Assessing High-Value Bioproducts from Seaweed Biomass: A Comparative Study of Wild, Cultivated and Residual Pulp Sources
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Biochemical Profiling Methodology
2.2.1. Ash Content and Moisture Percentage
2.2.2. Protein Content
2.2.3. Total Lipids
2.2.4. Fatty Acid Profile Determination
2.2.5. Pigment Analysis
2.2.6. Quantification of Elements
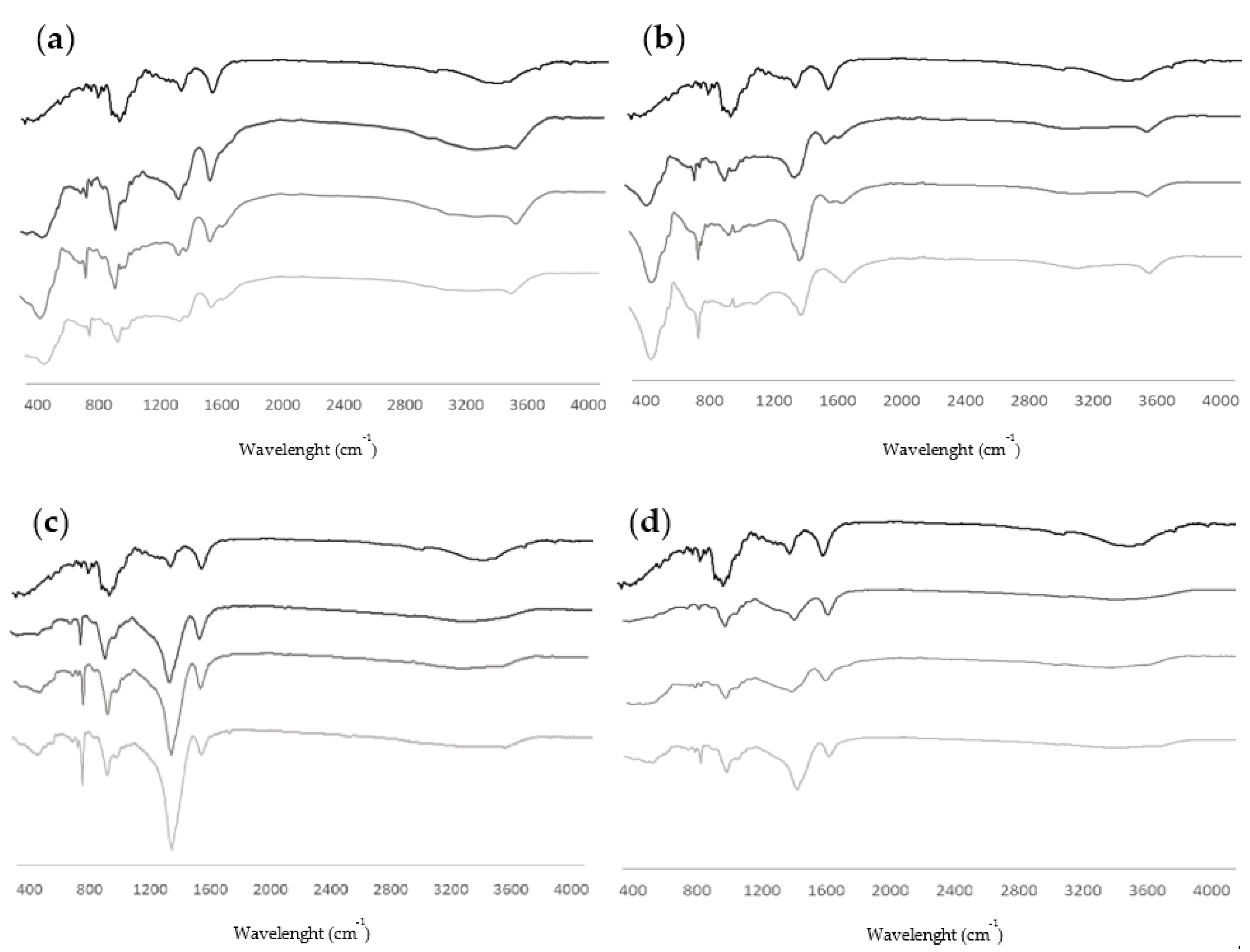
2.2.7. Alginate Extraction and Analysis
2.3. Antioxidant Activity
2.3.1. Hydroethanolic Extraction
2.3.2. Total Phenolic Content Assay (TPC)
2.3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4. Statistical Analysis
3. Results
3.1. Biochemical Profiling
3.1.1. Proximate Composition
3.1.2. Fatty Acid Profile
3.1.3. Pigment Contents
3.1.4. Elemental Composition
3.1.5. Alginate Content
3.2. Antioxidant Capacity
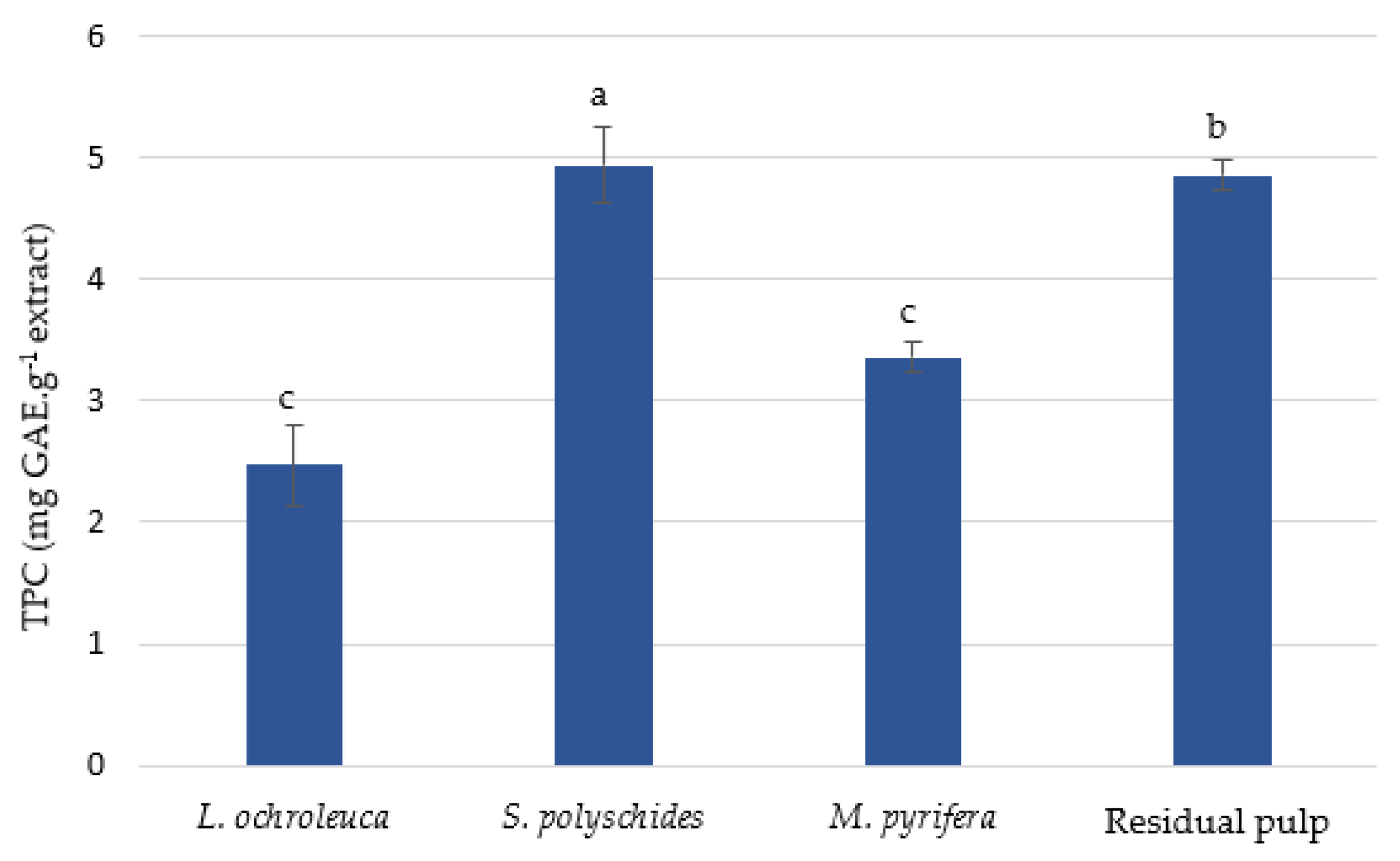
3.2.1. Total Phenolic Content (TPC)
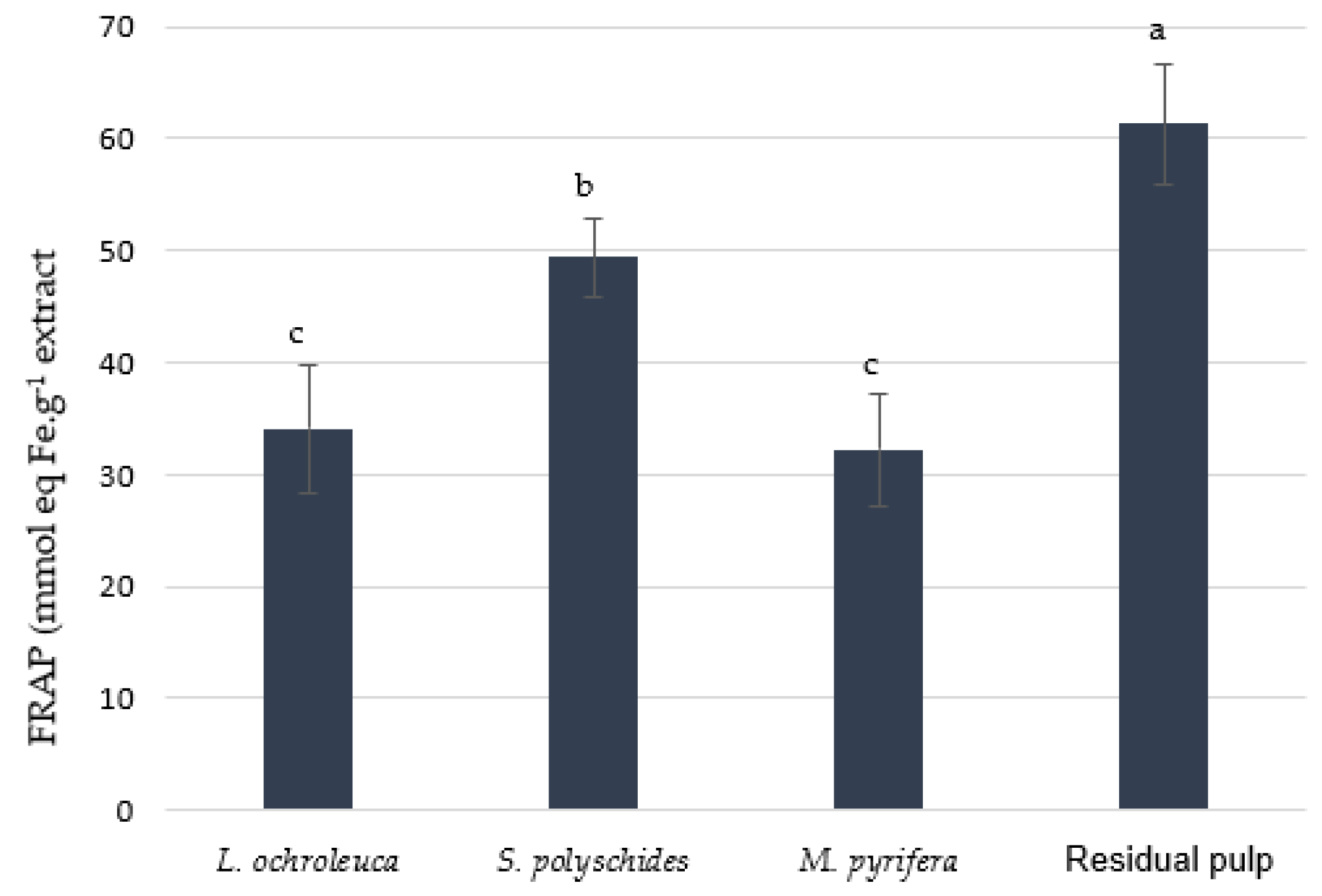
