Hand Dynamics in Healthy Individuals and Spinal Cord Injury Patients During Real and Virtual Box and Block Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Study
2.2. Data Preprocessing
2.2.1. Kinematic Data
2.2.2. sEMG Data
2.2.3. Data Segmentation and Selection
2.3. Kinematic Data Analysis
2.3.1. Cycle-Averaged Kinematic Parameters Definition
2.3.2. Cycle-Averaged Kinematic Parameters Analysis
2.3.3. Temporal Kinematic Analysis
2.4. Electromyographic Data Analysis
2.4.1. Cycle-Averaged sEMG Parameters Definition
2.4.2. Cycle-Averaged sEMG Parameters Analysis
2.4.3. Temporal sEMG Analysis
3. Results
3.1. Kinematic Data Analysis Results
3.1.1. Cycle-Averaged Kinematic Parameters Related to Trajectory: Smoothness and Efficiency
3.1.2. Cycle-Averaged Kinematic Parameters Related to Kinematic Synergies: Median and Ranges
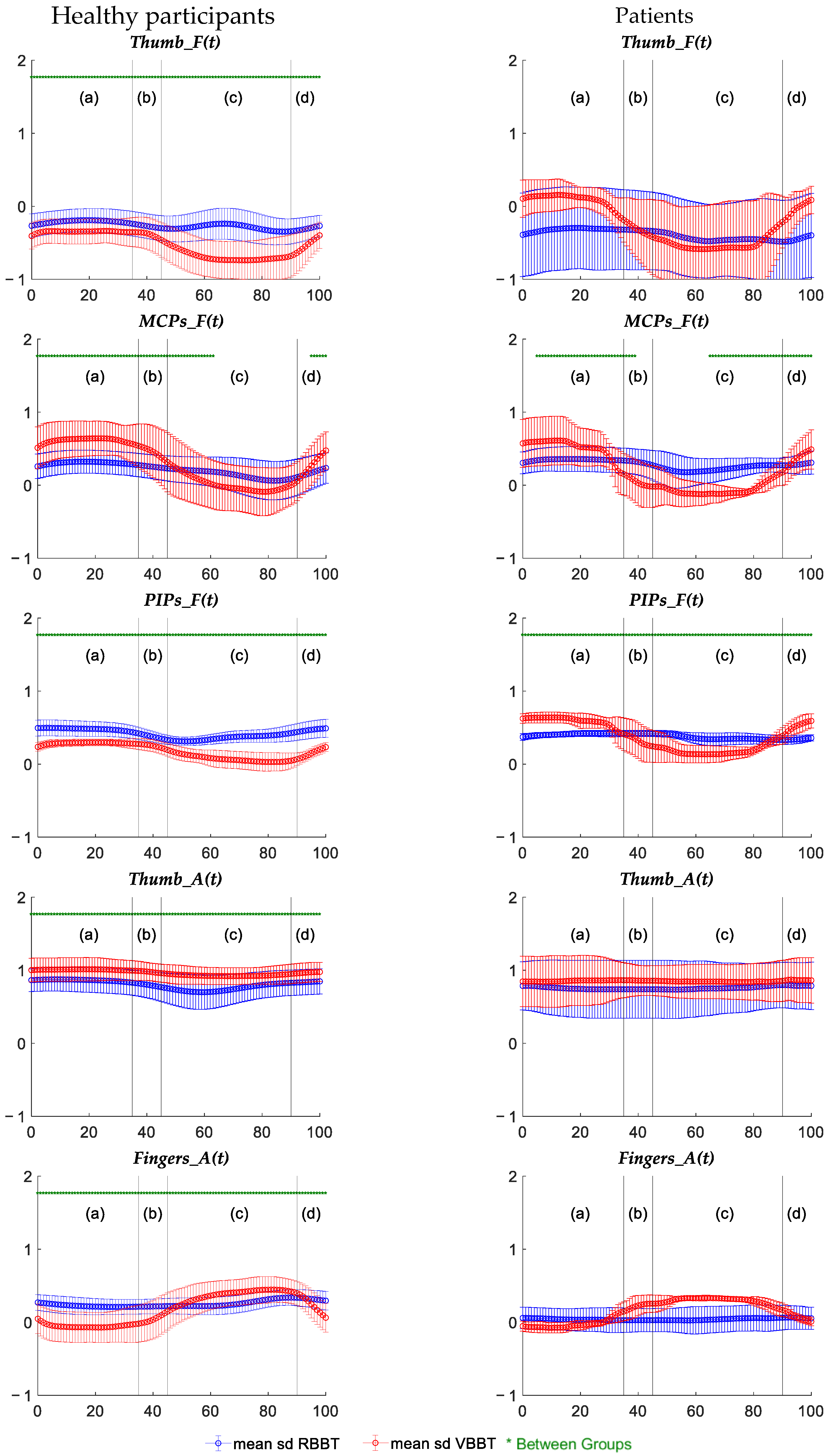
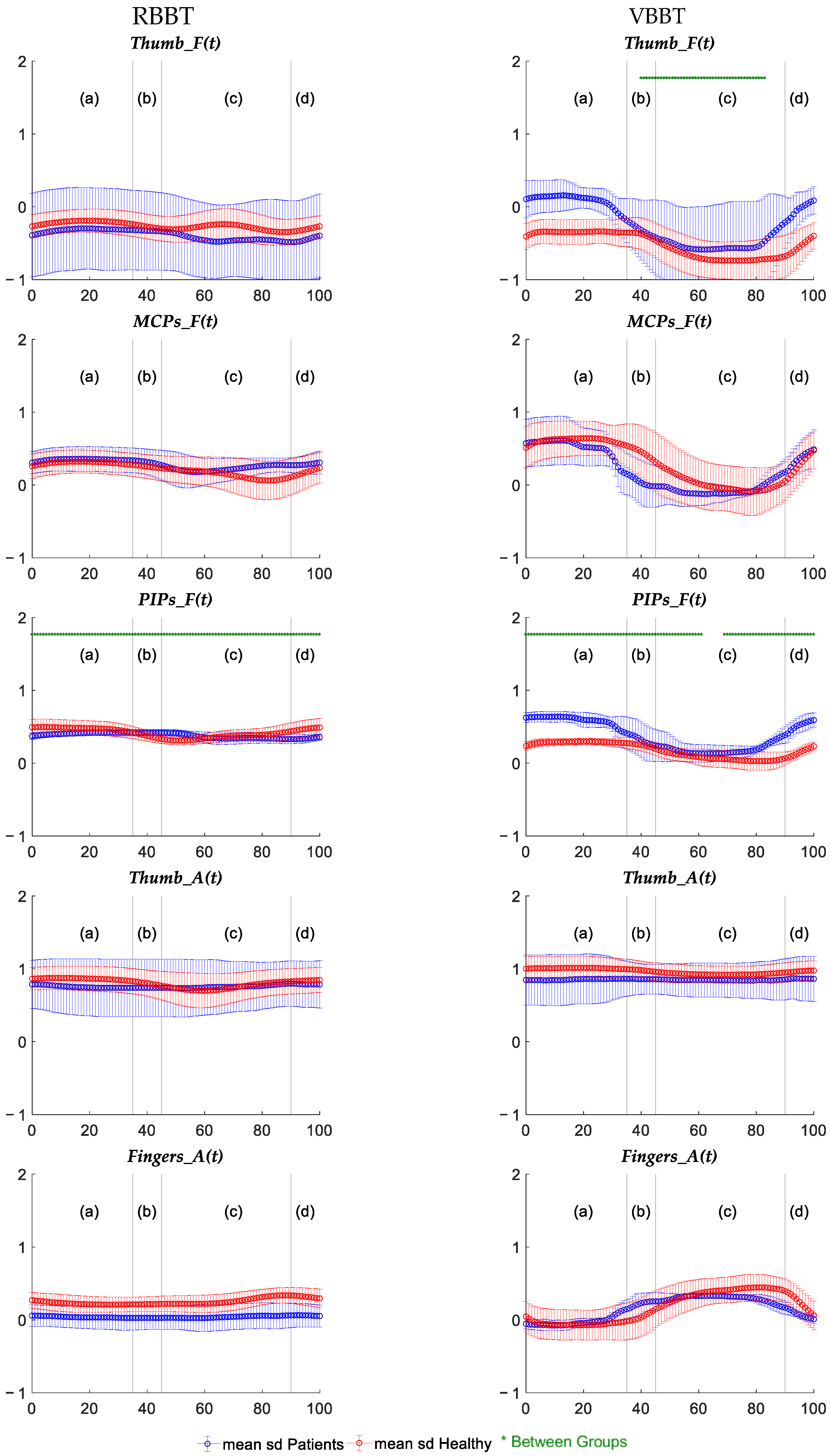
3.1.3. Temporal Kinematic Analysis Results
3.2. Electromyographic Data Analysis Results
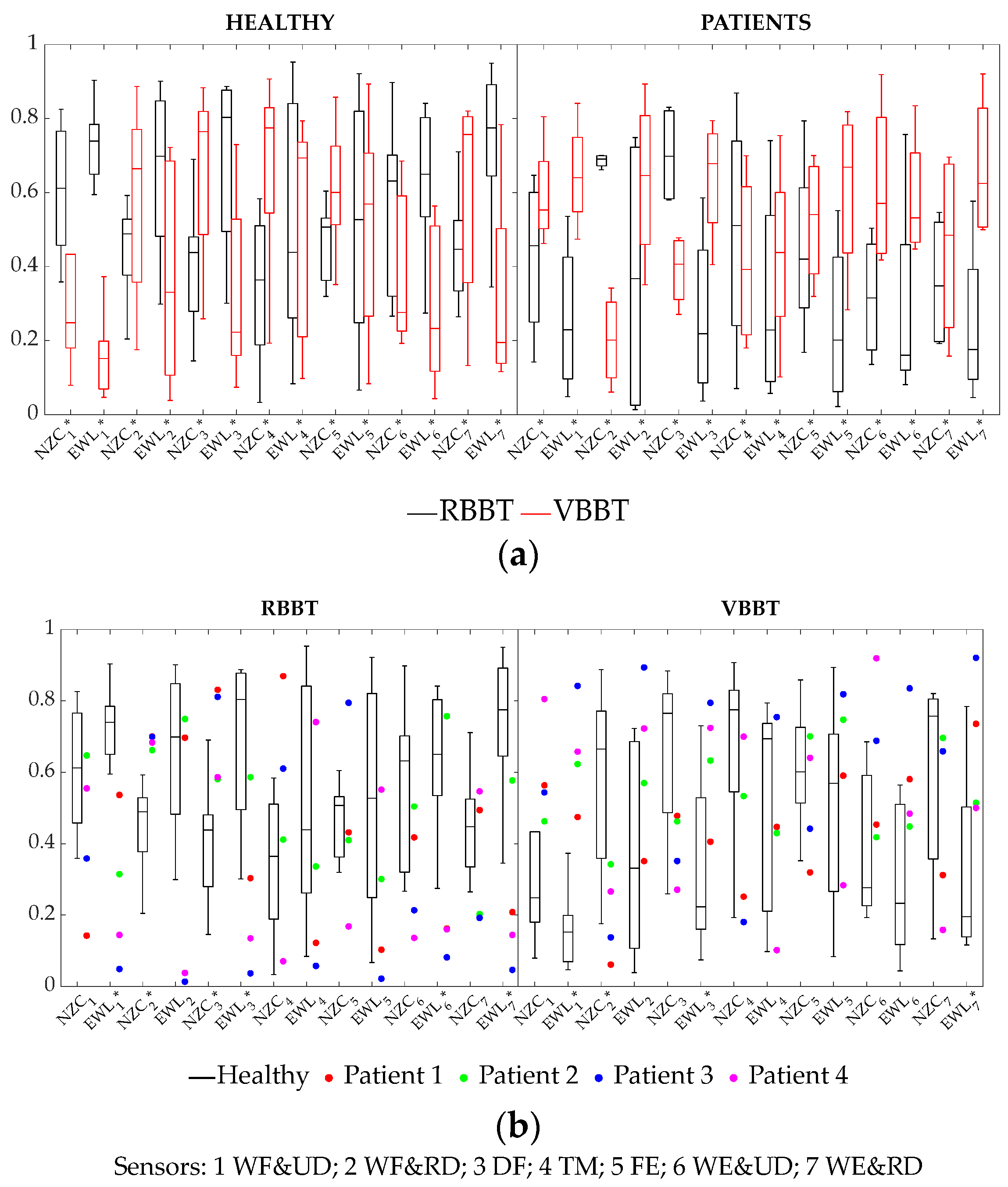
3.2.1. Cycle-Averaged sEMG Parameters Based on Signal Waveform
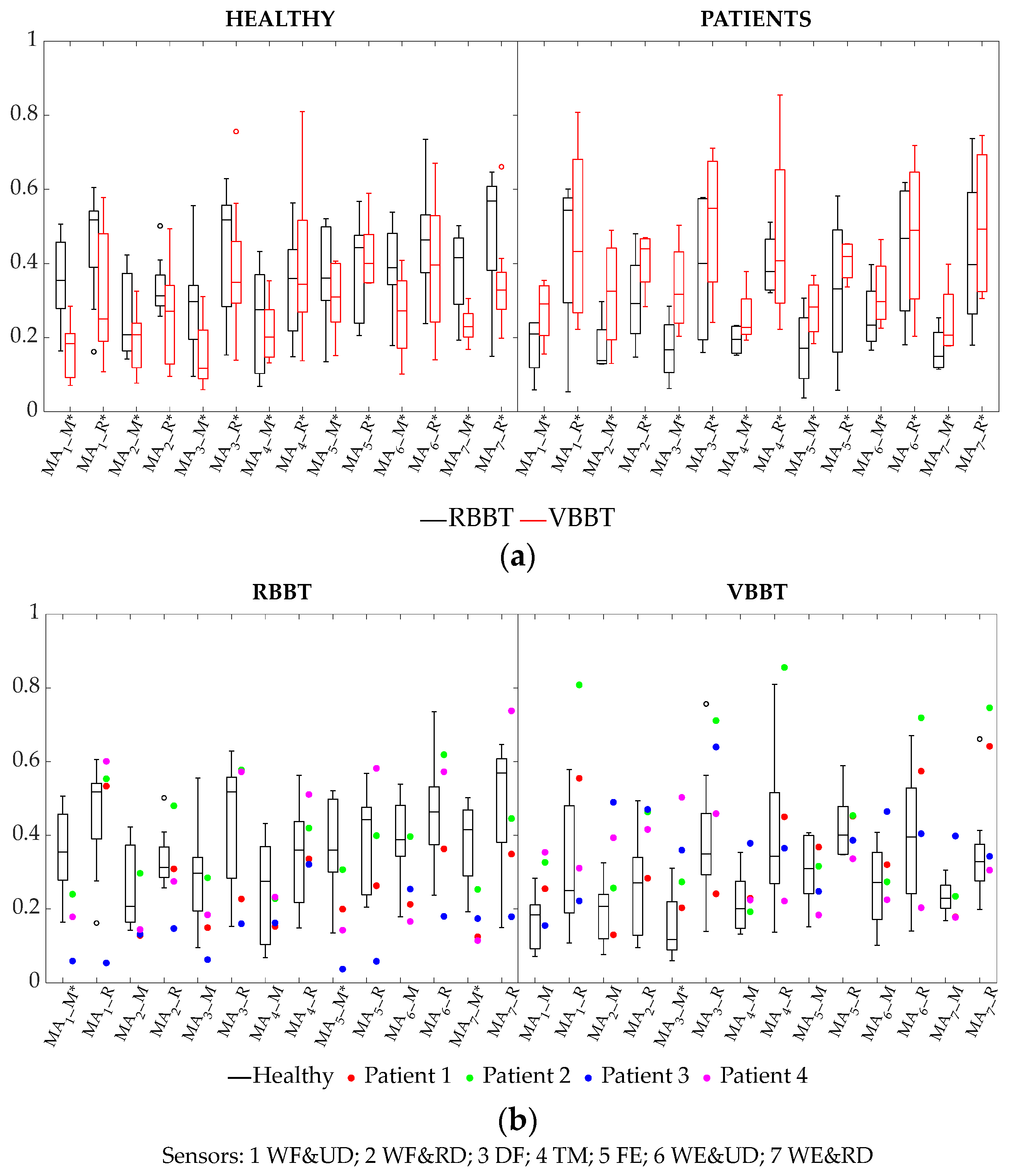
3.2.2. Cycle-Averaged Muscular Activation Parameters: Median and Ranges
3.2.3. Temporal Analysis of Muscular Activation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilera-Rubio, A.; Alguacil-Diego, I.M.; Mallo-López, A.; Cuesta-Gómez, A. Use of the Leap Motion Controller® System in the Rehabilitation of the Upper Limb in Stroke. A Systematic Review. J. Stroke Cerebrovasc. Dis. 2022, 31, 106174. [Google Scholar] [CrossRef] [PubMed]
- Kintschner, N.R.; Liporace, T.L.; Blascovi-Assis, S.M.; Corrêa, A.G.D. The Use of Leap Motion in Manual Dexterity Testing by the Box and Blocks Test: A Review Study; Open Access Book: The Hague, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Baranyi, R.; Körber, Y.; Galimov, P.; Parandeh, Z.; Grechenig, T. Rehafox–A therapeutical approach developing a serious game to support rehabilitation of stroke patients using a leap motion controller. Clin. eHealth 2023, 6, 85–95. [Google Scholar] [CrossRef]
