Abstract
The dairy industry generates substantial quantities of wastewater, primarily whey wastewater, posing environmental challenges. Current treatment methods involve physical, chemical, and biological processes, but efficient solutions are still sought. Biological treatments using microalgae are gaining attention due to their potential to remove pollutants from wastewater and generate valuable products, making them an alternative way to improve environmental sustainability. The physicochemical characterization of whey effluents reveals a high organic content, an acidic pH, and elevated nutrient levels. This study investigates the potential of Chromochloris zofingiensis (formerly known as Chlorella zofingiensis) for treating whey wastewater using three concentrations, 10%, 20%, and 50%, over a 7-day culture period. The optimal concentration of whey wastewater for biomass, nutrient removal, astaxanthin, and lipid production was found to be 10%. At this concentration, C. zofingiensis achieved a biomass of 3.86 g L−1 and a removal efficiency of nutrients between 77.08% and 99.90%. Analysis of pigment production revealed decreases in chlorophyll and carotenoid production with increasing whey wastewater concentration, while lipid and astaxanthin production peaked at the 10% dilution. The chlorophyll a, chlorophyll b, total carotenoid, astaxanthin, and lipid contents were, respectively, 11.49 mg g−1, 4.56 mg g−1, 4.04 mg g−1, 0.71 mg g−1, and 30.49% in 10% whey wastewater. The fatty acid profiles indicated the predominance of saturated and unsaturated fatty acids, enhancing the biofuel potential of C. zofingiensis cultivated in whey wastewater. These findings demonstrate the dual benefit of using C. zofingiensis for sustainable whey wastewater treatment and high-value bioproduct generation, supporting the development of circular biorefinery systems.
1. Introduction
The dairy industry encompasses various processes that transform raw milk into a range of products for consumption, such as pasteurized and sour milk, yogurt, different types of cheese, cream and butter products, ice cream, milk and whey powders, lactose, condensed milk, and various desserts [1,2]. In 2022, 160.0 million tons of raw milk were produced in Europe, with 149.9 million tons sent to dairies for processing into various fresh and manufactured dairy products [3]. Whey, or milk permeate, is the leftover liquid produced in the making of cheese and makes up around 85% (v/v) of the milk used. Based on the manufacture of cheese, about 8.7 L of whey are produced from 10 L of milk, with a total production of 145 million tons of whey each year [4,5]. Around 42% of whey is thrown away, fed to animals, or used as fertilizer [5].
Milk permeate is acknowledged as the primary wastewater source, posing a significant problem for the dairy sector in terms of efficient treatment. Releasing it into natural habitats may have major environmental impacts due to its high nitrogen and organic matter concentrations [6]. Consequently, whey wastewater treatment using physical-chemical-biological process combinations is essential prior to discharge into the environment according to ref. [7]. These authors effectively used the chemical-based coagulation approach, followed by aerobic microbial degradation, to efficiently remove pollutants from cheese whey effluent [7]. Ref. [8] found that physicochemical treatments (such as coagulation-flocculation) using iron salts, chitosan, and alginate may decrease indicators of contamination such as suspended particles, turbidity, and organic matter. Whey ultrafiltration is an industrial procedure utilized to extract whey protein, which is a dietary supplement with significant added value. However, this process additionally produces a byproduct known as whey permeate. The purification of these newly generated effluents is necessary due to their lactose content, which comprises 70% of the total particles [4].
Currently, various biological treatment techniques are under evaluation, and these methods are frequently recognized for their simplicity, low operational costs, and versatile applications [9]. Within the realm of biological treatments, there is rising interest in the utilization of microalgae. Algal microorganisms, when employed in wastewater treatment, contribute to a reduction in the need for chemical agents. This is attributed to the adaptability and thriving capability of efficient microalgae, which can easily acclimate to diverse wastewater environments owing to their high photosynthetic efficiency [10,11]. Microalgae cultivated in wastewater have the potential to be used in the synthesis of many products, such as lipids, proteins, carbohydrates, and carotenoids [10]. Chlorella and Scenedesmus genera are the predominant microalgal species used for cultivation because of their accelerated cell proliferation in comparison to other microalgae [12]. Additionally, under conditions with high carbon dioxide levels, they can eliminate ammonia, total nitrogen, total phosphate, and generate a significant quantity of biomass [13]. Chlorella zofingiensis, also named Chromochloris zofingiensis, has been used to treat various types of wastewaters, among them dairy wastewater [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. This species can remove 65–93% of TN (total nitrogen), 62–100% of TP (total phosphate), and 28–80% of COD (chemical oxygen demand) from wastewater and produce between 0.47 g L−1 and 3.8 g L−1 of biomass as well as a total lipid content between 19% and 44% under different culture conditions [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Chromochloris zofingiensis has the potential for dual lipid-astaxanthin biosynthesis [21,29]. To the best of our knowledge, no study has evaluated the potential of Chromochloris zofingiensis in the context of a full biorefinery approach involving simultaneous nutrient removal, biomass production, lipid accumulation, and astaxanthin biosynthesis in whey wastewater.
Additionally, ref. [16] used filtration to remove solids and autoclaving as pretreatment techniques of dairy wastewater, resulting in a significant biomass of 3.8 g L−1 ± 0.0 g L−1 and high lipid productivity of 132.1 mg L−1 d−1 ± 1.9 mg L−1 d−1. By contrast, ref. [24] used centrifugation to remove suspended particles before autoclaving and diluted the effluent prior to microalgal cultivation. Recovering waste nutrients from wastewater using microalgae without a high-cost pretreatment process and producing valuable bioproducts like lipid and astaxanthin would enhance the wastewater treatment process and align with the principles of a sustainable circular bioeconomy [31]. In this work, we intentionally avoided solid removal pretreatments to simplify the process and reduce energy costs. The only applied treatment was autoclaving to prevent bacterial contamination.
We hypothesize that Chromochloris zofingiensis can effectively grow in autoclaved, non-filtered whey wastewater, allowing for simultaneous pollutant removal and production of valuable biocompounds. Therefore, the main objectives of this study are to:
(1) Assess the growth and biomass productivity of C. zofingiensis in different dilutions of whey wastewater;
(2) Evaluate nutrient removal efficiency (total nitrogen (TN), nitrate (NO3−), ammonia (NH4+), phosphate (PO43−), Chemical oxygen demand (COD), total carbon (TC), total organic carbon (TOC), lactose, lactate, acetate);
(3) Quantify the accumulation of lipids, fatty acids, pigments, and astaxanthin;
(4) Analyze the feasibility of this process as an integrated, low-cost biorefinery strategy.
2. Materials and Methods
2.1. Strain and Preculture Conditions
Chromochloris zofingiensis CCAP 211/51 was obtained from the Culture Collection of Algae and Protozoa and cultivated in 500 mL Erlenmeyer flasks with 250 mL of Bold’s Basal Medium (BBM) [32] as the culture medium, containing (in g L−1): NaNO3 1.4, K2HPO4 0.075, NaCl 0.025, KH2PO4 0.175, CaCl2.2H2O 0.025, MgSO4.7H2O 1.75, H3BO3 0.01142, and NaEDTA 0.05. After sterilization by filtration using a Steritop filter (0.22 µm), 100 µL of trace elements solution and 1 mL of vitamin solution were added per liter of medium. The trace elements solution and vitamin solution contained, respectively, in 100 mL of water: MnSO4 0.15 g, (NH4)6Mo7O24 0.015 g, CoSO4 0.015 g, CuSO4 0.05g, ZnSO4 0.25 g, Fer-EDTA 10 g, and Vitamin B12 0.01 g, Thiamine 0.025 g, and Biotin 0.04 g. The pH of the medium was 6.5. Chromochloris zofingiensis stock cultures were maintained in an incubator (Innova S44i, Eppendorf, Fisher Scientific SAS, Illkirch, France) at 20 °C, with 100 rpm mixing and 150 µmol photons m−2 s−1 with a photoperiod of 16:8 h (light/dark).