3.2.2. Ferric Reducing Antioxidant Power (FRAP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becket, A.; Macgregor, G.; Duffy, P.; Finlay, N.; Miller, J.; Sheridan, A. Forest Grazing and Seaweed Foddering: Early Neolithic Occupation at Maybole, South Ayrshire. Proc. Soc. Antiqu. Scotl. 2009, 139, 105–122. [Google Scholar] [CrossRef]
- Yang, L.E.; Lu, Q.Q.; Brodie, J. A review of the bladed Bangiales (Rhodophyta) in China: History, culture and taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; pp. 354–355. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E. Phycology, 5th ed.; Cambridge University Press: New York, NY, USA, 2018. [Google Scholar]
- Cardoso, S.; Carvalho, L.; Silva, P.; Rodrigues, M.; Pereira, O.; Pereira, L. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Pereira, L.; Ribeiro-Claro, P. Analysis by vibrational spectroscopy of seaweeds with potential in food, pharmaceutical and cosmetic industries. In Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Neto, J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 228–239. [Google Scholar]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Open Access Libr. J. 2014, 1, e752. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Norton, T.A. Growth Form and Environment in Saccorhiza polyschides. J. Mar. Biol. Assoc. 1969, 49, 1025–1045. [Google Scholar] [CrossRef]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and Knowledge Gaps for Ecosystem Services Provided by Kelp Forests: A Northeast Atlantic Perspective. Ecol. Evol. 2013, 3, 4016–4038. [Google Scholar] [CrossRef]
- Salland, N.; Jensen, A.; Smale, D.A. The Structure and Diversity of Macroinvertebrate Assemblages Associated with the Understudied Pseudo-Kelp Saccorhiza polyschides in the Western English Channel (UK). Mar. Environ. Res. 2024, 198. [Google Scholar] [CrossRef] [PubMed]
- Biskup, S.; Bertocci, I.; Arenas, F.; Tuya, F. Functional Responses of Juvenile Kelps, Laminaria ochroleuca and Saccorhiza polyschides, to Increasing Temperatures. Aquat. Bot. 2014, 113, 117–122. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2013; Available online: http://www.algaebase.org (accessed on 31 January 2025).