- Iosa, M.; Fusco, A.; Castagnoli, M.; Fusco, F.R.; Pratesi, L. Leap motion controlled videogame-based therapy for rehabilitation of elderly patients with subacute stroke: A feasibility pilot study. Top. Stroke Rehabil. 2015, 22, 306–316. [Google Scholar] [CrossRef]
- Fong, K.N.K.; Tang, Y.M.; Sie, K.; Yu, A.K.H.; Lo, C.C.W.; Ma, Y.W.T. Task-specific virtual reality training on hemiparetic upper extremity in patients with stroke. Virtual Real. 2022, 26, 453–464. [Google Scholar] [CrossRef]
- Wu, Y.T.; Chen, K.H.; Ban, S.L.; Tung, K.Y.; Chen, L.R. Evaluation of leap motion control for hand rehabilitation in burn patients: An experience in the dust explosion disaster in Formosa Fun Coast. Burns 2019, 45, 157–164. [Google Scholar] [CrossRef]
- Fernández-González, P.; Carratalá-Tejada, M.; Monge-Pereira, E. Leap motion controlled video game-based therapy for upper limb rehabilitation in patients with Parkinson’s disease: A feasibility study. J. Neuroeng. Rehabil. 2019, 16, 133. [Google Scholar] [CrossRef]
- Reyes-Guzmán, A.D.L.; Lozano-Berrio, V.; Alvarez-Rodríguez, M.; López-Dolado, E.; Ceruelo-Abajo, S.; Talavera-Díaz, F.; Gil-Agudo, A. RehabHand: Oriented-tasks serious games for upper limb rehabilitation by using Leap Motion Controller and target population in spinal cord injury. NeuroRehabilitation 2021, 48, 365–373. [Google Scholar] [CrossRef]
- Nattah, M.M.A.A.; Tiberti, S.; Segaletti, L. Semi-Immersive Virtual Reality Exercise Therapy for Upper Limb Rehabilitation in Patients With Spinal Cord Injury Using the Leap Motion Controller. Cureus 2024, 16, e52261. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodríguez, M.; López-Dolado, E.; Salas-Monedero, M.; Lozano-Berrio, V. Concurrent Validity of a Virtual Version of Box and Block Test for Patients with Neurological Disorders. World J. Neurosci. 2019, 10, 79–89. [Google Scholar] [CrossRef]
