Effect of the Concentration of a Nitrite-Based Inhibitor and Chloride Ions on the Corrosion Behavior of FCD-500 in a Simulated Marine Engine Cooling Water System
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen and Corrosion Inhibitor
2.2. Immersion Test of FCD-500 Coupons
2.3. Surface Morphologies
2.4. Electrochemical Test
3. Results and Discussion
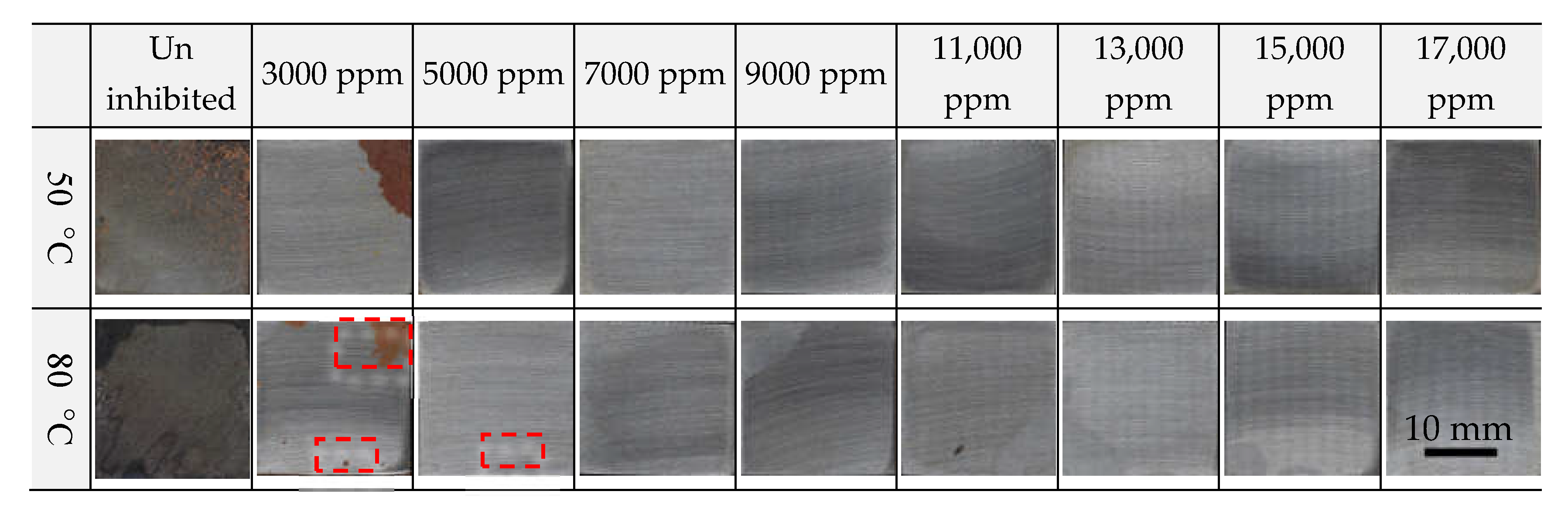
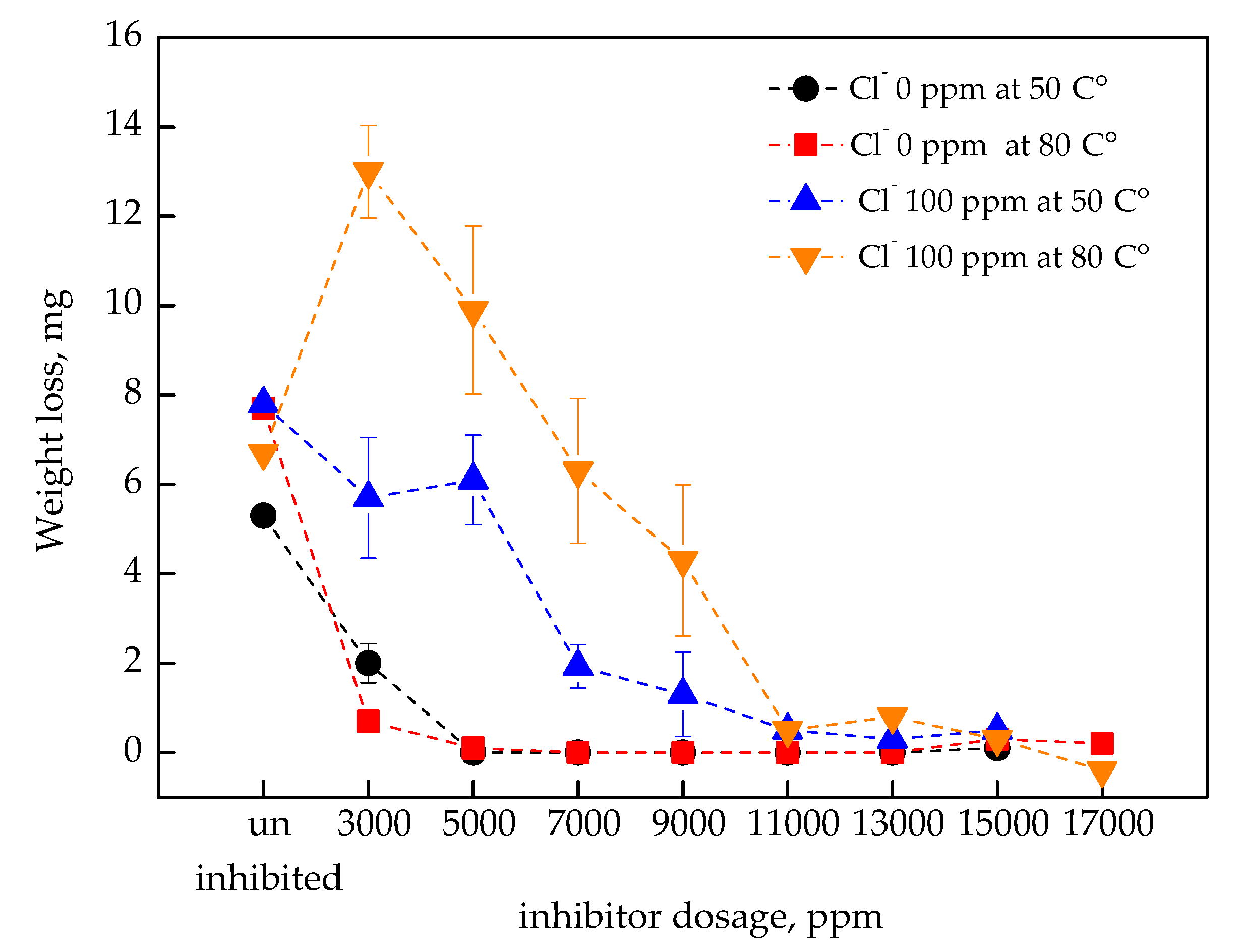
3.1. Corrosion Inhibition According to the Nitrite Dosage
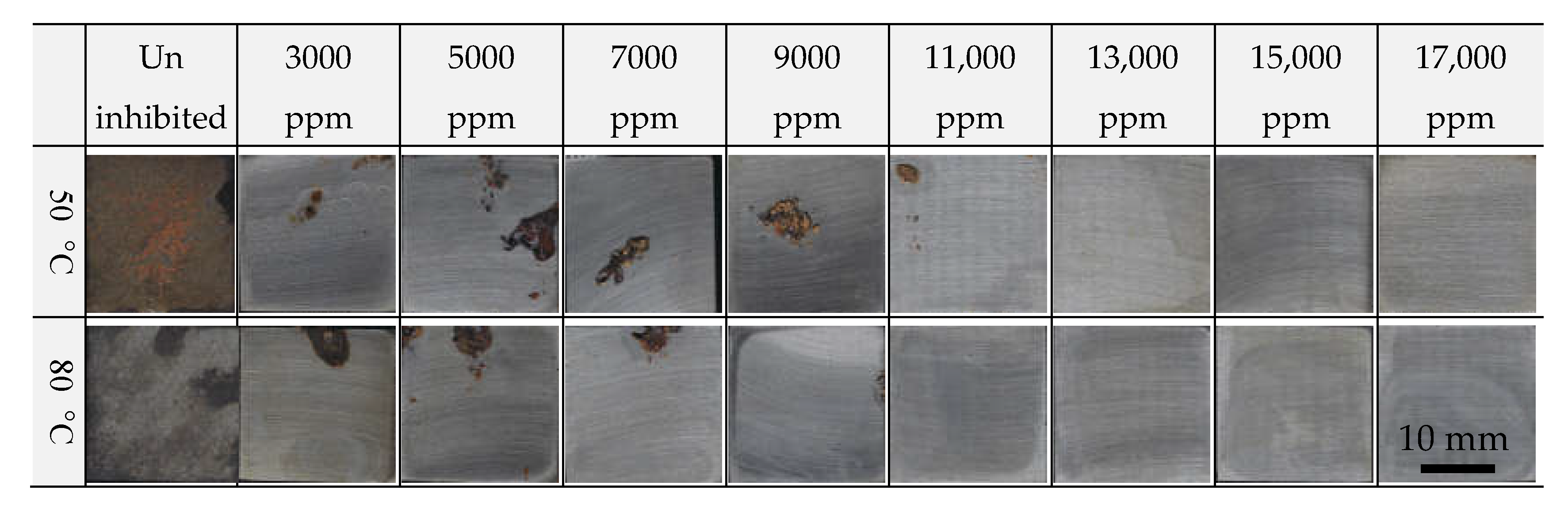
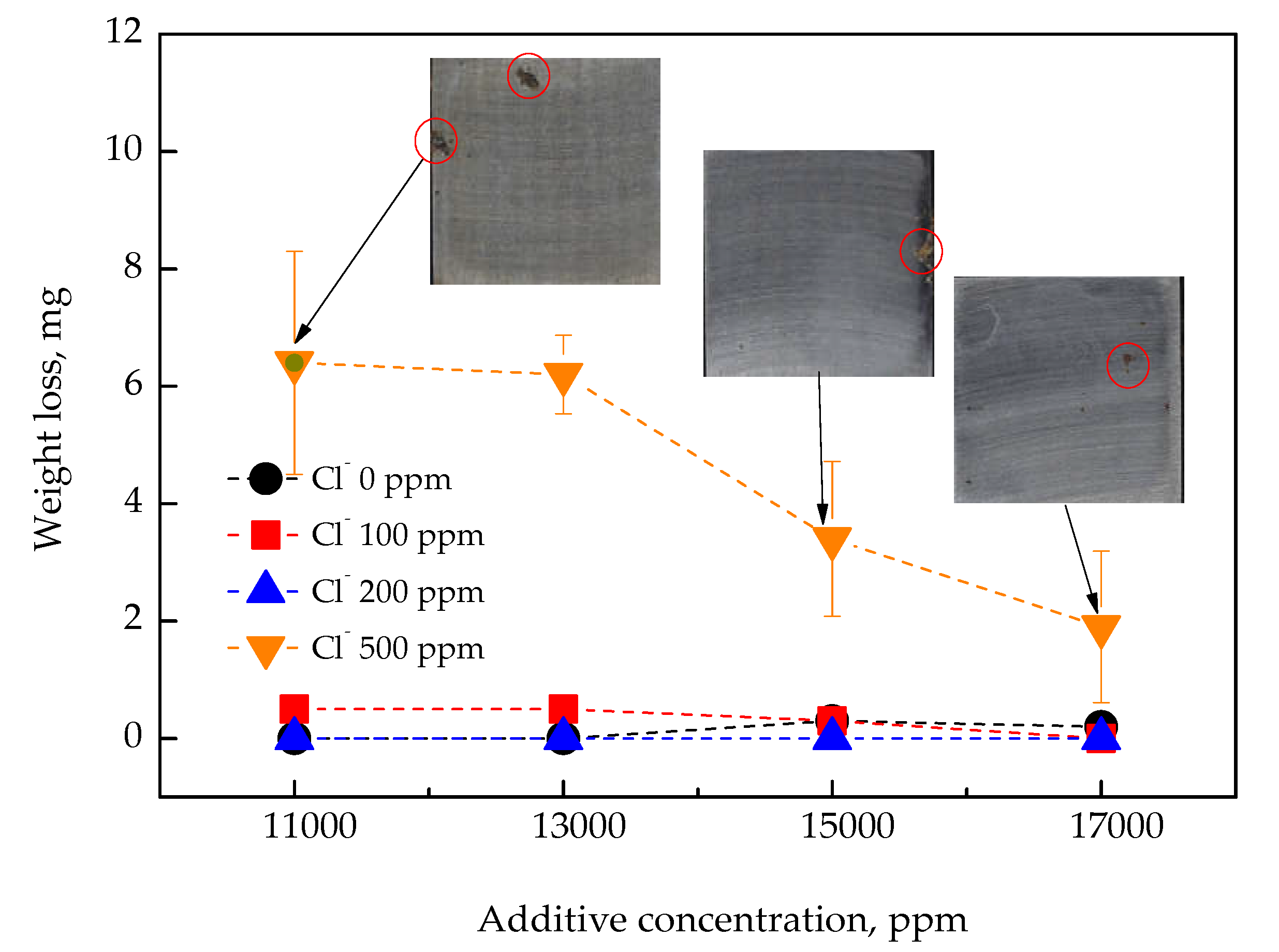
3.2. Corrosion Behavior Under Simulated Marine CWS Conditions
- Condition 1: well-maintained CW, with optimal additives and a negligible chloride level (Cl− free);
- Condition 2: adequate inhibitor concentration maintained, but chloride concentration has reached the recommended limit (Cl− 100 ppm);
- Condition 3: inhibitor concentration maintained, but the chloride concentration has exceeded the recommended limit due to insufficient management (Cl− 200 ppm);
- Condition 4: inhibitor concentration maintained, but with seawater intrusion, equivalent to 2% of the total coolant volume (Cl− 500 ppm).
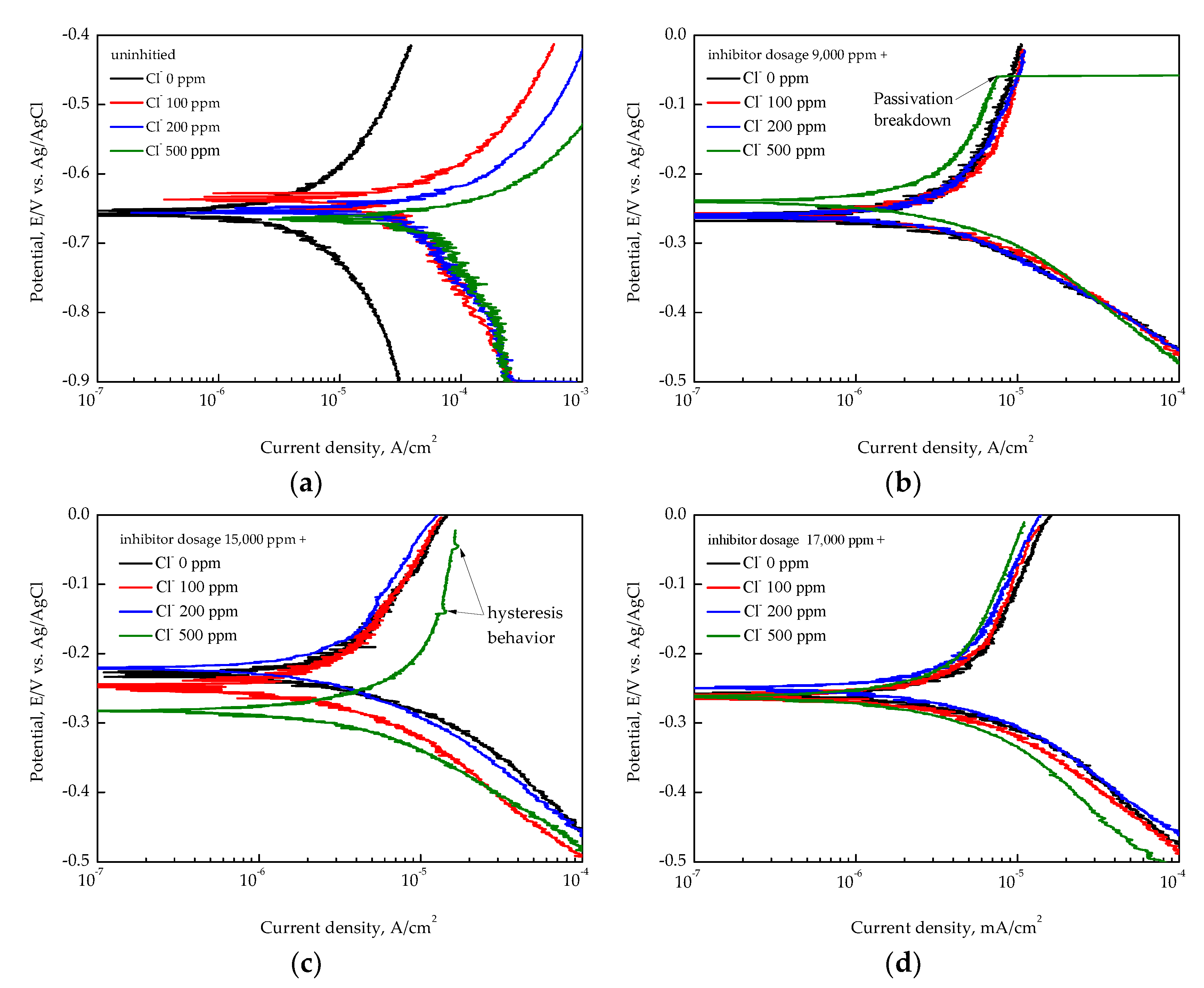
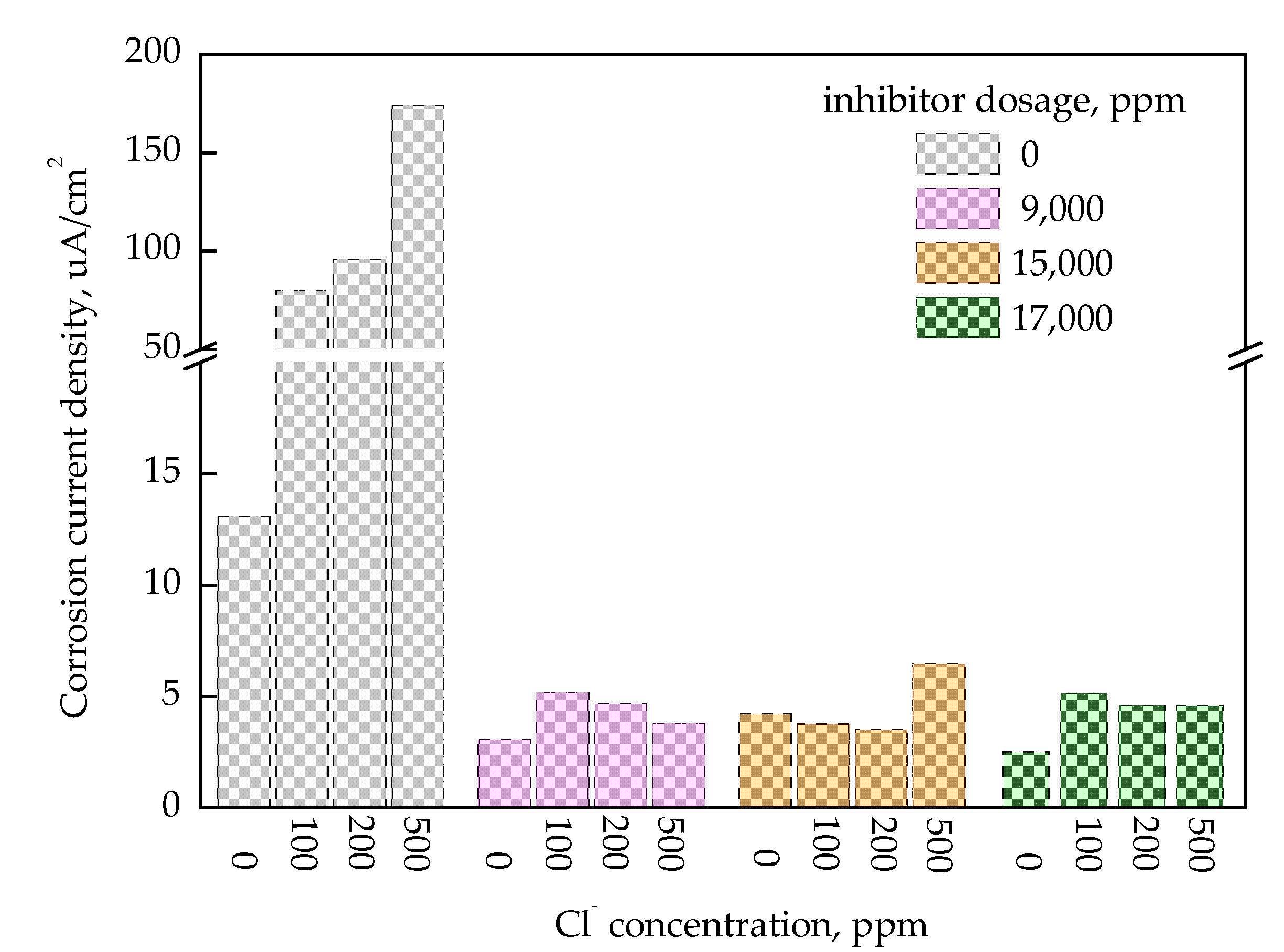
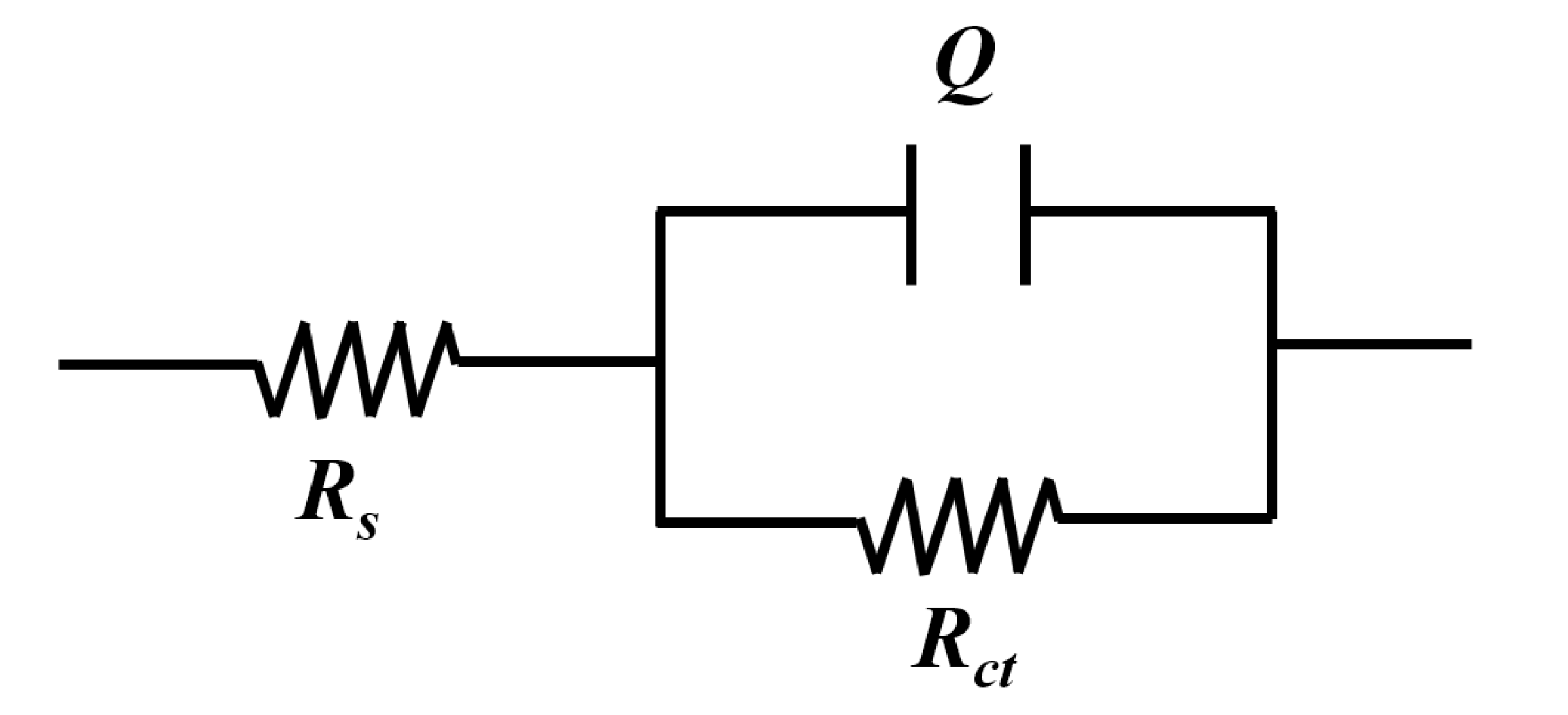
3.3. EIS Analysis of Inhibition Performance
4. Conclusions
- In the absence of Cl−, general and localized corrosion were effectively inhibited at both 50 °C and 80 °C when the inhibitor dosage exceeded 5000 ppm in the immersion coupon test;
- In 100 ppm of Cl−, the inhibitor required a concentration above 13,000 ppm to ensure comparable corrosion inhibition performance;