- Soares, C.; Švarc-Gajić, J.; Oliva-Teles, M.T.; Pinto, E.; Nastić, N.; Savić, S.; Almeida, A.; Delerue-Matos, C. Mineral Composition of Subcritical Water Extracts of Saccorhiza polyschides, a Brown Seaweed Used as Fertilizer in the North of Portugal. J. Mar. Sci. Eng. 2020, 8, 244. [Google Scholar] [CrossRef]
- Freitas, M.V.; Pereira, L.; Afonso, C.; Mouga, T. Sabores Do Mar; Politécnico Leiria: Leiria, Portugal, 2022. [Google Scholar]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective Effect of Seaweeds with High Antioxidant Activity from the Peniche Coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Neuroprotective Effects of Seaweeds against 6-Hydroxidopamine-Induced Cell Death on an in Vitro Human Neuroblastoma Model. BMC Complement. Altern. Med. 2018, 18, 58. [Google Scholar] [CrossRef]
- Assis, J.; Tavares, J.T.; Serrão, E.A.; Alberto, F.; Ferreira, C.; Tavares, D.; Paulos, L.; Tempera, F. Florestas Marinhas. As Espécies de Algas Castanhas Gigantes de Portugal, 1st ed.; Gobius Comunicação e Ciência: Oeiras, Portugal, 2011; Volume 1. [Google Scholar]
- Pereira, T.R.; Azevedo, I.C.; Oliveira, P.; Silva, D.M.; Sousa-Pinto, I. Life History Traits of Laminaria ochroleuca in Portugal: The Range-Center of Its Geographical Distribution. Aquat. Bot. 2019, 152, 1–9. [Google Scholar] [CrossRef]
- WoRMS, Editorial Board World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 19 February 2025).
- Pérez-Alva, A.; Ramírez-Rodrigues, M.A.; Baigts-Allende, D.K.; Martínez-Jiménez, L.A.; Ramírez-Rodrigues, M.M. Macrocystis pyrifera: A Potential Source of Bioactive Compounds. Curr. Nutr. Food Sci. 2024, 20, 505–512. [Google Scholar] [CrossRef]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in Food and Pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef]
- Gold, M.; Bae, Y.S.C.; Goldberg, D.J.; Jamal, S.; Lewis, A.B.; Marchbein, S.; Ros, A.; Santhanam, U.; DiNatale, L.; Emmetsberger, J. Macrocystis pyrifera Ferment-Containing Creams for Optimizing Facial Skin Rejuvenation. J. Cosmet. Dermatol. 2023, 22, 3313–3319. [Google Scholar] [CrossRef]
- Gojon-Baez, H.H.; Siqueiros-Beltrones, D.A.; Hernandez-Contreras, H. In Situ Ruminal Digestibility and Degradability of Macrocystis pyrifera and Sargassum spp. in Bovine Livestock. Cienc. Mar. 1998, 24, 463–481. [Google Scholar] [CrossRef]
- Mansilla, A.; Ávila, M. Using Macrocystis pyrifera (L.) C. Agardh from Southern Chile as a Source of Applied Biological Compounds. Rev. Bras. Farm. 2011, 21, 262–267. [Google Scholar] [CrossRef]
- Qin, Y. Alginate Fibres: An Overview of the Production Processes and Applications in Wound Management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) AOAC Official Methods of Analysis of AOAC International, 20th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2016; ISBN 0935584870. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The Use of Biomarkers in Daphnia Magna Toxicity Testing. IV. Cellular Energy Allocation: A New Methodology to Assess the Energy Budget of Toxicant-Stressed Daphnia Populations; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; Volume 6. [Google Scholar]
- Fernández, A.; Grienke, U.; Soler-Vila, A.; Guihéneuf, F.; Stengel, D.B.; Tasdemir, D. Seasonal and Geographical Variations in the Biochemical Composition of the Blue Mussel (Mytilus edulis L.) from Ireland. Food Chem. 2015, 177, 43–52. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Connan, S. Spectrophotometric Assays of Major Compounds Extracted from Algae. Methods Mol. Biol. 2015, 1308, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and Characterization of Alginate from an Edible Brown Seaweed (Cystoseira barbata) Harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Beratto-Ramos, A.; Agurto-Muñoz, C.; Pablo Vargas-Montalba, J.; Castillo, R. del P. Fourier-Transform Infrared Imaging and Multivariate Analysis for Direct Identification of Principal Polysaccharides in Brown Seaweeds. Carbohydr. Polym. 2020, 230, 115561. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carmona, G.; McHugh, D.J.; López-Gutiérrez, F. Pilot Plant Scale Extraction of Alginates from Macrocystis pyrifera. 2. Studies on Extraction Conditions and Methods of Separating the Alkaline-Insoluble Residue. J. Appl. Phycol. 1999, 11, 493–502. [Google Scholar] [CrossRef]