- Daliri, M.; Moradi, A.; Fatorehchy, S.; Bakhshi, E.; Moradi, E.; Sabbaghi, S. Investigating the Effect of Leap Motion on Upper Extremity Rehabilitation in Children with Cerebral Palsy: A Randomized Controlled Trial. Dev. Neurorehabil. 2023, 26, 244–252. [Google Scholar] [CrossRef]
- Everard, G.; Otmane-Tolba, Y.; Rosselli, Z.; Pellissier, T.; Ajana, K.; Dehem, S. Concurrent validity of an immersive virtual reality version of the Box and Block Test to assess manual dexterity among patients with stroke. J. Neuroeng. Rehabil. 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Oña, E.D.; Jardón, A.; Cuesta-Gómez, A.; Sánchez-Herrera-baeza, P.; Cano-De-la-cuerda, R.; Balaguer, C. Validity of a Fully-Immersive VR-Based Version of the Box and Blocks Test for Upper Limb Function Assessment in Parkinson’s Disease. Sensors 2020, 20, 2773. [Google Scholar] [CrossRef] [PubMed]
- Everard, G.; Burton, Q.; Sype, V.V.D.; Bibentyo, T.N. Extended reality to assess post-stroke manual dexterity: Contrasts between the classic box and block test, immersive virtual reality with controllers, with hand-tracking, and mixed-reality tests. J. Neuroeng. Rehabil. 2024, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, W.S.; Paik, N.J.; Bang, H. Upper-Limb Function Assessment Using VBBTs for Stroke Patients. IEEE Comput. Graph. Appl. 2016, 36, 70–78. [Google Scholar] [CrossRef]
- Salas-Monedero, M. Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients. Appl. Sci. 2023, 13, 4979. [Google Scholar] [CrossRef]
- Guna, J.; Jakus, G.; Pogačnik, M.; Tomažič, S.; Sodnik, J. An Analysis of the Precision and Reliability of the Leap Motion Sensor and Its Suitability for Static and Dynamic Tracking. Sensors 2014, 14, 3702–3720. [Google Scholar] [CrossRef]