2.2. Source and Characterization of Whey Wastewater
Whey wastewater (WW) was received from Lactalis, a French multinational in the dairy industry, and stored at 4 °C until its characterization and use. The untreated wastewater included particles or suspended materials as well as microorganisms that might affect the outcome of the experiment. In our study, the whey wastewater was autoclaved at 121 °C for 20 min to remove bacteria.
The pH was measured using a Mettler Toledo S20 Seven Easy™ pH meter (Mettler-Toledo SAS, Viroflay, France). Turbidity was measured in accordance with ISO 7027 (attenuated radiation method) [33]. Levels of nitrate, orthophosphates, ammonia, and chemical oxygen demand were determined using, respectively, the following colorimetric methods: the Cawse method [34] modified according to [35], the phosphomolybdate yellow method [36], the Berthelot method [37], and the dichromate method [38]. Levels of total nitrogen, total carbon, and total organic carbon were measured using a TOC-V CSN coupled with a Shimadzu TNM-1 (Shimadzu France, Marne la Vallée, France). Levels of lactose, lactate, and acetate were determined using HPLC analysis [39]. Using the spectrophotometric approach previously outlined by ref. [40], the protein content of the whey wastewater was determined. The characteristics of the raw and autoclaved whey wastewater are presented in Table 1.

Table 1.
Whey wastewater characterization.
2.3. Microalgal Experiment
Eight experimental tubes with a working volume of 80 mL were used in a Multi-Cultivator MC 1000 photobioreactor (Photon Systems Instruments, Drásov, Czech Republic) to conduct the experiments. All cultures were bubbled with filtered (0.45 µm) and humidified CO2 (5 mL min−1) and air (800 mL min−1) and exposed to cool-white LED illumination at 150 µmol m−2 s−1 over a 16/8 h light/dark cycle at a temperature of 20 °C regulated by a thermostatic water bath during 7 days of culture.
A total of 0.15 g L−1 of Chromochloris zofingiensis biomass was inoculated into pH-adjusted (7.5) and autoclaved whey wastewater (WW) with various concentrations (10%, 20%, and 50% of WW) diluted in BBM medium, compared to the control (BBM medium).
Daily samples with a fixed amount of 3 mL were collected using syringes under sterile conditions. These samples were used to monitor the cell density and pH, before centrifugation at 10,000× g for 10 min. The pellets were discarded, and the supernatants were kept in a freezer for physicochemical characterization and nutrient removal.
2.4. Microalgal Growth and Biomass Productivity
The extreme turbidity of the wastewater, caused by the presence of many suspended particles, made it challenging to estimate the biomass concentration accurately using direct dry weight measurement or spectrophotometry. Cell growth was assessed by observing cell density with a microscope (Olympus BX41TF, Olympus Corporation, Tokyo, Japan) and a hemacytometer. The dry cell weight (DCW, g L−1) was determined by establishing a linear link between cell density and dry cell weight using Equation (1) and biomass productivity (Pb) using Equation (2):
Cell density is in cells mL−1. DCW0 represents the initial dry cell weight in g L−1, whereas DCWx represents the dry cell weight in g L−1 at time tx in days.
2.5. Supernatant Characterization and Nutrient Removal
Samples of 3 mL were collected every day. After centrifugation at 10,000× g for 10 min, the supernatant was filtered through a 0.45 µm syringe filter and then analyzed as described in Section 2.2. The removal efficiency of nutrients was determined using Equation (3):
where Cf (g L−1) represent concentration of nutrients on the final day of culture and Ci (g L−1) represents the concentration of nutrients on the initial day of culture.
2.6. Biochemical Analysis
2.6.1. Lipid and Fatty Acid Profiles
The lipid content was assessed using a chloroform-methanol-water combination, following a protocol previously described [41], modified from ref. [42]. A total of 30 mg of biomass was transferred into a 15 mL centrifuge polypropylene tube. Next, a solution containing water (0.5 mL), chloroform (2 mL), and methanol (1 mL) was introduced into the tube and stirred for 2 min. Afterward, another solution of water (2 mL) and chloroform (2 mL) was added to the mixture, which was mixed for an additional 2 min.
The mixture was centrifuged for 10 min at 8000× g, and the organic phase (lipids) was transferred to a pre-weighed tube. The remaining liquid phase and solid biomass were then extracted two more times. The extracts were combined before evaporation using a Speed-Vac centrifugal evaporator (Savant™ SPD111 SpeedVac™, Thermo Fisher Scientific, Waltham, MA, USA) to eliminate the solvent at 40 °C under a vacuum pressure of 0.95 bar. The residue was weighed to calculate the lipid content and suspended in 200 µL of chloroform for fatty acid composition analysis [41].
Equation (4) was used to calculate the lipid content %:
The fatty acid profile of C. zofingiensis was analyzed using gas chromatography after converting the lipid extract into methyl esters, as previously described [41], based on Morrison and Smith’s method [43].
In a screw-capped tube, 800 µL of 14% boron trifluoride-methanol solution was mixed with 100 µL of lipid extract. The mixture was heated at 100 °C for 15 min in a dry bath and then cooled to room temperature. Subsequently, 1500 μL of MilliQ water and 750 μL of hexane containing 1% (v/v) heptadecane as an internal standard were added. After vortexing for 2 min, the solution was allowed to settle, and the methylated fatty acids were collected from the upper phase.
The analysis was performed using an Agilent 6850 Series II gas chromatographic system (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID), a G4513A autosampler, and a polar HP 88 column (112-88A7E) with dimensions of 100 m × 0.25 mm × 0.2 μm. One microliter of the product was injected into the system. The oven was preheated to 140 °C for 5 min, and the temperature was increased by 4 °C per minute until reaching 240 °C, where it was maintained for 5 min. The FID and injector temperatures were set at 260 °C, and nitrogen was used as the carrier gas.
Data collection and analysis were conducted using ChemStation B.04.03 software. Fatty acid methyl esters were identified and quantified using calibration curves of standards methylated following the same protocol.
2.6.2. Pigment Analysis
To determine the total amount of carotenoids and chlorophylls, 40 mg of dried biomass was weighed and transferred into a 15 mL centrifuge polypropylene tube. The extraction was performed using the chloroform-methanol-water combination as described in Section 2.6.1 [41]. The residue was then suspended in 1 mL of acetone and thoroughly mixed to dissolve the contents.
The photosynthetic pigments were quantified using Lichtenthaler’s equations [44]:
where chlorophyll a is represented by Ca, chlorophyll b by Cb, total carotenoids by C(x+c), and A is the absorbance at 470, 644.8, and 661.6 nm.
Ca (mg∕L) = 11.24A661.6 − 2.04A644.8
Cb (mg∕L) = 20.13A644.8 − 4.19A661.6
C(x+c) (mg∕L) = (1000A470 − 1.9Ca − 63.14Cb)/214
The result was then expressed on a weight basis (mg gbiomass−1), taking into account the biomass amount used for the extraction and volume of solvent.
2.6.3. Astaxanthin Analysis
The method described by Li et al. [45] and Liyanaarachchi et al. [46] was followed to determine the astaxanthin content. A 15 mL centrifuge tube containing 20 mg of dried biomass was filled with 5 mL of dimethyl sulfoxide (DMSO). The mixture was heated for ten minutes at 70 °C while being gently shaken on a regular basis. The sample was then centrifuged at 4000× g for 5 min, and the supernatant was removed. The extraction process was repeated until the supernatant lost all color and the extracted supernatants were combined. The extracts were further diluted with DMSO before the absorbance was measured at 530 nm using a UV–Vis spectrophotometer. The astaxanthin (Cast) concentration in the extracts was determined using Equation (8):
where A530 represents the extract’s absorbance at 530 nm.
