Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Samples
2.1.2. Chemicals
2.2. Methods
2.2.1. Water Content
2.2.2. Electrical Conductivity
2.2.3. Diastase Number
2.2.4. Specific Rotation
2.2.5. Carbohydrate Profile
2.2.6. HMF Analysis
2.2.7. Color Measurements
2.2.8. Electron Paramagnetic Resonance Measurements of Antioxidant Activities
2.2.9. Total Phenolic Compounds Content (TPC)
2.2.10. DPPH Free Radical Scavenging Ability
2.2.11. Chromatographic Analysis of Polyphenol Profile
2.2.12. Statistical Analysis
Cluster Analysis
Ordinary Least Squares (OLS) Regression
3. Results and Discussion
3.1. Physicochemical and Antioxidant Features of Honeys
3.2. Statistical Discrimination of Honeys
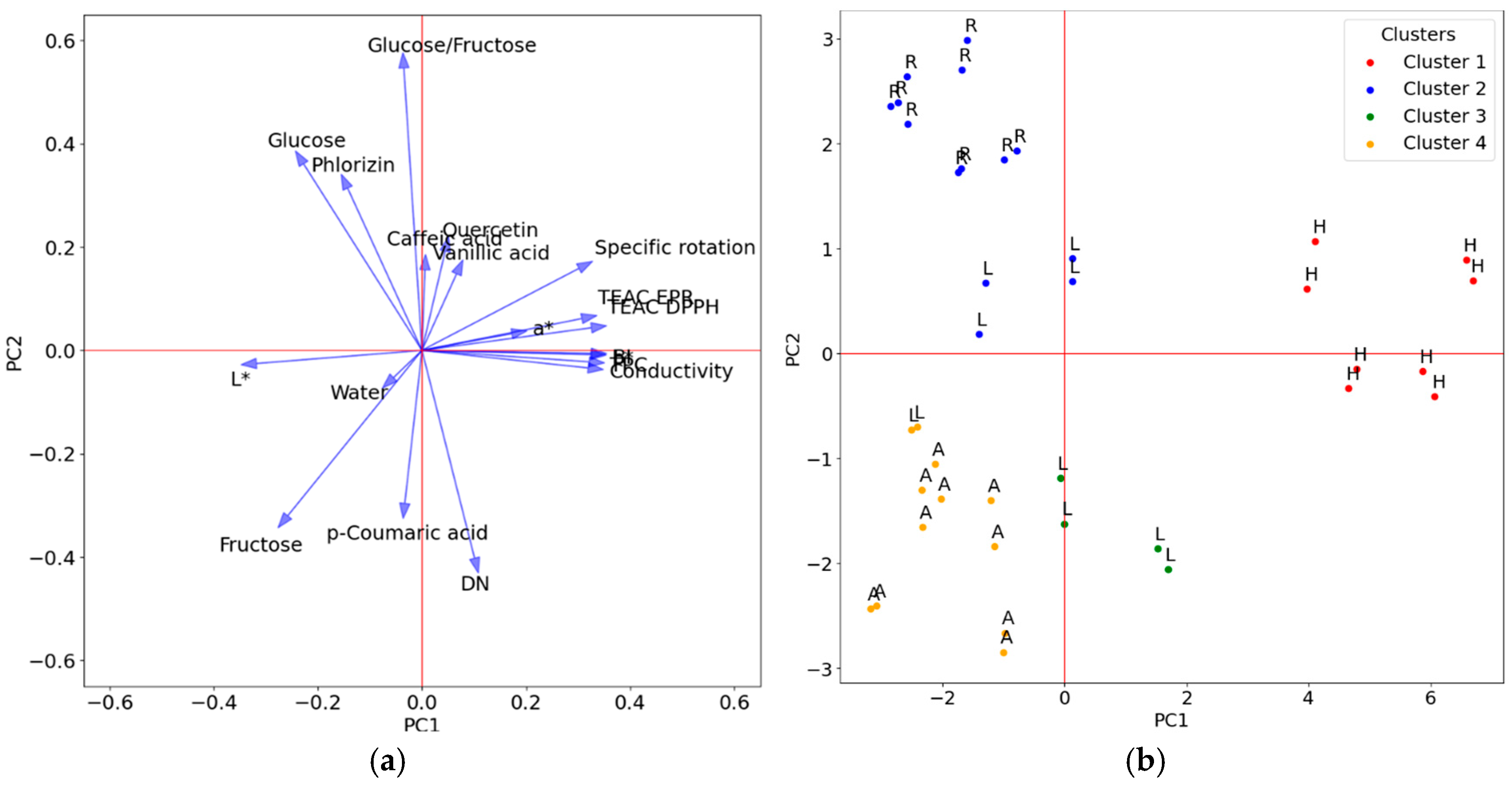
3.2.1. Cluster Analysis
3.2.2. Assessment of OLS Regression Model Fit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biluca, F.C.; Della Betta, F.; de Oliveira, G.P.; Pereira, L.M.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. 5-HMF and Carbohydrates Content in Stingless Bee Honey by CE before and after Thermal Treatment. Food Chem. 2014, 159, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A Review of Volatile Analytical Methods for Determining the Botanical Origin of Honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Zielińska, S.; Wesołowska, M.; Bilek, M.; Kaniuczak, J.; Dżugan, M. The Saccharide Profile of Polish Honeys Depending on Their Botanical Origin. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 387–390. [Google Scholar]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Hermosin, I.; Chicon, R.M.; Dolores Cabezudo, M. Free Amino Acid Composition and Botanical Origin of Honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Khalil, M.I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.A.; Islam, M.N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef]
- Kačániová, M.; Borotová, P.; Galovičová, L.; Kunová, S.; Štefániková, J.; Kowalczewski, P.Ł.; Šedík, P. Antimicrobial and Antioxidant Activity of Different Honey Samples from Beekeepers and Commercial Producers. Antibiotics 2022, 11, 1163. [Google Scholar] [CrossRef]
- Popek, S. Identification of Honey Types. Nahrung 2003, 47, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free Amino Acids Profile of Polish and Slovak Honeys Based on LC–MS/MS Method without the Prior Derivatisation. J. Food Sci. Technol. 2017, 54, 3716–3723. [Google Scholar] [CrossRef]
- Bose, D.; Padmavati, M. Honey Authentication: A Review of the Issues and Challenges Associated with Honey Adulteration. Food Biosci. 2024, 61, 105004. [Google Scholar] [CrossRef]
- Devillers, J.; Morlot, M.; Pham-Delegue, M.H.; Dore, J.C. Classification of Monofloral Honeys Based on Their Quality Control Data. Food Chem. 2004, 86, 305–312. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic Acids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus Honeys and Their Potential for Floral Authentication. Food Res. Int. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Lund, B.; Ma, J. A Review of Cluster Analysis Techniques and Their Uses in Library and Information Science Research: K-Means and k-Medoids Clustering. Perform. Meas. Metr. 2021, 22, 161–173. [Google Scholar] [CrossRef]