- Teruel, M.A. Picking cubes: A rehabilitation tool for improving the rehabilitation of gross manual dexterity. Adv. Intell. Syst. Comput. 2019, 806, 265–273. [Google Scholar] [CrossRef]
- Reyes-Guzmán, A.D.L.; García, F.; Alvarez-Rodríguez, M.; Lozano-Berrio, V.; Domingo-García, A.M.; Ceruelo-Abajo, S. Low-cost virtual reality. A new application for upper extremity motor rehabilitation in neurological pathology: Pilot study. Ann. Rehabil. Medicine 2022, 56, 173–181. [Google Scholar] [CrossRef]
- Rupp, R. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef]
- Gracia-Ibáñez, V.; Vergara, M.; Buffi, J.H.; Murray, W.M.; Sancho-Bru, J.L. Across-subject calibration of an instrumented glove to measure hand movement for clinical purposes. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 587–597. [Google Scholar] [CrossRef]
- Jarque-Bou, N.J.; Vergara, M.; Sancho-Bru, J.L.; Roda-Sales, A.; Gracia-Ibáñez, V. Identification of forearm skin zones with similar muscle activation patterns during activities of daily living. J. Neuroeng. Rehabil. 2018, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Ibáñez, V.; Vergara, M.; Sancho-Bru, J.L.; Mora, M.C.; Piqueras, C. Functional range of motion of the hand joints in activities of the International Classification of Functioning, Disability and Health. J. Hand Ther. 2017, 30, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Ibáñez, V.; Sancho-Bru, J.L.; Vergara, M.; Jarque-Bou, N.J.; Roda-Sales, A. Sharing of hand kinematic synergies across subjects in daily living activities. Sci. Rep. 2020, 10, 11097. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Schluter, P.J.; Macey, K.E.; Harper, R.M. Detecting variable responses in time-series using repeated measures ANOVA: Application to physiologic challenges. F1000Research 2016, 5, 563. [Google Scholar] [CrossRef]