- Pacheco, D.; Miranda, G.; Rocha, C.P.; Pato, R.L.; Cotas, J.; Gonçalves, A.M.M.; Dias Santos, S.M.; Bahcevandziev, K.; Pereira, L. Portuguese Kelps: Feedstock Assessment for the Food Industry. Appl. Sci. 2021, 11, 10681. [Google Scholar] [CrossRef]
- Garcia, J.S.; Palacios, V.; Roldán, A. Nutritional Potential of Four Seaweed Species Collected in the Barbate Estuary (Gulf of Cadiz, Spain). J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar] [CrossRef]
- Milinovic, J.; Fernando, A.L.; Campos, B.; Leite, B.; Mata, P.; Diniz, M.; Sardinha, J.; Noronha, J.P. Nutritional Benefits of Edible Macroalgae from the Central Portuguese Coast: Inclusion of Low-Calorie ‘Sea Vegetables’ in Human Diet. Int. J. Environ. Sci. Nat. Res. 2021, 28, 556250. [Google Scholar] [CrossRef]
- Fennel, W. Theory of the Benguela Upwelling System. J. Phys. Oceanogr. 1999, 29, 177–190. [Google Scholar] [CrossRef]
- Rixen, T.; Lahajnar, N.; Lamont, T.; Koppelmann, R.; Martin, B.; van Beusekom, J.E.E.; Siddiqui, C.; Pillay, K.; Meiritz, L. Oxygen and Nutrient Trapping in the Southern Benguela Upwelling System. Front. Mar. Sci. 2021, 8, 730591. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of Alginate-Extracted Seaweed Biomass for the Development of Cellulose-Based Packaging Films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional and functional properties. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese Coast as a Source of Proteinaceous Material: Total and Free Amino Acid Composition Profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Bolton, J.J.; Rothman, M.D. The Potential for Kelp (Order Laminariales) Aquaculture in South Africa: A Biological Review. Bot. Mar. 2024, 67, 525–541. [Google Scholar] [CrossRef]
- Biparva, P.; Mirzapour-Kouhdasht, A.; Valizadeh, S.; Garcia-Vaquero, M. Advanced Processing of Giant Kelp (Macrocystis Pyrifera) for Protein Extraction and Generation of Hydrolysates with Anti-Hypertensive and Antioxidant Activities In Vitro and the Thermal/Ionic Stability of These Compounds. Antioxidants 2023, 12, 775. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.; Hayes, M.; Sheldrake, G.; Tiwari, B.; Walsh, P. Macroalgal Proteins: A Review. Foods 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Macroalgae Specialized Metabolites: Evidence for Their Anti-Inflammatory Health Benefits. Mar. Drugs 2022, 20, 789. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Macroalgae farming for sustainable future: Navigating opportunities and driving innovation. Heliyon 2024, 10, e28208. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Sokovic, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Macroalgae as an Alternative Source of Nutrients and Compounds with Bioactive Potential. Proceedings 2021, 70, 46. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Sousa, S.; Machado, S.; Vieira, E.; Carvalho, A.P.; Ramalhosa, M.J.; Morais, S.; Correia, M.; Oliva-Teles, T.; Domingues, V.F.; et al. Bioactive Lipids of Seaweeds from the Portuguese North Coast: Health Benefits versus Potential Contamination. Foods 2021, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal Variability of the Biochemical Composition and Antioxidant Properties of Fucus spiralis at Two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty Acids, Total Lipid, Protein and Ash Contents of Processed Edible Seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Sustainable Bio-Based Materials from Minimally Processed Red Seaweeds: Effect of Composition and Cell Wall Structure. J. Polym. Environ. 2023, 31, 886–899. [Google Scholar] [CrossRef]
- Mouga, T. Integridade membranar: A resposta da membrana plasmática ao stress térmico e aos solventes orgânicos em células de beterraba. Captar Ciência e Ambiente Para Todos 2024, 13, 1–13. [Google Scholar]
- Belattmania, Z.; Engelen, A.H.; Pereira, H.; Serrão, E.A.; Custódio, L.; Varela; Zrid; Reani; Sabour, B. Fatty Acid Composition and Nutraceutical Perspectives of Brown Seaweeds from the Atlantic Coast of Morocco. Int. Food Res. J. 2018, 25, 1520–1527. [Google Scholar]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of Pressurized Liquid Extraction (PLE) to Obtain Bioactive Fatty Acids and Phenols from Laminaria ochroleuca Collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Horta, A.; Barroso, S.; Mendes, S.; Gil, M.M. Study of the Seasonal Variations of the Fatty Acid Profiles of Selected Macroalgae. Molecules 2021, 26, 5807. [Google Scholar] [CrossRef] [PubMed]