The result was then expressed on a weight basis (mg gbiomass−1), taking into account the biomass amount used for the extraction and volume of solvent.
2.7. Statistical Analysis
A one-way analysis of variance (ANOVA) and Tukey’s post hoc test were used in the statistical study to see if there were any significant differences between the tested media. A 95% confidence interval was maintained throughout the analytical process. SPSS 22, a statistical program developed by SPSS Ltd., (Woking, UK) was used for the investigation.
3. Results and Discussion
3.1. Whey Wastewater Characterization
One of the primary industries in Europe that produces industrial wastewater is the dairy industry [47]. The properties of dairy wastewater may vary considerably based on factors such as the final products, the type of system, and the processes used in the production plant [7]. The results of the physicochemical characterization of the raw and autoclaved whey effluents used in this study are shown in Table 1. Whey is a dairy byproduct obtained after the coagulation of raw milk during the cheese manufacturing process. It consists of 94% water, 4 to 5% lactose, 0.5 to 1% proteins, and small amounts of minerals and vitamins [48]. As shown in Table 1, the raw and autoclaved whey wastewaters had an acidic pH (<5), high turbidity, and significant total solids (much greater than reported in the literature), limiting their direct use for microalgal culture. The lactose concentration in raw whey wastewater was 66.75 g L−1, whereas autoclaved whey wastewater had a lactose level of 61.57 g L−1, consistent with the scientific literature (ranging from 10 g L−1 to 92 g L−1 [4,8,9]). Some species of microalgae can use lactose as a suitable carbon source to support growth [49]. High levels of chemical oxygen demand (COD) and total carbon (TC) were observed in the raw and autoclaved whey effluents, with values of 100.36 g L−1 and 96.00 g L−1 for COD and 39.85 g L−1 and 39.07 g L−1 for TC, respectively. These values were similar to those reported in the literature [6,8,9]. The concentrations of phosphate, total nitrogen (TN), nitrate, and ammonia were 795.3 mg L−1, 2.81 g L−1, 0.51 g L−1, and 74.53 mg L−1, respectively, in raw whey wastewater, and 756.31 mg L−1, 2.54 g L−1, 0.32 g L−1, and 109.04 mg L−1, respectively, in autoclaved whey effluent. The phosphate and TN contents were higher than those reported in the literature.
It is noteworthy that the composition of whey wastewater was slightly modified after autoclaving. This thermal pretreatment induced the denaturation of milk proteins, including β-lactoglobulin and α-lactalbumin, resulting in structural changes such as thiol-disulfide bond rearrangement and protein aggregation. These effects may explain the observed increases in turbidity and ammonia levels after autoclaving, as previously reported by Qian et al. in their study on the thermal denaturation of whey proteins [50]. Despite these modifications, the nutrient profile observed in this study remained consistent with typical whey wastewater characteristics. Moreover, the selected pretreatment method was appropriate for investigating microalgal cultivation, as it effectively eliminated bacterial contaminants that could otherwise interfere with algal growth, metabolism, and experimental reproducibility.
3.2. Biomass Production of C. zofingiensis in Whey Wastewater
The high nutrient content in most wastewater necessitates expensive chemical treatments to eliminate it. Table 1 confirms that the raw and autoclaved whey wastewaters (WW) required treatment due to their significant pollutant potential caused by high levels of organic matter, nitrogen, and phosphorus. In this study, whey wastewater was diluted without any chemical treatment to reduce the effects of solids and high nutrient content on the growth of Chromochloris zofingiensis. Figure 1 and Figure 2 show the growth curves and pH of Chromochloris zofingiensis cultivated separately in different concentrations of whey wastewater (10%, 20%, and 50% v/v) diluted in BBM medium (control).
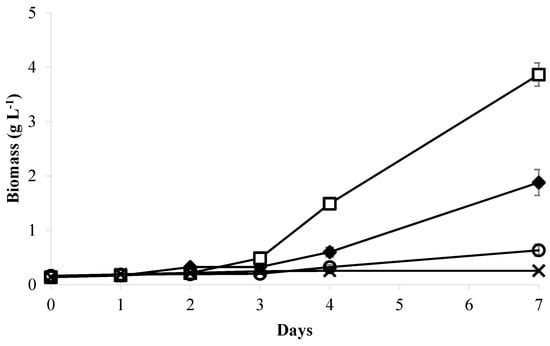
Figure 1.
Biomass production of C. zofingiensis cultivated in various concentrations of whey wastewater (WW) compared to BBM medium. –◆– BBM medium; –□– 10% WW; –O– 20% WW; –×– 50% WW.
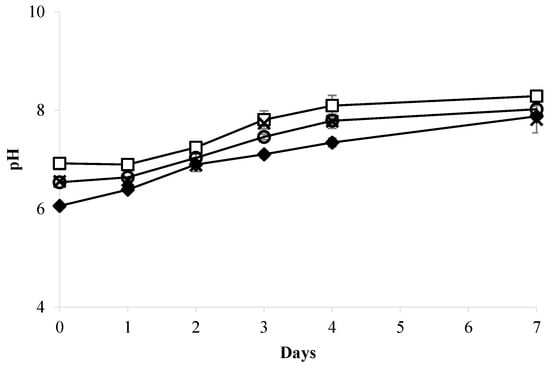
Figure 2.
pH assessment during the culture of C. zofingiensis cultivated in various concentrations of whey wastewater (WW) compared to BBM medium. –◆– BBM medium; –□– 10% WW; –O– 20% WW; –×– 50% WW.
As seen in the figures, biomass production decreased as the proportion of whey wastewater increased from 0% to 50%, and throughout the observation period, the pH in all treatments increased daily. A similar finding was observed by Mohammad et al. [6] using mozzarella cheese whey. By contrast, Wang et al. [51] and Zimermann et al. [4] reported that increasing the proportion of whey wastewater led to a significant increase in cell number, demonstrating the varying effects of different wastewater compositions on microalgae. The maximum biomass production of 3.86 ± 0.17 g L−1 and productivity of 0.53 ± 0.02 g L−1 d−1 were achieved in cultures containing 10% WW, followed by 20% WW (0.63 ± 0.11 g L−1; 0.25 ± 0.03 g L−1 d−1) and 50% WW (0.26 ± 0.00 g L−1; 0.02 ± 0.00 g L−1 d−1). Non-diluted raw whey wastewater resulted in total inhibition of cell growth. Biomass obtained with 10% WW was approximately double that of the BBM medium control (1.88 ± 0.24 g L−1), indicating that the addition of this concentration of whey wastewater could enhance the growth of C. zofingiensis. The use of mixotrophic cultivation, which combines light and both organic and inorganic carbon sources, is regarded as the most effective method for producing microalgal biomass [52,53]. The increase in biomass production of C. zofingiensis using 10% whey wastewater was likely due to the utilization of lactose as an organic carbon source and the availability of other nutrients (Table 1). This fraction of whey wastewater produced a biomass comparable to that found by Ribeiro et al. [54] when using 30% whey wastewater to cultivate C. protothecoides. Mohammad et al. [6] used 20% mozzarella cheese whey as a substrate for Dunaliella salina and reported a maximum biomass of 0.41 g L−1, which was higher than that obtained using 50% and 75% whey wastewater. The use of 50% whey wastewater for C. zofingiensis resulted in a biomass similar to that produced by D. salina. The production of biomass by Chromochloris zofingiensis varies depending on the type of wastewater used [15,16,21]. Ref. [15] reported that the microalgae C. zofingiensis could produce 2.21 g L−1 biomass using 80% municipal wastewater as a culture medium. In another experiment, this species achieved 0.48 g L−1 biomass when grown in 2.5% palm oil mill effluent [21]. In the present study, using 10% whey wastewater, C. zofingiensis produced 3.86 g L−1 biomass, consistent with the findings of Shu et al. [16], who used dairy wastewater. Differences in wastewater composition, pretreatment techniques, and culture conditions could account for variations in biomass production. Shu et al. [16] cultivated C. zofingiensis at 25 ± 1 °C for 8 days in dairy wastewater pretreated by sedimentation, filtration with two-layered gauze, and autoclaving for 20 min at 121 °C. The wastewater used in their study had significantly lower levels of solids (20 times lower), chemical oxygen demand (COD, 7 times lower), and total nitrogen (3 times lower). It exhibited a high pH of 9.31, indicating that it was not the same dairy wastewater used in our study. In addition, it contained 580 mg/L suspended solids, which were reduced by prior sedimentation and filtration. The chemical oxygen demand (COD) was 1428 mg/L, which was considerably lower than the values observed in our undiluted whey wastewater. The total phosphorus (TP) and total nitrogen (TN) concentrations were 48.0 mg/L and 75.5 mg/L, respectively, while ammonia (NH3-N) was present at 31.4 mg/L. By contrast, the whey wastewater used in our study was substantially richer in organic matter and nutrients, necessitating dilution to 10–50% to avoid inhibitory effects on microalgal growth. The observed difference in optimal growth conditions does not reflect a contradiction but rather highlights the importance of dairy wastewater type and pretreatment in influencing algal performance.