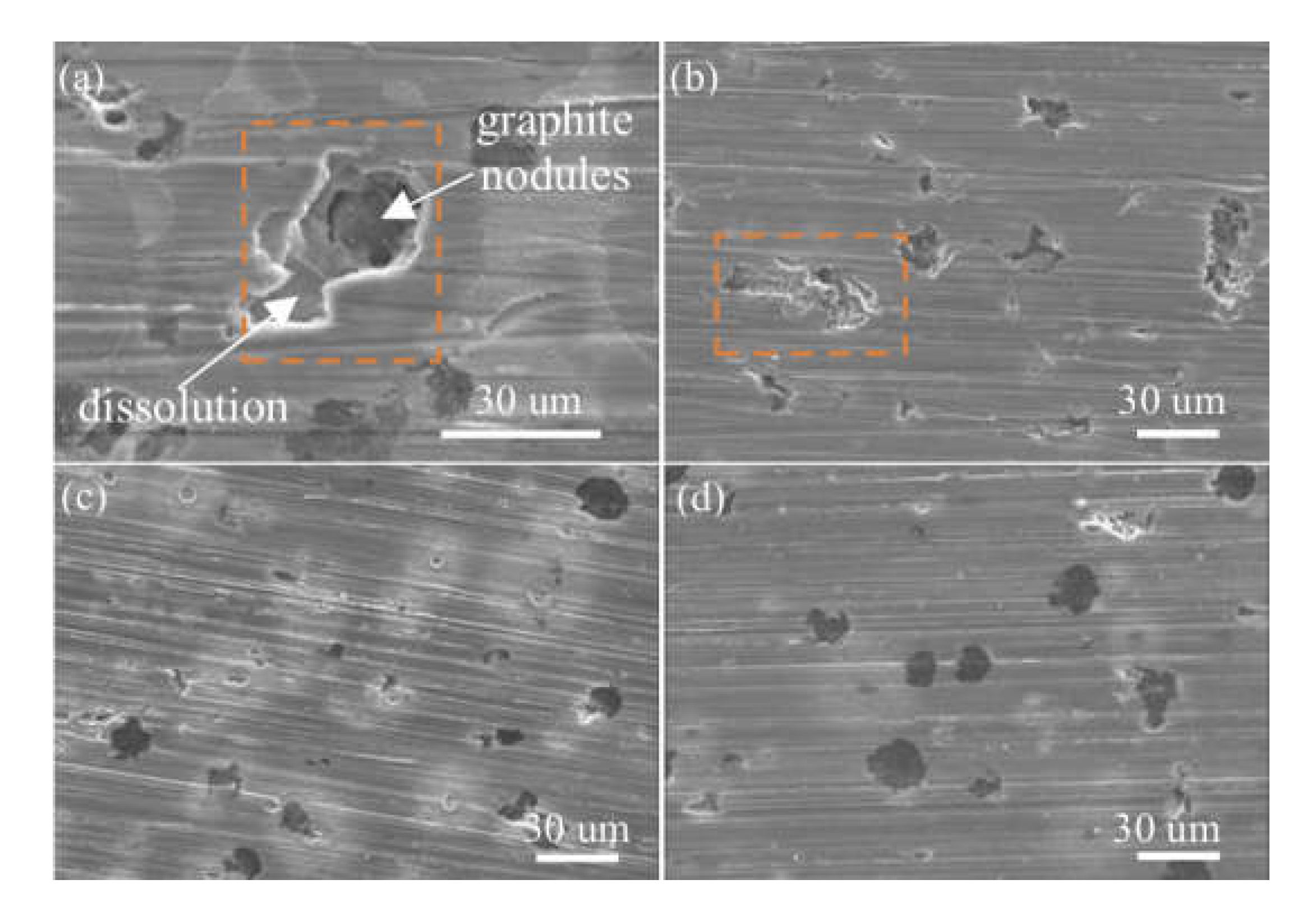
- FCD-500 showed uniform corrosion under uninhibited conditions, whereas insufficient inhibitor concentrations led to localized corrosion. It was primarily observed around the matrix near the graphite nodules;
- With a high level of Cl− (500 ppm), visible rust was observed even at 17,000 ppm, although the corrosion current density remained below 6.42 μA/cm2 in the electrochemical reaction;
- Electrochemical impedance spectroscopy (EIS) confirmed the formation of a stable passive film with an increasing inhibitor concentration. The highest inhibition efficiency, calculated from the EIS parameter, was approximately 97.3% at 17,000 ppm inhibitor dosage under 100 ppm of Cl−;
- Overall, the inhibition performance was well-maintained within the inhibitor concentration range from 11,000 to 17,000 ppm under chloride ion concentrations below 200 ppm. However, in high-chloride environments (500 ppm), the inhibition performance became unstable or declined.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSW | Cooling Water System |
| CW | Cooling Water |
| FCD | Ductile Cast Iron |
| CPE | Constant Phase Element |
References
- Xue, F.; Wei, X.; Dong, J.; Wang, C.; Ke, W. Effect of chloride ion on corrosion behavior of low carbon steel in 0.1 M NaHCO3 solution with different dissolved oxygen concentrations. J. Mater. Sci. Technol. 2019, 35, 596–603. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Coffetti, D.; Coppola, L.; Pastore, T. Inhibition effect of tartrate ions on the localized corrosion of steel in pore solution at different chloride concentrations. Buildings 2020, 10, 105. [Google Scholar] [CrossRef]