- World Health Organization. Towards a Common Language for Functioning, Disability and Health ICF The International Classification of Functioning, Disability and Health; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Gohritz, A.; Fridén, J. Management of Spinal Cord Injury-Induced Upper Extremity Spasticity. Hand Clin. 2018, 34, 555–565. [Google Scholar] [CrossRef]
- Miall, R.C.; Rosenthal, O.; Ørstavik, K.; Cole, J.D.; Sarlegna, F.R. Loss of haptic feedback impairs control of hand posture: A study in chronically deafferented individuals when grasping and lifting objects. Exp. Brain Res. 2019, 237, 2167–2184. [Google Scholar] [CrossRef]
- Calabro, F.J.; Perez, M.A. Bilateral reach-to-grasp movement asymmetries after human spinal cord injury. J. Neurophysiol. 2016, 115, 157–167. [Google Scholar] [CrossRef]
- Razavian, R.S.; Mehrabi, N.; McPhee, J. A model-based approach to predict muscle synergies using optimization: Application to feedback control. Front. Comput. Neurosci. 2015, 9, 121. [Google Scholar] [CrossRef]





| Variables | Sample Analysed | |
|---|---|---|
| Healthy (n = 9) | SCI Patients (n = 4) | |
| Sex (Male) * | 1 (11.11%) | 4 (100.00%) |
| Age (Years) + | 33.33 ± 13.12 | 34.75 ± 13.07 |
| Etiology Injury (Traumatic) | - | 4 (100%) |
| Time Since Injury (Months) | - | 7.50 ± 3.00 |
| Injury Level | - | C4:1 (25.00%) |
| - | C5:1 (25.00%) | |
| - | C6:1 (25.00%) | |
| - | C8:1 (25.00%) | |
| AIS Classification | ||
| A | - | 2 (50.00%) |
| B | - | 1 (25.00%) |
| C | - | - |
| D | - | 1 (25.00%) |
| SCIM-III | 100.00 ± 00.00 a | 53.50 ± 25.30 a |
| Upper Extremity Motor Score | 25.00 ± 00.00 a | 17.50 ± 6.45 a |
| Kinematics—Cycle-Averaged Parameters | ||
|---|---|---|
| Differences Between Tests Within Each Sample | ||
| Sample | Tests | |
| Trajectory Smoothness (SM) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| Trajectory Length (EM) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| Thumb Flexion (Median) | H | RBBT > VBBT |
| P | VBBT > RBBT | |
| Thumb Flexion (Range) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| MCP Flexion (Median) | H | VBBT > RBBT |