- Zdaniuk, B. Ordinary Least-Squares (OLS) Model. In Encyclopedia of Quality of Life and Well-Being Research; Maggino, F., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 4867–4869. ISBN 978-3-031-17299-1. [Google Scholar]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The Influence of Geographical Origin on Honey Composition Studied by Polish and Slovak Honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Czigle, S.; Filep, R.; Balažová, E.; Szentgyörgyi, H.; Balázs, V.L.; Kocsis, M.; Purger, D.; Papp, N.; Farkas, Á. Antioxidant Capacity Determination of Hungarian-, Slovak-, and Polish-Origin Goldenrod Honeys. Plants 2022, 11, 792. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lullmann, C. Harmonised Methods of the International Honey Commission; International Honey Commission; Swiss Bee Research Centre: Bern, Switzerland, 2002; Volume 5, pp. 1–62. [Google Scholar]
- Maskan, M. Kinetics of Colour Change of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Polovka, M.; Sťavíková, L.; Hohnová, B.; Karásek, P.; Roth, M. Offline Combination of Pressurized Fluid Extraction and Electron Paramagnetic Resonance Spectroscopy for Antioxidant Activity of Grape Skin Extracts Assessment. J. Chromatogr. A 2010, 1217, 7990–8000. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Poyrazoglu, E.S.; Velioglu, Y.S. Effects of Prolonged Heating on Antioxidant Activity and Colour of Honey. Food Chem. 2006, 95, 653–657. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of Storage on the Content of Polyphenols, Vitamin C and the Antioxidant Activity of Orange Juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, Ł.; Tabaszewska, M.; Wywrocka-Gurgul, A. Hemp Flour as a Valuable Component for Enriching Physicochemical and Antioxidant Properties of Wheat Bread. LWT 2019, 102, 164–172. [Google Scholar] [CrossRef]
- EEC. Council Directive of 20 December relating to honey 2001/110/EC. Off. J. Eur. Communities 2001, 10, 47–52. [Google Scholar]
- Kowalski, S. Changes of Antioxidant Activity and Formation of 5-Hydroxymethylfurfural in Honey during Thermal and Microwave Processing. Food Chem. 2013, 141, 1378–1382. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Plos, C.; Stelbrink, N.; Römermann, C.; Knight, T.M.; Hensen, I. Abiotic Conditions Affect Nectar Properties and Flower Visitation in Four Herbaceous Plant Species. Flora 2023, 303, 152279. [Google Scholar] [CrossRef]
- Dmitruk, M.; Denisow, B.; Chrzanowska, E.; Dąbrowska, A.; Bożek, M. Comparison of Nectar and Pollen Resources in Various Tilia Species. A Case Study from Southern Poland. Trees 2024, 38, 953–967. [Google Scholar] [CrossRef]