- Otani, K.; Sakairi, M. Effects of metal cations on corrosion of mild steel in model fresh water. Corros. Sci. 2016, 111, 302–312. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Zhang, D.; Wang, X.; Ju, C.; Jiang, B. A superior strength and sliding-wear resistance combination of ductile iron with nanobainitic matrix. J. Mater. Res. Technol. 2021, 11, 1175–1183. [Google Scholar] [CrossRef]
- Zhuang, H.; Shen, J.; Yu, M.; An, X.; Hu, J. Mechanism analysis for the enhancement of low-temperature impact toughness of nodular cast iron by heat treatment. Materials 2024, 17, 513. [Google Scholar] [CrossRef]
- Şeker, U.; Hasirci, H. Evaluation of machinability of austempered ductile irons in terms of cutting forces and surface quality. J. Mater. Process. Technol. 2006, 173, 260–268. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Deng, Y.; Yang, L.; Zhang, J. Nitrate on localized corrosion of carbon steel and stainless steel in aqueous solutions. Electrochim. Acta 2021, 369, 137660. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Martin, U.; Bastidas, J.M.; Ress, J. Corrosion inhibition mechanism of steel reinforcements in mortar using soluble phosphates: A critical review. Materials 2021, 14, 6168. [Google Scholar] [CrossRef]
- Li, J.; Lai, T.; Chen, Y.; Yan, H.; Song, H.; Luo, C.; Hu, Z. Effect of the sodium silicate inhibitor on the corrosion protection of AZ31 magnesium alloy. Materials 2024, 17, 5533. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Corrosion inhibitors: Natural and synthetic organic inhibitors. Lubricants 2023, 11, 174. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Seyeux, A.; Zanna, S.; Pailleret, A.; Nesic, S.; Marcus, P. Adsorption mechanism of quaternary ammonium corrosion inhibitor on carbon steel surface using ToF-SIMS and XPS. Corros. Sci. 2023, 213, 110952. [Google Scholar] [CrossRef]
- Zhou, J.; Niu, X.; Cui, Y.; Wang, Z.; Wang, J.; Wang, R. Study on the film forming mechanism, corrosion inhibition effect and synergistic action of two different inhibitors on copper surface chemical mechanical polishing for GLSI. Appl. Surf. Sci. 2020, 505, 144507. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Nitrate as corrosion inhibitor. In Inorganic Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–296. [Google Scholar]
- Gaidis, J.M. Chemistry of corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 181–189. [Google Scholar] [CrossRef]
- Berke, N.S.; Hicks, M.C. Predicting long-term durability of steel reinforced concrete with calcium nitrite corrosion inhibitor. Cem. Concr. Compos. 2004, 26, 191–195. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.; Olek, J. Differentiating seawater and groundwater sulfate attack in Portland cement mortars. Cem. Concr. Res. 2006, 36, 2132–2137. [Google Scholar] [CrossRef]
- Touir, R.; Dkhireche, N.; Touhami, M.E.; Lakhrissi, M.; Lakhrissi, B.; Sfaira, M. Corrosion and scale processes and their inhibition in simulated cooling water systems by monosaccharides derivatives: Part I: EIS study. Desalination 2009, 249, 922–928. [Google Scholar] [CrossRef]