- Méndez, F.; Rivero, A.; Bahamonde, F.; Gallardo, P.; Frangopulos, M.; Zolezzi, J.; Inestrosa, N.C.; Mansilla, A. Fatty Acid Composition of Different Morphological Structures in the Sub-Antarctic Kelps Macrocystis pyrifera (L.) C. Agardh and Lessonia Flavicans Bory of the Magellan Ecoregion: Nutritional and Biomedical Potentials. J. Appl. Phycol. 2023, 36, 831–844. [Google Scholar] [CrossRef]
- Carboni, S.; Hughes, A.D.; Atack, T.; Tocher, D.R.; Migaud, H. Fatty Acid Profiles during Gametogenesis in Sea Urchin (Paracentrotus lividus): Effects of Dietary Inputs on Gonad, Egg and Embryo Profiles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 376–382. [Google Scholar] [CrossRef]
- Gomes, A.S.; Lourenço, S.; Santos, P.M.; Neves, M.; Adão, P.; Tecelão, C.; Pombo, A. High Dietary Protein, n − 3/n − 6 Ratio and β-Carotene Enhances Paracentrotus lividus (Lamarck, 1816) Larval Development. Aquac. Res. 2022, 53, 5398–5412. [Google Scholar] [CrossRef]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importancia Del Equilibrio Del Índice Omega-6/Omega-3 En El Mantenimiento de Un Buen Estado de Salud. Recomendaciones Nutricionales. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Fatty Acid Contents and Profiles of 16 Macroalgae Collected from the Irish Coast at Two Seasons. J. Appl. Phycol. 2014, 26, 451–463. [Google Scholar] [CrossRef]
- McHugh, D.J. Production, Properties and Uses of Alginates. In Production and Utilization of Products from Commercial Seaweeds; McHugh, D.J., Ed.; FAO: Rome, Italy, 1987. [Google Scholar]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.Y.; Ward, K. Multistage Extraction and Purification of Waste Sargassum natans to Produce Sodium Alginate: An Optimization Approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kaidi, S.; Bentiss, F.; Jama, C.; Khaya, K.; Belattmania, Z.; Reani, A.; Sabour, B. Isolation and Structural Characterization of Alginates from the Kelp Species Laminaria ochroleuca and Saccorhiza polyschides from the Atlantic Coast of Morocco. Colloids Interfaces 2022, 6, 51. [Google Scholar] [CrossRef]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid Pretreatment and Enzymatic Saccharification of Brown Seaweed for Polyhydroxybutyrate (PHB) Production Using Cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Ward, A.A.; Mostafa, D.M.; Abd-El-Messieh, S.L.; Abdel-Nour, K.N.; Darwish, M.M.; Khalil, W.A. Drug–polymer interaction between glucosamine sulfate and alginate nanoparticles: FTIR, DSC and dielectric spectroscopy studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 02501. [Google Scholar] [CrossRef]
- Miguel, S.P.; D’Angelo, C.; Ribeiro, M.P.; Simões, R.; Coutinho, P. Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Mar. Drugs 2023, 21, 589. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.K.; Chopin, T.; Robinson, S.M.C.; Azevedo, P.; Quinton, M.; Belyea, E. Weight Ratios of the Kelps, Alaria esculenta and Saccharina latissima, Required to Sequester Dissolved Inorganic Nutrients and Supply Oxygen for Atlantic Salmon, Salmo salar, in Integrated Multi-Trophic Aquaculture Systems. Aquaculture 2013, 408–409, 34–46. [Google Scholar] [CrossRef]
- Cavaco, M.; Duarte, A.; Freitas, M.V.; Afonso, C.; Bernardino, S.; Pereira, L.; Martins, M.; Mouga, T. Seasonal Nutritional Profile of Gelidium corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods 2021, 10, 2394. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 465/2023 of the European Parliament and of the Council. In Official Journal of the European Union; European Commission: Bruxelles, Belgium, 2023. [Google Scholar]
- Yu, Y.; Morales-Rodriguez, A.; Zhou, G.; Barrón, D.; Sahuquillo, À.; López-Sánchez, J.F. Survey of Arsenic Content in Edible Seaweeds and Their Health Risk Assessment. Food Chem. Toxicol. 2024, 187, 114603. [Google Scholar] [CrossRef]
- Wei, C.; Li, W.; Zhang, C.; Van Hulle, M.; Cornelis, R.; Zhang, X. Safety Evaluation of Organoarsenical Species in Edible Porphyra from the China Sea. J. Agric. Food Chem. 2003, 51, 5176–5182. [Google Scholar] [CrossRef]