- d’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting Flowering Phenology in Oil Seed Rape Parcels with Sentinel-1 and -2 Time Series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef]
- Martin, G.D. Addressing Geographical Bias: A Review of Robinia Pseudoacacia (Black Locust) in the Southern Hemisphere. S. Afr. J. Bot. 2019, 125, 481–492. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Futur. Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
| Honey | Acacia | Lime | Rapeseed | Honeydew | |
|---|---|---|---|---|---|
| Cluster | |||||
| 1 | 0 | 0 | 0 | 8 | |
| 2 | 0 | 4 | 10 | 0 | |
| 3 | 0 | 4 | 0 | 0 | |
| 4 | 10 | 2 | 0 | 0 | |
| Variable | Coefficient | Standard Error | t-Statistic | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| const | −0.2466 | 0.1500 | −1.6410 | 0.1100 | −0.5520 | 0.0590 |
| a* | −0.1588 | 0.0570 | −2.8090 | 0.0080 | −0.2740 | −0.0440 |
| b* | 0.0377 | 0.0030 | 12.8410 | 0.0000 | 0.0320 | 0.0440 |
| p-coumaric acid | −0.0800 | 0.0180 | −4.4140 | 0.0000 | −0.1170 | −0.0430 |
| vanillic acid | 0.0435 | 0.0160 | 2.6660 | 0.0120 | 0.0100 | 0.0770 |
| Variable | Coefficient | Standard Error | t-Statistic | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| const | 0.1590 | 0.0220 | 7.2400 | 0.0000 | 0.1140 | 0.2040 |
| BI | 0.0163 | 0.0010 | 21.5180 | 0.0000 | 0.0150 | 0.0180 |
| Variable | Coefficient | Standard Error | t-Statistic | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| const | 166.9172 | 16.3000 | 10.2400 | 0.0000 | 133.8260 | 200.0080 |
| b* | 8.3961 | 0.4150 | 20.2190 | 0.0000 | 7.5530 | 9.2390 |
| caffeic acid | 11.2498 | 5.3470 | 2.1040 | 0.0430 | 0.3940 | 22.1050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, S.; Ciesarová, Z.; Kukurová, K.; Tobolková, B.; Polovka, M.; Skoczylas, Ł.; Tabaszewska, M.; Mikulec, K.; Mikulec, A.; Buksa, K. Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys. Appl. Sci. 2025, 15, 5810. https://doi.org/10.3390/app15115810
Kowalski S, Ciesarová Z, Kukurová K, Tobolková B, Polovka M, Skoczylas Ł, Tabaszewska M, Mikulec K, Mikulec A, Buksa K. Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys. Applied Sciences. 2025; 15(11):5810. https://doi.org/10.3390/app15115810
Chicago/Turabian StyleKowalski, Stanisław, Zuzana Ciesarová, Kristína Kukurová, Blanka Tobolková, Martin Polovka, Łukasz Skoczylas, Małgorzata Tabaszewska, Karolina Mikulec, Anna Mikulec, and Krzysztof Buksa. 2025. "Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys" Applied Sciences 15, no. 11: 5810. https://doi.org/10.3390/app15115810
APA StyleKowalski, S., Ciesarová, Z., Kukurová, K., Tobolková, B., Polovka, M., Skoczylas, Ł., Tabaszewska, M., Mikulec, K., Mikulec, A., & Buksa, K. (2025). Physicochemical and Antioxidant Properties of Selected Polish and Slovak Honeys. Applied Sciences, 15(11), 5810. https://doi.org/10.3390/app15115810