- Coelho, L.B.; Lukaczynska-Anderson, M.; Clerick, S.; Buytaert, G.; Lievens, S.; Terryn, H.A. Corrosion inhibition of AA6060 by silicate and phosphate in automotive organic additive technology coolants. Corros. Sci. 2022, 199, 110188. [Google Scholar] [CrossRef]
- Al-Sodani, K.A.A.; Al-Amoudi, O.S.B.; Maslehuddin, M.; Shameem, M. Efficiency of corrosion inhibitors in mitigating corrosion of steel under elevated temperature and chloride concentration. Constr. Build. Mater. 2018, 163, 97–112. [Google Scholar] [CrossRef]
- Emregül, K.C.; Kurtaran, R.; Atakol, O. An investigation of chloride-substituted Schiff bases as corrosion inhibitors for steel. Corros. Sci. 2003, 45, 2803–2817. [Google Scholar] [CrossRef]
- Kozaderov, O.; Shikhaliev, K.; Prabhakar, C.; Tripathi, A.; Shevtsov, D.; Kruzhilin, A.; Kuznetsov, Y. Corrosion of α-brass in solutions containing chloride ions and 3-mercaptoalkyl-5-amino-1H-1,2,4-triazoles. Appl. Sci. 2019, 9, 2821. [Google Scholar] [CrossRef]
- Zunita, M.; Wahyuningrum, D.; Buchari; Bundjali, B.; Wenten, I.G.; Boopathy, R. Corrosion inhibition performances of imidazole derivatives-based new ionic liquids on carbon steel in brackish water. Appl. Sci. 2020, 10, 7069. [Google Scholar] [CrossRef]
- Ogundare, O.; Babatope, B.; Adetunji, A.; Olusunle, O. Atmospheric Corrosion Studies of Ductile Iron and Austenitic Stainless Steel in an Extreme Marine Environment. J. Miner. Mater. Charact. Eng. 2012, 11, 914–918. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Akinwamide, S.O.; Ajibola, O.; Obadele, B.A.; Olusunle, S.O.; Olubambi, P.A. Corrosion behavior of ductile and austempered ductile cast iron in 0.01 M and 0.05 M Nacl Environments. Procedia Manuf. 