| P | RBBT > VBBT | |
| MCP Flexion (Range) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| PIP Flexion (Median) | H | RBBT > VBBT |
| P | - | |
| PIP Flexion (Range) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| Thumb Abduc. (Median) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| Thumb Abduc. (Range) | H | RBBT > VBBT |
| P | - | |
| Fingers Abduc. (Median) | H | RBBT > VBBT |
| P | - | |
| Fingers Abduc. (Range) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| Differences Between Samples Within Each Test | ||
| Test | Samples | |
| Trajectory Smoothness (SM) | RBBT | P > H |
| VBBT | P > H | |
| Trajectory Length (EM) | RBBT | P > H |
| VBBT | P > H | |
| PIP Flexion (Median) | RBBT | - |
| VBBT | P > H | |
| PIP Flexion (Range) | RBBT | H > P |
| VBBT | - | |
| Finger Abduction (Range) | RBBT | H > P |
| VBBT | - | |
| Kinematics—Temporal Patterns | ||
| Patterns Across Participants Within Each Sample | ||
| MCP Flexion (t) PIP Flexion (t) Thumb Abduc. (t) Fingers Abduc. (t) | H | Distinct temporal patterns per test type (VBBT ≠ RBBT) |
| P | - | |
| Patterns Across Participants Within Each Test | ||
| Thumb Flexion (t) | RBBT | Distinct temporal patterns |
| VBBT | Distinct temporal patterns per sample (H ≠ P) | |
| MCP Flexion (t) | RBBT | Distinct temporal patterns |
| VBBT | Distinct temporal patterns | |
| PIP Flexion (t) | RBBT | Distinct temporal patterns per sample (H ≠ P) |
| VBBT | Distinct temporal patterns per sample (H ≠ P) | |
| Fingers Abduc. (t) | RBBT | - |
| VBBT | Distinct temporal patterns | |
| EMG—Cycle-Averaged Parameters | ||
| Differences Between Tests Within Each Sample | ||
| Sample | Tests | |
| EWL (sensors 1, 2, 3, 6, 7) | H | RBBT > VBBT |
| P | VBBT > RBBT | |
| EWL (sensors 4, 5) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| NCZ (sensors 1, 6) | H | RBBT > VBBT |
| P | VBBT > RBBT | |
| NCZ (sensors 2, 3, 4) | H | VBBT > RBBT |