- Chen, J.; Cao, W.; Wei, P.; Li, T.; Weng, W. Speciation Transformation of Arsenic in Abalone Viscera Hydrolysate Fraction: In Vitro Digestion and in Vivo Metabolism. Food Res. Int. 2019, 123, 340–345. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EU) No 1169/2011 of the European Parliament and of the Council. In Official Journal of the European Union; European Commission: Bruxelles, Belgium, 2011. [Google Scholar]
- Beattie, S.W.; Morançais, M.; Déléris, P.; Fleurence, J.; Dumay, J. Extraction of Phycocyanin and Phycoerythrin Pigments. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 249–265. [Google Scholar]
- Gomez, I.; Huovinen, P. Morpho-functional patterns and zonation of South Chilean seaweeds: The importance of photosynthetic and bio-optical traits. Mar. Ecol. Prog. Ser. 2011, 422, 77–91. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Tormena, C.D.; Pauli, E.D.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Experimental Mixture Design Solvent Effects on Pigment Extraction and Antioxidant Activity from Coffea arabica L. Leaves. Microchem. J. 2019, 146, 713–721. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of Fucoxanthin Extraction from Irish Seaweeds by Response Surface Methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The Pigments of Kelps (Ochrophyta) as Part of the Flexible Response to Highly Variable Marine Environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Zabochnicka, M.; Krzywonos, M.; Romanowska-Duda, Z.; Szufa, S.; Darkalt, A.; Mubashar, M. Algal Biomass Utilization toward Circular Economy. Life 2022, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Helgadóttir, J.K.; Jónsdóttir, R. Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals. Phycology 2024, 4, 576–588. [Google Scholar] [CrossRef]
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae Fucus vesiculosus with Antioxidant and Photo-Protecting Properties. Marine Drugs 2022, 20, 687. [Google Scholar] [CrossRef]
- van Hees, D.H.; Olsen, Y.S.; Wernberg, T.; Van Alstyne, K.L.; Kendrick, G.A. Phenolic concentrations of brown seaweeds and relationships to nearshore environmental gradients in Western Australia. Mar. Biol. 2017, 164, 74. [Google Scholar] [CrossRef]
- Lopes, C.; Obando, J.M.C.; Santos, T.C.D.; Cavalcanti, D.N.; Teixeira, V.L. Abiotic Factors Modulating Metabolite Composition in Brown Algae (Phaeophyceae): Ecological Impacts and Opportunities for Bioprospecting of Bioactive Compounds. Mar. Drugs 2024, 22, 544. [Google Scholar] [CrossRef]
- Beratto-Ramos, A.; Castillo-Felices, R.d.P.; Troncoso-Leon, N.A.; Agurto-Muñoz, A.; Agurto-Muñoz, C. Selection criteria for high-value biomass: Seasonal and morphological variation of polyphenolic content and antioxidant capacity in two brown macroalgae. J. Appl. Phycol. 2019, 31, 653–664. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Ravanal, M.C.; Pezoa-Conte, R.; von Schoultz, S.; Hemming, J.; Salazar, O.; Anugwom, I.; Jogunola, O.; Mäki-Arvela, P.; Willför, S.; Mikkola, J.-P.; et al. Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Res. 2016, 13, 141–147. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of Bioactive Properties in Brown Algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2017, 53, 174–181. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 13193. [Google Scholar] [CrossRef]
| Species | Order | Harvest Season | Harvest Location | Coordinates |
|---|---|---|---|---|
| Macrocystis pyrifera (L.) C. Agardh 1820 | Laminariales | Winter | Lüderitz, Namibia | 26°38′15.8” S 15°06′17.5” E |
| Laminaria ochroleuca Bachelot de la Pylaie 1824 | Laminariales | Summer | Peniche Coast, Peniche, Portugal | 39°21′31.00″ N 9°21′59.05″ W |
| Saccorhiza polyschides (Lightfoot) Batters 1902 | Tilopteridales | Summer | Casais do Baleal, Peniche, Portugal | 39°22′29.61” N 9°19′36.00” W |
| L. ochroleuca | S. polyschides | M. pyrifera | Residual Pulp | |
|---|---|---|---|---|
| Moisture (% fw) | 73.35 ± 1.36 b | 79.74 ± 0.50 a | - | - |
| Ash (% dw) | 22.03 ± 0.60 c | 20.01 ± 0.05 d | 48.94 ± 0.70 a | 28.56 ± 0.25 b |
| Proteins (% dw) | 8.93 ± 0.16 | 8.97 ± 0.27 | 9.20 ± 0.04 | 9.08 ± 0.02 |