2019, 30, 167–172. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of Chloride Ions on Corrosion of Ductile Iron and Carbon Steel in Soil Environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, H.W.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. Corrosion Inhibiting Mechanism of Nitrite Ion on the Passivation of Carbon Steel and Ductile Cast Iron for Nuclear Power Plants. Adv. Mater. Sci. Eng. 2015, 2015, 408138. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Chen, T.; Hao, L.; Wu, J.; Liu, C. The Effect of Corrosion Inhibitors on the Corrosion Behavior of Ductile Cast Iron. Metals 2025, 15, 70. [Google Scholar] [CrossRef]
- Ahn, J.K.; So, M.O. Load-dependent PID temperature control of jacket cooling-water system for two stroke low speed marine main diesel engine. J. Korean Soc. Mar. Eng. 2018, 42, 572–579. [Google Scholar]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- Guo, L.; Ou, Y.; Shen, X.; Kaya, S.; Shi, W.; Zhang, R.; Wang, J. Specific adsorption of halide ions on iron surface: A combined electrochemical and Monte Carlo simulation investigation. Int. J. Electrochem. Sci. 2017, 12, 7064–7074. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.P.; Normand, B. Hydrodynamic effect on the behaviour of a corrosion inhibitor film: Characterization by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 4011–4020. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Abdo, H.S.; Zein El Abedin, S. Corrosion inhibition of cast iron in Arabian gulf seawater by two different ionic liquids. Materials 2015, 8, 3883–3895. [Google Scholar] [CrossRef] [PubMed]