3.3. Nutrient Removal Efficiency
The effectiveness of a microalgal wastewater treatment system is primarily determined by its nutrient removal efficiency. The concept of using microalgae to remove nutrients from wastewater is receiving significant global attention [10]. This study investigated the phycoremediation of unfiltered and sterilized whey wastewater by assessing the removal of TN (total nitrogen), NO3-N (nitrate), NH4-N (ammonia), PO4-P (phosphate), COD (chemical oxygen demand), TC (total carbon), TOC (total organic carbon), lactose, lactate, and acetate using Chromochloris zofingiensis (Figure 3 and Figure 4, and Table 2).
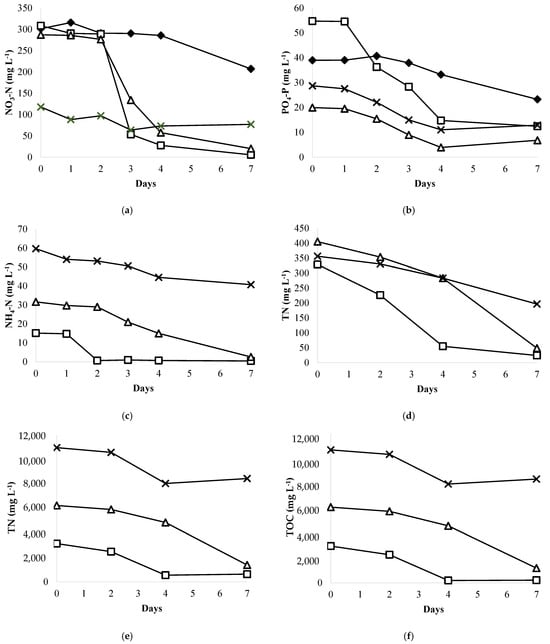
Figure 3.
Nutrient uptake from whey wastewater diluted in BBM medium by Chromochloris zofingiensis: (a) Nitrate removal efficiency; (b) Phosphate removal efficiency; (c) Ammonia removal efficiency; (d) TN removal efficiency; (e) TC removal efficiency; (f) TOC removal efficiency. –◆– BBM medium; –□– 10% WW; –△– 20% WW; –×– 50% WW.
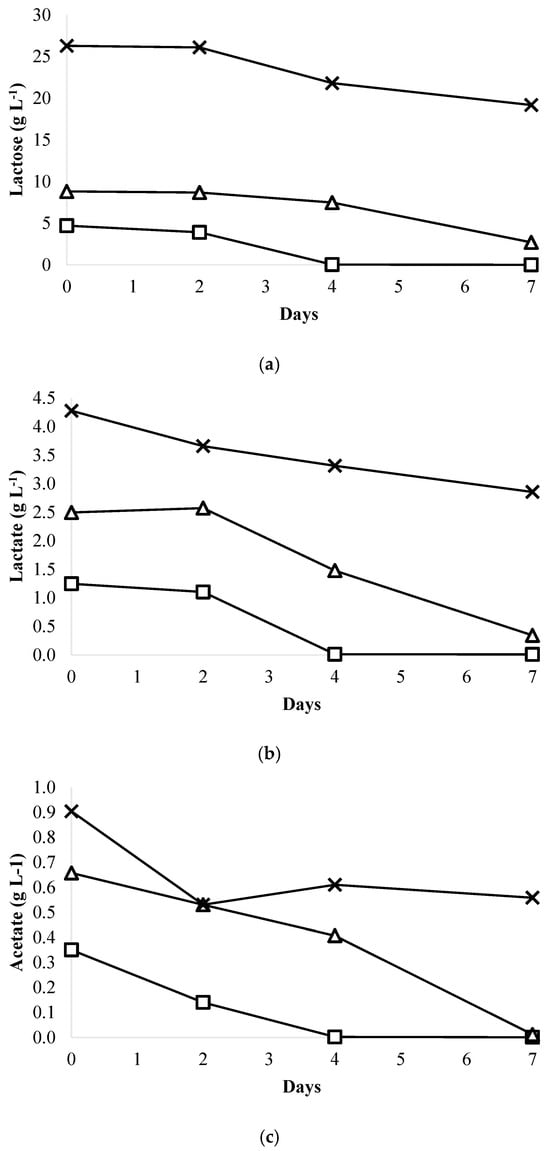
Figure 4.
Lactose, lactate, and acetate uptake from whey wastewater diluted in BBM medium by Chromochloris zofingiensis: (a) Lactose removal efficiency; (b) Lactate removal efficiency; (c) Acetate removal efficiency. –□– 10% WW; –△– 20% WW; –×– 50% WW.

Table 2.
Nutrient removal efficiency (%) of C. zofingiensis cultivated in different concentrations of whey wastewater (WW) compared to BBM medium (mean ± standard deviation of three different tests, n = 3). Letters indicate statistical order of differences after one way ANOVA and post hoc tests (p < 0.05): a > b > c > d.
