applied Sciences 15, no. 11: 5923. https://doi.org/10.3390/app15115923
APA StyleHidalgo, D., Garrote, L., Infante, F., Martín-Marroquín, J. M., Pérez-Zapatero, E., & Corona, F. (2025). Targeted Acidogenic Fermentation of Waste Streams for the Selective Production of Volatile Fatty Acids as Bioplastic Precursors. Applied Sciences, 15(11), 5923. https://doi.org/10.3390/app15115923