- Khaled, K.F. Experimental and theoretical study for corrosion inhibition of mild steel in hydrochloric acid solution by some new hydrazine carbodithioic acid derivatives. Appl. Surf. Sci. 2006, 252, 4120–4128. [Google Scholar] [CrossRef]
- Khani, H.; Arefinia, R. Inhibition mechanism of nitrite on the corrosion of carbon steel in simulated cooling water systems. Mater. Corros. 2018, 69, 337–345. [Google Scholar] [CrossRef]
- Rizvi, M.; Gerengi, H.; Kaya, S.; Uygur, I.; Yıldız, M.; Sarıoglu, I.; El Ibrahimi, B. Sodium nitrite as a corrosion inhibitor of copper in simulated cooling water. Sci. Rep. 2021, 11, 8353. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Lu, M.; Chai, C.; Wu, Y. Evaluation of inhibition efficiency of an imidazoline derivative in CO2-containing aqueous solution. Mater. Chem. Phys. 2007, 105, 331–340. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Shaker, L.M.; Isahak, W.N.R.W.; Takriff, M.S. Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution. Sci. Rep. 2022, 12, 4705. [Google Scholar] [CrossRef]


| FCD-500 | Y.S (N/mm2) | T.S (N/mm2) | E.L (%) | Hardness (HB) 10/3000 |
| 389 | 563 | 7 | 192~201 |
| FCD-500 | C | Si | Mn | P | S | Mg | Sn |
| 2.90~ 3.90 | 2.20~ 3.40 | Max. 0.70 | Max. 0.10 | Max. 0.02 | Max. 0.065 | Max. 0.10 | |
| 3.57 | 2.75 | 0.21 | 0.047 | 0.006 | 0.035 | - |
| Dosage per 500 mL D.I Water | Nitrite Concentration, ppm | pH | Manufacturer’s Recommendation |
|---|---|---|---|
| Uninhibited | 0 | 6.4 | - |
| 1 mL | 3000 | 10.4 | Lower Control Limit |
| 2 mL | 5000 | 10.6 | |
| 3 mL | 7000 | 10.8 | |
| 4 mL | 9000 | 11.0 | Within Control Limits |