Microalgal cells absorb nutrients such as carbon, nitrogen, and phosphorus from wastewater to synthesize essential compounds like proteins, phospholipids, and nucleic acids [55]. The whey wastewater used in this study exhibited elevated levels of these nutrients, requiring their removal before environmental discharge. The changes in nutrient concentrations (NO3-N, NH4-N, TN, PO4-P, COD, TC, TOC, lactose, lactate, and acetate) in sterilized whey wastewater compared to BBM medium during the cultivation period are shown in Figure 3 and Figure 4. Figure 3a illustrates that nitrate (NO3-N) concentrations decreased daily in all experiments, with the degree of reduction varying among them. Cultivation of C. zofingiensis in 10% whey wastewater diluted in BBM medium (90%) achieved the highest removal efficiency of 98.19% ± 0.06%, followed by 20% WW (92.98% ± 2.08%), 50% (34.58% ± 1.69%), and BBM alone (31.34% ± 4.02%). Remarkably, C. zofingiensis removed over 80% of NO3-N within 3 days compared to BBM medium (3.66%), demonstrating that the addition of whey wastewater enhanced the nitrate removal efficiency. For ammonia (NH4-N), the removal efficiencies were similarly high, with 97.11% ± 1.44% and 91.92% ± 1.50% achieved for 10% and 20% whey wastewater, respectively. However, for 50% whey wastewater, the reduction was significantly lower at 38.47% ± 7.94%. Total nitrogen (TN), total carbon (TC), and total organic carbon (TOC) were measured using a TOC-V CSN analyzer coupled with a Shimadzu TNM-1 instrument on days 0, 2, 4, and 7. These measurements provided insights into nutrient utilization by C. zofingiensis and its potential for effective wastewater treatment. The amounts of these parameters in all variants of whey wastewater steadily declined until the final stage of culture, as seen in Figure 3d–f. The final total nitrogen (TN) contents in 10%, 20%, and 50% whey wastewater were reduced to 24.07 mg L−1 ± 1.09 mg L−1, 48.59 mg L−1 ± 2.17 mg L−1, and 196.40 mg L−1 ± 3.15 mg L−1, respectively. The highest total nitrogen removal efficiency of about 92.69% ± 0.29% was achieved in 10% whey wastewater. This finding was followed by 88.00% ± 0.50% and 44.78% ± 0.50% in 20% and 50% effluent, respectively. For phosphate removal, Figure 3b shows that the PO4-P concentrations dropped and reached 23.26 mg L−1 ± 5.62 mg L−1, 12.43 mg L−1 ± 1.88 mg L−1, 6.80 mg L−1 ± 1.42 mg L−1, and 12.88 mg L−1 ± 0.02 mg L−1, respectively, in BBM medium and 10%, 20%, and 50% whey wastewater. The removal efficiencies of PO4-P were higher, about 77.08% ± 3.98%, 65.40% ± 8.02%, and 55.14% ± 0.02%, respectively, in 10%, 20% and 50% whey wastewater compared to BBM medium (48.00 ± 5.47%). The N/P ratio should have a significant impact on phosphorus removal [56]. Redfield’s Ratio is widely recognized as a worldwide average that varies significantly between phytoplankton species [57]. Although there are clear taxon-specific variances, it is generally acknowledged that this ratio may represent the ideal nutrient ratio needed for growth. For example, the average optimum N/P ratio for eukaryotic algae is 16–23 N/1 P, but for cyanobacteria it can vary from 10 to 16 N to 1 P [58]. In this investigation, 10% whey wastewater diluted in BBM medium resulted in a higher N/P ratio of 25. Depending on the biological parameters in the wastewater, ref. [56] proposed that the ideal N/P ratio for phosphorus removal from municipal wastewater treatment utilizing microalgae ranges from 5 to 30.
The total carbon (TC) and total organic carbon (TOC) levels were decreased with removal efficiencies of 79.75% ± 2.73%, 77.84% ± 0.93%, and 23.03% ± 1.75% for TC and 92.78% ± 3.21%, 80.54% ± 1.22%, and 21.96% ± 1.76% for TOC in 10%, 20%, and 50% whey wastewater, respectively. For chemical oxygen demand (COD), as observed in Table 2, the removal efficiency was higher using 10% and 20% whey wastewater (85.47% ± 0.45% and 86.27% ± 0.85%, respectively) compared to 50% effluent (24.75% ± 1.64%).
The concentrations of lactose, lactate, and acetate were reduced by Chromochloris zofingiensis (Figure 4a–c).
The removal efficiencies of lactose, lactate, and acetate were more than 99% in 10% whey wastewater compared to 20% (69.37% ± 0.06%, 86.25% ± 0.13%, and 98.16% ± 1.09%, respectively) and 50% whey wastewater (27.03% ± 0.05%, 33.24% ± 0.45%, and 38.29% ± 1.96%, respectively). The observed reductions in COD, TC, TOC, lactose, lactate, and acetate contents in this research indicated that Chromochloris zofingiensis has the ability to use the organic matter found in whey wastewater as an energy source and substrate for its development, in addition to CO2. Chromochloris zofingiensis achieved the highest removal efficiencies of NO3-N, NH4-N, TN, PO4-P, COD, TC, TOC, lactose, lactate, and acetate using 10% whey wastewater, at about 98.19%, 97.11%, 92.69%, 77.08%, 85.47%, 79.75%, 92.78%, 99.90%, 99.35%, and 99.81%, respectively. Presumably, under these conditions, algal cells exhibited enhanced metabolic (catabolism) efficiency due to the low concentration of total solids in the medium [9].
There is a scarcity of research that has assessed the efficacy of nutrient removal (such as NO3-N, NH4-N, TN, PO4-P, COD, TC, TOC, lactose, lactate, and acetate) from whey wastewater using microalgae. Almeida Pires et al. [9] cultivated Chlorella vulgaris in 10% cheese whey wastewater diluted in BBM medium to evaluate the potential of this species for wastewater treatment. They observed removal efficiencies for TC, TN, and COD of approximately 46%, 59%, and 17%, respectively, which were lower than the results of this current study using the microalgae Chromochloris zofingiensis. Similarly, Zimermann et al. [4] reported lower COD removal (60%) using Galdieria sulphuraria grown in 20% whey permeate. Chromochloris zofingiensis has been used to treat various types of wastewaters [15,20,27,28]. For example, Zhou et al. [20] found approximately (93%) the same removal efficiency of TN using Chromochloris zofingiensis cultivated in 8% pig biogas slurry diluted in municipal wastewater. In another study, C. zofingiensis cultivated using 10% dairy wastewater diluted in municipal wastewater achieved a lower removal of TN at approximately 90% [15]. Chen et al. [27] explored six microalgal species, including Chromochloris zofingiensis, for the potential generation of lipids and the removal of nutrients from anaerobically digested swine effluent. Their findings showed that the TN and NH4-N removal efficiencies (74.87% and 64.71%, respectively) were higher in 20% anaerobically digested swine wastewater as opposed to TP removal (62.05%); it was higher in 100% anaerobically digested swine effluent. Chromochloris zofingiensis was grown by Qin et al. [28] in dairy wastewater with Scenedesmus spp. and Chlorella sp. to determine the consortium’s potential for treating dairy wastewater. They found that the microalgal consortia achieved the maximum removal efficiencies of chemical oxygen demand and total phosphorus (57.01–62.86% and 91.16–95.96%, respectively) compared to the Chlorella sp. monoalgal cultivation system (44.76% and 86.74%, respectively). These investigations demonstrated that different wastewater types, pretreatment setups, and Chromochloris zofingiensis culture types resulted in different levels of nutrient removal.
3.4. Pigments and Astaxanthin Production
The process of photosynthesis preserves energy input and provides supplies of carbon for life. Chlorophylls a and b as well as carotenoids are the main pigments used in photosynthetic processes. Carotenoids are often found in environments with high levels of stress [14]. Table 3 displays the pigment amounts (chlorophyll a and b, total carotenoids, and astaxanthin) produced by C. zofingiensis grown in various concentrations of whey wastewater diluted in BBM medium.

Table 3.
C. zofingiensis biochemical composition in whey wastewater (WW) compared to BBM medium (mean ± standard deviation of three different tests, n = 3). Letters indicate statistical order of differences after one way ANOVA and post hoc tests (p < 0.05): a > b > c > d.
The amounts of chlorophyll a and b obtained in BBM medium (control) were higher than those obtained in whey wastewater. According to the data, chlorophyll a was the highest prevalent pigment discovered in C. zofingiensis, followed by chlorophyll b and total carotenoids. The reason for this outcome may be attributed to the fact that chlorophyll a serves as the primary pigment in microalgae, while chlorophyll b acts as an auxiliary pigment that absorbs energy and transfers it to chlorophyll a. In this study, C. zofingiensis grown in BBM medium achieved high levels of chlorophyll a and b at about 36.59 mg g−1 ± 0.26 mg g−1 and 31.85 mg g−1 ± 0.17 mg g−1, respectively, compared to the results found by Azaman et al. [19] (chlorophyll a: 15.69 mg g−1 and chlorophyll b: 7.31 mg g−1). This difference can be attributed to the light intensity, which was 15 times higher in the current research than that used in the study by Azaman et al. [19]. As the quantity of whey wastewater rose throughout the cell growth process, the levels of chlorophyll a and b decreased. Notably, 10%, 20%, and 50% whey wastewater displayed 11.49 mg g−1 ± 0.01 mg g−1, 4.10 mg g−1 ± 0.09 mg g−1, and 0.47 mg g−1 ± 0.01 mg g−1 of chlorophyll a and 4.56 mg g−1 ± 0.05 mg g−1, 1.77 mg g−1 ± 0.13 mg g−1, and 0.33 mg g−1 ± 0.01 mg g−1 of chlorophyll b, respectively. High pollutant concentrations and contact time may have been responsible for the decreases in chlorophyll concentrations [23]. Similar findings were obtained by Onay et al. [14] using C. zofingiensis grown in various concentrations of municipal wastewater.