| P | RBBT > VBBT | |
| NCZ (sensors 5, 7) | H | VBBT > RBBT |
| P | VBBT > RBBT | |
| MA median (sensors 1, 2, 3, 4, 5, 6, 7) | H | RBBT > VBBT |
| P | VBBT > RBBT | |
| MA range (sensors 1, 2, 3, 4, 5, 6, 7) | H | RBBT > VBBT |
| P | VBBT > RBBT | |
| Differences Between Samples Within Each Test | ||
| Test | Samples | |
| EWL (sensors 1, 3, 7) | RBBT | H > P |
| VBBT | P > H | |
| EWL (sensor 6) | RBBT | H > P |
| VBBT | - | |
| NCZ (sensor 2) | RBBT | P > H |
| VBBT | H > P | |
| NCZ (sensor 3) | RBBT | P > H |
| VBBT | - | |
| MA median (sensors 1, 5, 7) | RBBT | H > P |
| VBBT | - | |
| MA median (sensor 3) | RBBT | - |
| VBBT | P > H | |
| EMG—Temporal Patterns | ||
| Patterns Across Participants Within Each Sample | ||
| MA1 (t), MA2 (t), MA7 (t) | H | Distinct temporal patterns per test type (VBBT ≠ RBBT) |
| P | - | |
| MA3 (t), MA5 (t), MA6 (t) | H | Distinct temporal patterns |
| P | - | |
| Patterns Across Participants Within Each Test | ||
| MA2 (t), MA3 (t) | RBBT | - |
| VBBT | Distinct temporal patterns per sample (H ≠ P) | |
| MA4 (t) | RBBT | - |
| VBBT | Distinct temporal patterns | |
| MA7 (t) | RBBT | Distinct temporal patterns per sample (H ≠ P) |
| VBBT | Distinct temporal patterns | |
| Kinematic Synergy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thumb_F(t) | MCPs_F(t) | PIPs_F(t) | Thumb_A(t) | Fingers_A(t) | |||||||||||
| Health condition groups | Factors | Factors | Factors | Factors | Factors | ||||||||||
| t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | |
| Healthy | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Patient | * | * | * | * | * | * | * | ||||||||
| Test condition groups | Factors | Factors | Factors | Factors | Factors | ||||||||||
| t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | |
| RBBT | * | * | * | * | * | * | * | * | * | * | |||||
| VBBT | * | * | * | * | * | * | * | * | * | * | |||||