| 5 mL | 11,000 | 11.1 | |
| 6 mL | 13,000 | 11.2 | |
| 7 mL | 15,000 | 11.2 | |
| 8 mL | 17,000 | 11.3 | Upper Control Limit |
| Inhibitor Dosage, ppm | Rs, Ω | Yo × 10−4, S·sn | n | Rct, Ω | IE, % | x2 × 10−3 |
|---|---|---|---|---|---|---|
| Blank (0) | 528.1 | 25.81 | 0.86 | 991.6 | 0 | 1.08 |
| 7000 | 182.8 | 6.44 | 0.59 | 3492 | 71.6 | 0.3 |
| 9000 | 161.0 | 0.65 | 0.88 | 30,280 | 96.7 | 1.04 |
| 15,000 | 99.75 | 0.62 | 0.9 | 35,990 | 97.2 | 0.33 |
| 17,000 | 95.87 | 0.71 | 0.88 | 36,100 | 97.3 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, W.-S.; Jung, K.-H. Effect of the Concentration of a Nitrite-Based Inhibitor and Chloride Ions on the Corrosion Behavior of FCD-500 in a Simulated Marine Engine Cooling Water System. Appl. Sci. 2025, 15, 5883. https://doi.org/10.3390/app15115883
Jeon W-S, Jung K-H. Effect of the Concentration of a Nitrite-Based Inhibitor and Chloride Ions on the Corrosion Behavior of FCD-500 in a Simulated Marine Engine Cooling Water System. Applied Sciences. 2025; 15(11):5883. https://doi.org/10.3390/app15115883
Chicago/Turabian StyleJeon, Woo-Seok, and Kwang-Hu Jung. 2025. "Effect of the Concentration of a Nitrite-Based Inhibitor and Chloride Ions on the Corrosion Behavior of FCD-500 in a Simulated Marine Engine Cooling Water System" Applied Sciences 15, no. 11: 5883. https://doi.org/10.3390/app15115883
APA StyleJeon, W.-S., & Jung, K.-H. (2025). Effect of the Concentration of a Nitrite-Based Inhibitor and Chloride Ions on the Corrosion Behavior of FCD-500 in a Simulated Marine Engine Cooling Water System. Applied Sciences, 15(11), 5883. https://doi.org/10.3390/app15115883