Table 3 depicts the increase in total carotenoid levels in C. zofingiensis cultivated in 10% whey wastewater compared to BBM medium (control) and their decrease in 20% and 50% whey wastewater. Additionally, the content of astaxanthin was lower than in the control medium and decreased with increasing whey wastewater concentration. The maximum astaxanthin content using whey wastewater was obtained in the 10% concentration at about 0.71 mg g−1 ± 0.14 mg g−1.
It is important to note that wastewater bioremediation studies with C. zofingiensis have not thoroughly examined astaxanthin accumulation simultaneously. Fernando et al. [21] found a high amount of astaxanthin, approximately 5.65 mg g−1, in C. zofingiensis growing in 7.5% palm oil mill effluent. Nguyen and Dinh [29] reported that C. zofingiensis cultivated in ozonated domestic wastewater could accumulate astaxanthin at 0.009 mg L−1.
3.5. Lipid Production and Fatty Acids Profiles
The Chromochloris zofingiensis biomass produced from three concentrations of whey wastewater (10%, 20%, and 50%) in comparison to BBM medium was tested for total lipid content and fatty acid profiles (Table 3 and Table 4). C. zofingiensis accumulated lipid contents of 30.49% ± 1.65%, 24.72% ± 2.08%, and 19.19% ± 1.43% when cultivated in 10%, 20%, and 50% whey wastewater, respectively, compared to the BBM medium experiment (34.59% ± 4.10% total lipids). The lipid content achieved in 10% whey wastewater was higher than that obtained in 20% and 50% whey wastewater. There was no significant difference between the total lipids contents achieved in BBM medium and that in 10% whey wastewater.

Table 4.
Fatty acid profiles of C. zofingiensis grown in whey wastewater (WW) compared to BBM medium (mean ± standard deviation of three different tests, n = 3). Letters indicate statistical order of differences after one way ANOVA and post hoc tests (p < 0.05): a > b > c > d.
The lipid content of C. zofingiensis differs across various types of wastewater and even using the same type of wastewater [16,24,25,26,27,28,29,30,59]. In this study, using 10% whey wastewater diluted in BBM medium, C. zofingiensis accumulated almost the same amount of lipids as reported in previous studies using dairy wastewater [24] and piggery wastewater [25]. However, Cheng et al. [59] reported that C. zofingiensis achieved a higher lipid content of 44.12% in anaerobically digested swine wastewater diluted with fishery wastewater (20:80). Recently, Vitali et al. [30] found a total lipid content of 38% using C. zofingiensis cultivated in molasses wastewater (2 g L−1). On the other hand, Zhu et al. [60] and Qin et al. [28] reported lower total lipid contents of approximately 20% when using synthetic wastewater and dairy wastewater, respectively. Shu et al. [16] compared raw dairy wastewater (DWW) with blue-green medium (BG11 medium) for biofuel production using three microalgal species (Chlorella sp., Scenedesmus sp., and Chromochloris zofingiensis). They demonstrated that, after eight days of cultivation, Chromochloris zofingiensis and Scenedesmus sp. exhibited the best lipid content using raw dairy wastewater as the culture medium compared to BG11 medium, with lipid contents of around 27.7% and 15.3%, respectively. Conversely, Chlorella sp. grown on BG11 medium exhibited a higher total lipid content (28.2%) [16]. Differences in the lipid content of Chromochloris zofingiensis cultivated in various types of wastewater can be attributed to factors such as nutrient content, light intensity, photoperiod, culture conditions, and CO2 concentration [61]. Among these, the two most important parameters influencing lipid content in microalgae cultivated in wastewater are nutrient availability and light intensity [62]. The fatty acid profile of lipids is a critical factor for evaluating the potential of microalgae for biofuel and other bioproduct applications [63].
Table 4 lists the FAME (fatty acid methyl ester) compositions of Chromochloris zofingiensis grown in 10%, 20%, and 50% whey wastewater compared to BBM medium. In this study, eight fatty acids were detected in C. zofingiensis, primarily palmitic acid (C16:0), oleic acid (C18:1 cis), linoleic acid (C18:2), and linolenic acid (C18:3). Similar findings were reported in previous studies [16,64,65]. For instance, Chlorella pyrenoidosa grown in diluted primary piggery wastewater exhibited nine fatty acids, with palmitic acid, linoleic acid, and linolenic acid being the most prevalent [65]. Likewise, Chlorella vulgaris grown in dairy wastewater pretreated by UV irradiation and sodium hypochlorite showed a similar fatty acid profile [64]. Shu et al. [16] found comparable results for Chlorella sp., Scenedesmus sp., and Chromochloris zofingiensis grown in raw dairy wastewater, where the most abundant fatty acids were palmitic acid, linoleic acid, and linolenic acid. These findings highlight the consistency of fatty acid profiles across different wastewater types and microalgal species, underscoring their potential as feedstocks for biofuel production. Compared to BBM medium, there were notable increases in the relative contents of C16:0, C18:0, and C18:1 (cis) and notable decreases in the relative contents of C18:1 (trans), C18:2, and C18:3 in the whey wastewater experiments. Chlorella pyrenoidosa exhibited variations in fatty acid composition when grown in diluted piggery wastewater, with notable decreases in the relative contents of oleic acid (C18:1) and hexadecadienoic acid (C16:2), and an increase in the relative content of hexadecanoic acid (C16:0) compared to the standard medium [65]. Fatty acids with 16–18 carbon atoms are considered the best components for biodiesel production due to their ideal chain length and properties [22]. In this study, the percentage of C16–C18 fatty acids reached 97.23%, 96.11%, and 96.72% in 10%, 20%, and 50% whey wastewater, respectively, compared to 97.20% in BBM medium. Furthermore, the concentration of linolenic acid (C18:3), which is restricted to a maximum of 12% in biodiesel according to the European standard EN 14214 [16,66], was about 6.80% in 10% whey wastewater, meeting the biodiesel quality requirements. These results suggest that Chromochloris zofingiensis cultivated in 10% whey wastewater diluted in BBM medium has the potential to produce high-quality biofuel. The fatty acid profile aligned well with the standards for biodiesel production, making this cultivation method an attractive option for sustainable biofuel development.
This study focused on a low-cost approach using autoclaved, non-filtered whey wastewater. Future research may explore the integration of sustainable enzymatic pretreatment methods to enhance nutrient bioavailability. As demonstrated by Bonanno et al. [67], enzymatic hydrolysis of whey can significantly increase the release of bioavailable compounds, potentially improving microalgal growth, nutrient uptake, and the overall production of valuable metabolites within an integrated biorefinery framework [67].
4. Conclusions
This study demonstrates the efficacy of Chromochloris zofingiensis in the treatment of whey wastewater while concurrently producing valuable biomass, pigments, and lipids. Through physicochemical characterization, it was observed that whey wastewater contains high levels of organic matter and nutrients, necessitating treatment before disposal. Cultivation experiments revealed that C. zofingiensis can efficiently remove more than 90% of nitrates and ammonia and up to 70% of phosphorus and organic carbon from whey wastewater at a 10% dilution. At this concentration, the chlorophyll a, chlorophyll b, total carotenoid, astaxanthin, and lipid contents of C. zofingiensis were, respectively, 11.49 mg g−1, 4.56 mg g−1, 4.04 mg g−1, 0.71 mg g−1, and 30.49%. Overall, these findings underscore the feasibility of utilizing C. zofingiensis for whey wastewater treatment and valorization, offering a sustainable and economically viable solution for dairy industry wastewater management. Further research is warranted to optimize the cultivation conditions and scale-up processes for industrial applications.