| Muscular Amplitude | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MA1(t) | MA2(t) | MA3(t) | MA4(t) | MA5(t) | MA6(t) | MA7(t) | |||||||||||||||
| Health condition groups | Factors | Factors | Factors | Factors | Factors | Factors | Factors | ||||||||||||||
| t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | t | TC | t:TC | |
| Healthy | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |||||||
| Patient | * | * | |||||||||||||||||||
| Test condition groups | Factors | Factors | Factors | Factors | Factors | Factors | Factors | ||||||||||||||
| t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | t | HC | t:HC | |
| RBBT | * | * | * | * | * | * | * | * | * | ||||||||||||
| VBBT | * | * | * | * | * | * | * | * | * | * | * | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gracia-Ibáñez, V.; de los Reyes-Guzmán, A.; Vergara, M.; Jarque-Bou, N.J.; Sancho-Bru, J.-L. Hand Dynamics in Healthy Individuals and Spinal Cord Injury Patients During Real and Virtual Box and Block Test. Appl. Sci. 2025, 15, 5842. https://doi.org/10.3390/app15115842
Gracia-Ibáñez V, de los Reyes-Guzmán A, Vergara M, Jarque-Bou NJ, Sancho-Bru J-L. Hand Dynamics in Healthy Individuals and Spinal Cord Injury Patients During Real and Virtual Box and Block Test. Applied Sciences. 2025; 15(11):5842. https://doi.org/10.3390/app15115842
Chicago/Turabian StyleGracia-Ibáñez, Verónica, Ana de los Reyes-Guzmán, Margarita Vergara, Néstor J. Jarque-Bou, and Joaquín-Luis Sancho-Bru. 2025. "Hand Dynamics in Healthy Individuals and Spinal Cord Injury Patients During Real and Virtual Box and Block Test" Applied Sciences 15, no. 11: 5842. https://doi.org/10.3390/app15115842
APA StyleGracia-Ibáñez, V., de los Reyes-Guzmán, A., Vergara, M., Jarque-Bou, N. J., & Sancho-Bru, J.-L. (2025). Hand Dynamics in Healthy Individuals and Spinal Cord Injury Patients During Real and Virtual Box and Block Test. Applied Sciences, 15(11), 5842. https://doi.org/10.3390/app15115842