Author Contributions
Conceptualization, C.L., M.S. and H.E.B.; methodology, C.L. and H.E.B.; validation, C.L.; investigation, H.E.B.; writing—original draft preparation, H.E.B. and A.M.; writing—review and editing, C.L.; supervision, C.L. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marazzi, F.; Bellucci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions Between Microalgae and Bacteria in the Treatment of Wastewater from Milk Whey Processing. Water 2020, 12, 297. [Google Scholar] [CrossRef]
- Slavov, A.K. Dairy Wastewaters—General Characteristics and Treatment Possibilities—A Review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Popescu, A.; Stoian, E.; Șerban, V. The EU-28 milk sector trends in the period 2009–2018. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2019, 19, 249–263. [Google Scholar]
- Zimermann, J.D.F.; Sydney, E.B.; Cerri, M.L.; De Carvalho, I.K.; Schafranski, K.; Sydney, A.C.N.; Vitali, L.; Gonçalves, S.; Micke, G.A.; Soccol, C.R.; et al. Growth Kinetics, Phenolic Compounds Profile and Pigments Analysis of Galdieria sulphuraria Cultivated in Whey Permeate in Shake-Flasks and Stirred-Tank Bioreactor. J. Water Process Eng. 2020, 38, 101598. [Google Scholar] [CrossRef]
- De Almeida, M.P.G.; Mockaitis, G.; Weissbrodt, D.G. Got Whey? The Significance of Cheese Whey at the Confluence of Dairying, Environmental Impacts, Energy and Resource Biorecovery. Fermentation. 2023, 9, 897. [Google Scholar] [CrossRef]
- Mohammad, H.Y.; Tawfeek, F.E.-Z.; Eltanahy, E.; Mansour, T.A.; Khalil, Z. Enhancement of Growth, Lipid, and Carbohydrate Production of the Egyptian Isolate Dunaliella salina SA20 Using Mozzarella Cheese Whey as a Growth Supplement. Egypt. J. Bot. 2022, 63, 101–111. [Google Scholar] [CrossRef]
- Rivas, J.; Prazeres, A.R.; Carvalho, F.; Beltrán, F. Treatment of Cheese Whey Wastewater: Combined Coagulation-Flocculation and Aerobic Biodegradation. J. Agric. Food Chem. 2010, 58, 7871–7877. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- De Almeida Pires, T.; Cardoso, V.L.; Batista, F.R.X. Feasibility of Chlorella vulgaris to Waste Products Removal from Cheese Whey. Int. J. Environ. Sci. Technol. 2022, 19, 4713–4722. [Google Scholar] [CrossRef]
- El Bakraoui, H.; Slaoui, M.; Mabrouki, J.; Hmouni, D.; Laroche, C. Recent Trends on Domestic, Agricultural and Industrial Wastewaters Treatment Using Microalgae Biorefinery System. Appl. Sci. 2022, 13, 68. [Google Scholar] [CrossRef]
- El Bakraoui, H.; Rahali, K.; Slaoui, M.; Rouichat, I.; Allalat, F.; Bouyahia, C.; Aamri, F.E.; Hmouni, D. Domestic Wastewater as Nutrient Media and Its Effect on Biochemical Composition of a Marine Diatom Chaetoceros calcitrans. Int. J. Chem. Biochem. Sci. 2023, 24, 493–502. [Google Scholar]
- Bohutskyi, P.; Keller, T.A.; Phan, D.; Parris, M.L.; Li, M.; Richardson, L.; Kopachevsky, A.M. Co-Digestion of Wastewater-Grown Filamentous Algae With Sewage Sludge Improves Biomethane Production and Energy Balance Compared to Thermal, Chemical, or Thermochemical Pretreatments. Front. Energy Res. 2019, 7, 47. [Google Scholar] [CrossRef]
- Mathimani, T.; Pugazhendhi, A. Utilization of Algae for Biofuel, Bio-Products and Bio-Remediation. Biocat. Agric. Biotechnol. 2019, 17, 326–330. [Google Scholar] [CrossRef]
- Onay, M. The Effects of Indole-3-Acetic Acid and Hydrogen Peroxide on Chlorella zofingiensis CCALA 944 for Bio-Butanol Production. Fuel 2020, 273, 117795. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Zhu, S.; Huo, S.; Yuan, Z.; Xie, J. Culture of Four Microalgal Strains for Bioenergy Production and Nutrient Removal in the Meliorative Municipal Wastewater. Energy Sources A Recovery Util. Environ. Eff. 2016, 38, 670–679. [Google Scholar] [CrossRef]
- Shu, Q.; Qin, L.; Yuan, Z.; Zhu, S.; Xu, J.; Xu, Z.; Feng, P.; Wang, Z. Comparison of Dairy Wastewater and Synthetic Medium for Biofuels Production by Microalgae Cultivation. Energy Sources Part A Recovery Util. Environ. Effects. 2018, 40, 751–758. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, H.; Ren, Y.; Wu, T.; He, Y.; Chen, F. Chlorella zofingiensis as a Promising Strain in Wastewater Treatment. Bioresour. Technol. 2018, 268, 286–291. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Ren, Y.; Wu, T.; Chen, F. Cost-Effective Wastewater Treatment in a Continuous Manner by a Novel Bio-Photoelectrolysis Cell (BPE) System. Bioresour. Technol. 2019, 273, 297–304. [Google Scholar] [CrossRef]
- Azaman, S.N.A.; Nagao, N.; Yusoff, F.M.; Tan, S.W.; Yeap, S.K. A Comparison of the Morphological and Biochemical Characteristics of Chlorella sorokiniana and Chlorella zofingiensis Cultured under Photoautotrophic and Mixotrophic Conditions. PeerJ 2017, 5, e3473. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xu, J.; Ma, L. Cultivation of Microalgae Chlorella zofingiensis on Municipal Wastewater and Biogas Slurry Towards Bioenergy. J. Biosci. Bioeng. 2018, 126, 644–648. [Google Scholar] [CrossRef]
- Fernando, J.S.R.; Premaratne, M.; Dinalankara, D.M.S.D.; Perera, G.L.N.J.; Ariyadasa, T.U. Cultivation of Microalgae in Palm Oil Mill Effluent (POME) for Astaxanthin Production and Simultaneous Phycoremediation. J. Environ. Chem. Eng. 2021, 9, 105375. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae Biofuels: Illuminating the Path to a Sustainable Future amidst Challenges and Opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, M.; Ozaki, N.; Kazeroon, R.A.; Rezania, S.; Baharlooeian, M.; Vakili, M.; Farraji, H.; Ohashi, A.; Kindaichi, T.; Zhou, J.L. Contaminant Removal from Wastewater by Microalgal Photobioreactors and Modeling by Artificial Neural Network. Water 2022, 14, 4046. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Zhu, S.; Zhou, W.; Dong, R.; Yuan, Z. Cultivation of Chlorella zofingiensis in Bench-Scale Outdoor Ponds by Regulation of pH Using Dairy Wastewater in Winter, South China. Bioresour. Technol. 2012, 121, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Takala, J.; Hiltunen, E.; Qin, L.; Xu, Z.; Qin, X.; Yuan, Z. Scale-up Potential of Cultivating Chlorella zofingiensis in Piggery Wastewater for Biodiesel Production. Bioresour. Technol. 2013, 137, 318–325. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient Removal and Biodiesel Production by Integration of Freshwater Algae Cultivation with Piggery Wastewater Treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Liu, T.; Yuan, M.; Liu, G.; Fang, J.; Yang, B. Exploration of Microalgal Species for Nutrient Removal from Anaerobically Digested Swine Wastewater and Potential Lipids Production. Microorganisms 2021, 9, 2469. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae Consortia Cultivation in Dairy Wastewater to Improve the Potential of Nutrient Removal and Biodiesel Feedstock Production. Environ. Sci. Pollut. Res. 2016, 23, 8379–8387. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Dinh, T.T.D. Ozonation and Cultivation of Green Algae Chlorella zofingiensis in Domestic Wastewater. Hong Duc Univ. J. Sci. 2019, 10, 5–12. [Google Scholar]
- Vitali, L.; Lolli, V.; Sansone, F.; Concas, A.; Lutzu, G.A. Effect of Mixotrophy on Lipid Content and Fatty Acids Methyl Esters Profile by Chlorella zofingiensis Grown in Media Containing Sugar Cane Molasses. Bioenergy Res. 2023, 16, 1851–1861. [Google Scholar] [CrossRef]
- Wichuk, K.; Brynjolfsson, S.; Fu, W. Biotechnological Production of Value-Added Carotenoids from Microalgae. Bioengineered 2014, 5, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Stein-Taylor, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973; 472p. [Google Scholar]
- Ruban, G.; Joannis, C.; Gromaire, M.-C.; Bertrand-Krajewski, J.-I.; Chebbo, G. Mesurage de la turbidité sur échantillons: Application aux eaux résiduaires urbaines. TSM 2008, 4, 61–74. [Google Scholar] [CrossRef][Green Version]
- Cawse, P.A. The Determination of Nitrate in Soil Solutions by Ultraviolet Spectrophotometry. Analyst 1967, 92, 311–315. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1971; pp. 237–239. [Google Scholar]
- Heinonen, J.K.; Lahti, R.J. A New and Convenient Colorimetric Determination of Inorganic Orthophosphate and Its Application to the Assay of Inorganic Pyrophosphatase. Anal. Biochem. 1981, 113, 313–317. [Google Scholar] [CrossRef]
- Patton, C.J.; Crouch, S.R. Spectrophotometric and Kinetics Investigation of the Berthelot Reaction for the Determination of Ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Dedkov, Y.M.; Elizarova, O.V.; Kel’ina, S.Y. Dichromate Method for the Determination of Chemical Oxygen Demand. J. Anal. Chem. 2000, 55, 777–781. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Improvement and Modeling of Culture Parameters to Enhance Biomass and Lipid Production by the Oleaginous Yeast Cryptococcus curvatus Grown on Acetate. Bioresour. Technol. 2015, 192, 582–591. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ba, F.; Foissard, A.; Lebert, A.; Djelveh, G.; Laroche, C. Polyethyleneimine as a Tool for Compounds Fractionation by Flocculation in a Microalgae Biorefinery Context. Bioresour. Technol. 2020, 315, 123857. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride-Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Li, Y.; Miao, F.; Geng, Y.; Lu, D.; Zhang, C.; Zeng, M. Accurate Quantification of Astaxanthin from Haematococcus Crude Extract Spectrophotometrically. Chin. J. Oceanol. Limnol. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin Accumulation in the Green Microalga Haematococcus pluvialis: Effect of Initial Phosphate Concentration and Stepwise/Continuous Light Stress. Biotechnol. Rep. 2020, 28, e00538. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic Treatment of Dairy Wastewaters: A Review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and Whey Proteins—From ‘Gutter-to-Gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic Cultivation of Green Microalgae Scenedesmus obliquus on Cheese Whey Permeate for Biodiesel Production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Qian, F.; Sun, J.; Cao, D.; Tuo, Y.; Jiang, S.; Mu, G. Experimental and modelling study of the denaturation of milk protein by heat treatment. Korean J. Food Sci. Anim. Resour. 2017, 37, 44–51. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, X.; Miao, J.; Tian, Y.-T. Tofu Whey Wastewater Is a Promising Basal Medium for Microalgae Culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Ding, S.-Y.; Hoe, C.-H.; Low, C.-S. Mixotrophic Growth of Chlorella sorokiniana in Outdoor Enclosed Photobioreactor. J. Appl. Phycol. 1996, 8, 163–169. [Google Scholar] [CrossRef]
- Andrade, A.F.D.; Silva, P.E.D.C.E.; Melo, R.G.D.; Ferreira, M.P.D.N.; Porto, A.L.F.; Bezerra, R.P. Microalgal Production under Mixotrophic Conditions Using Cheese Whey as Substrate. Acta Sci. Biol. Sci. 2022, 44, e62512. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; Martini, M.; Altomonte, I.; Salari, F.; Nardoni, S.; Sorce, C.; Silva, F.L.H.D.; Andreucci, A. Production of Chlorella protothecoides Biomass, Chlorophyll and Carotenoids Using the Dairy Industry By-Product Scotta as a Substrate. Biocat. Agric. Biotechnol. 2017, 11, 207–213. [Google Scholar] [CrossRef]
- Kumar, P.K.; Krishna, S.V.; Naidu, S.S.; Verma, K.; Bhagawan, D.; Himabindu, V. Biomass Production from Microalgae Chlorella Grown in Sewage, Kitchen Wastewater Using Industrial CO₂ Emissions: Comparative Study. Carbon Res. Conv. 2019, 2, 126–133. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P Ratio on Biomass Productivity and Nutrient Removal from Municipal Wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The Influence of Organisms on the Composition of Seawater. Sea Ideas Obs. Prog. Study Seas 1963, 2, 26–77. [Google Scholar]
- Figler, A.; Márton, K.; Béres, V.; Bácsi, I. Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal, and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale. Energies 2021, 14, 2112. [Google Scholar] [CrossRef]
- Cheng, P.; Cheng, J.J.; Cobb, K.; Zhou, C.; Zhou, N.; Addy, M.; Chen, P.; Yan, X.; Ruan, R. Tribonema sp. and Chlorella zofingiensis Co-Culture to Treat Swine Wastewater Diluted with Fishery Wastewater to Facilitate Harvest. Bioresour. Technol. 2020, 297, 122516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hiltunen, E.; Shu, Q.; Zhou, W.; Li, Z.; Wang, Z. Biodiesel Production from Algae Cultivated in Winter with Artificial Wastewater Through pH Regulation by Acetic Acid. Appl. Energy 2014, 128, 103–110. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Mohd Yasin, N.H. Microalgae Acclimatization in Industrial Wastewater and Its Effect on Growth and Primary Metabolite Composition. Algal Res. 2021, 53, 102163. [Google Scholar] [CrossRef]
- Yirgu, Z.; Asfaw, S.L.; Dekebo, A.H.; Khan, M.M.; Aragaw, T. Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent. Sustainability 2023, 15, 15403. [Google Scholar] [CrossRef]
- Carneiro, M.; Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Kozhan, D.M.; Toribio, A.; Moreno, J.; Otero, A.; Varela, J.; et al. Growth and Bioactivity of Two Chlorophyte (Chlorella and Scenedesmus) Strains Co-Cultured Outdoors in Two Different Thin-Layer Units Using Municipal Wastewater as a Nutrient Source. Algal Res. 2021, 56, 102299. [Google Scholar] [CrossRef]
- Qin, L.; Shu, Q.; Wang, Z.; Shang, C.; Zhu, S.; Xu, J.; Li, R.; Zhu, L.; Yuan, Z. Cultivation of Chlorella vulgaris in Dairy Wastewater Pretreated by UV Irradiation and Sodium Hypochlorite. Appl. Biochem. Biotechnol. 2014, 172, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic Cultivation of Chlorella pyrenoidosa with Diluted Primary Piggery Wastewater to Produce Lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing Biodiesel: Standards and Other Methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Bonanno, A.; Poma, I.; Calabrese, V.; Di Stefano, V.; Barone, R. Enzymatic hydrolysis of dairy by-products as a source of marine bioactive peptides: Potential and challenges. Mar. Drugs 2023, 21, 190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).