| Lipids (% dw) | 2.79 ± 0.25 a, b | 3.41 ± 0.46 a | 1.28 ± 0.19 c | 2.37 ± 0.21 b |
| Carbohydrates 1 (% dw) | 56.03 ± 1.33 a | 55.42 ± 0.30 a | 29.17 ± 0.71 c | 46.41 ± 0.28 b |
| L. ochroleuca | S. polyschides | M. pyrifera | Residual Pulp | |
|---|---|---|---|---|
| SFA (% Total FA) | ||||
| C14:0 | 6.53 ± 0.56 c | 5.31 ± 0.50 c | 13.29 ± 0.66 a | 9.71 ± 0.82 b |
| C15:0 | 0.73 ± 0.05 a | 0.49 ± 0.02 b | 0.42 ± 0.02 b | 0.43 ± 0.03 b |
| C16:0 | 33.9 ± 0.40 b | 40.25 ± 1.37 a | 21.50 ± 0.34 c | 18.54 ± 0.47 d |
| C17:0 | 0.16 ± 0.01 b | 0.26 ± 0.00 a | 0.19 ± 0.04 a, b | 0.19 ± 0.04 a, b |
| C18:0 | 1.43 ± 0.04 b | 3.07 ± 0.12 a | 1.70 ± 0.78 b | 1.46 ± 0.53 b |
| C20:0 | 0.32 ± 0.01 b | 1.97 ± 0.22 a | 0.27 ± 0.03 b | 0.32 ± 0.03 b |
| ∑ Total SFA | 43.45 ± 0.59 | 51.57 ± 1.57 | 37.66 ± 1.52 | 30.91 ± 0.79 |
| MUFA (% Total FA) | ||||
| C16:1 n-7 | 3.58 ± 0.03 a | 1.49 ± 0.06 d | 1.82 ± 0.06 b | 1.65 ± 0.06 c |
| C18:1 n-9 | 17.74 ± 2.09 a | 14.81 ± 0.33 b | 12.11 ± 0.55 b | 8.64 ± 0.11 c |
| C20:1 n-9 | 6.23 ± 0.06 c | 3.13 ± 0.04 d | 9.07 ± 0.15 b | 15.56 ± 0.10 a |
| ∑ Total MUFA | 30.79 ± 0.97 | 23.35 ± 0.35 | 28.14 ± 0.94 | 31.07 ± 0.30 |
| PUFA (% Total FA) | ||||
| C18:2 n-6 cis | 6.27 ± 0.12 b | 9.41 ± 0.24 a | 5.59 ± 0.06 c | 5.53 ± 0.05 c |
| C18:3 n-3 (ALA) | 3.38 ± 0.10 c | 2.54 ± 0.06 d | 5.68 ± 0.04 b | 8.54 ± 0.06 a |
| C20:4 n-6 (ARA) | 8.64 ± 0.17 c | 9.24 ± 0.72 c | 14.55 ± 0.50 a | 10.70 ± 0.52 b |
| C20:5 n-3 (EPA) | 5.90 ± 0.18 c | 2.49 ± 0.15 d | 6.80 ± 0.16 b | 11.14 ± 0.50 a |
| C22:6 n-3 (DHA) | n.d. | 0.02 ± 0.01 a | 0.14 ± 0.02 a | 0.11 ± 0.02 a |
| ∑ Other PUFA | 0.27 ± 0.03 | 0.22 ± 0.01 | 0.59 ± 0.07 | 0.92 ± 0.08 |
| ∑ Total PUFA | 25.77 ± 0.60 | 25.08 ± 1.24 | 34.20 ± 0.68 | 38.01 ± 1.05 |
| n-3/n-6 | 1.75 ± 0.02 | 3.92 ± 0.03 | 1.66 ± 0.02 | 0.87 ± 0.01 |
| L. ochroleuca | S. polyschides | M. pyrifera | Residual Pulp | |
|---|---|---|---|---|
| Fresh Samples (μg·g−1 fw) | ||||
| Fucoxanthin | 439.93 ± 30.32 b | 695.48 ± 22.51 a | - | - |
| Chlorophyll a | 2599.38 ± 701.51 b | 4218.74 ± 93.53 a | - | - |
| Chlorophyll c | 681.34 ± 159.54 b | 965.62 ± 127.39 a | - | - |
| Dried Samples (μg·g−1 dw) | ||||
| Fucoxanthin | 142.12 ± 3.31 b | 111.34 ± 4.99 c | 56.71 ± 1.38 d | 157.52 ± 2.71 a |
| Chlorophyll a | 26.59 ± 1.93 a | 22.18 ± 2.47 a, b | 19.27 ± 3.17 b | 16.63 ± 2.33 b |
| Chlorophyll c | 2.95 ± 0.09 a | 2.66 ± 0.32 a | 0.69 ± 0.07 b | 0.15 ± 0.08 c |
| L. ochroleuca | S. polyschides | M. pyrifera | Residual Pulp | |
|---|---|---|---|---|
| Macroelements (mg·g −1) | ||||
| Phosphorus (P) | 1.18 ± 0.05 b, c | 0.83 ± 0.02 c | 5.29 ± 0.03 a | 4.05 ± 0.26 a, b |
| Sulphur (S) | 8.40 ± 0.18 a | 7.50 ± 0.15 a, b | 0.59 ± 0.08 c | 2.55 ± 0,13 b, c |
| Chlorine (Cl) | 24.84 ± 0.86 b | 22.67 ± 1.95 b | 77.97 ± 0.65 a | 39.84 ± 0.62 a, b |
| Potassium (K) | 22.34 ± 0.35 b, c | 6.03 ± 0.44 c | 67.72 ± 0.62 a | 56.07 ± 0.70 a, b |
| Calcium (Ca) | 7.80 ± 0.32 a, b | 11.72 ± 0.74 a | 3.61 ± 0.05 c | 7.18 ± 0.11 b, c |
| Microelements (μg·g−1) | ||||
| Iron (Fe) | 72.67 ± 15.1 c | 137.67 ± 10.50 b, c | 646.33 ± 143.79 a | 229.00 ± 6.56 a, b |
| Zinc (Zn) | 15.67 ± 1.15 c | 33.33 ± 2.52 a | 24.67 ± 0.58 a, b | 22.00 ± 1.00 b, c |
| Copper (Cu) | 5.00 ± 0,00 b | 3.67 ± 0.58 b, c | 8.00 ± 1.00 a | 3.67 ± 0.58 c |
| Arsenic (As) | 40.00 ± 1.73 a, b | 12.67 ± 1.53 c | 55.67 ± 4.16 a | 26.00 ± 1.00 b, c |
| L. ochroleuca | S. polyschides | M. pyrifera | Residual Pulp | |
|---|---|---|---|---|
| 25 °C | 7.33 ± 0.29 d | 22.67 ± 0.74 b | 13.34 ± 0.66 c | 41.37 ± 0.50 a |
| 60 °C | 13.61 ± 0.71 d | 33.32 ± 0.73 b | 28.32 ± 1.05 c | 51.04 ± 0.75 a |
| 80 °C | 29.91 ± 0.75 c | 46.71 ± 1.02 a | 31.63 ± 0.93 c | 38.85 ± 0.43 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, H.S.; Mouga, T.; Lourenço, S.; Matias, M.H.; Freitas, M.V.; Afonso, C.N. Assessing High-Value Bioproducts from Seaweed Biomass: A Comparative Study of Wild, Cultivated and Residual Pulp Sources. Appl. Sci. 2025, 15, 5745. https://doi.org/10.3390/app15105745
Ferreira HS, Mouga T, Lourenço S, Matias MH, Freitas MV, Afonso CN. Assessing High-Value Bioproducts from Seaweed Biomass: A Comparative Study of Wild, Cultivated and Residual Pulp Sources. Applied Sciences. 2025; 15(10):5745. https://doi.org/10.3390/app15105745
Chicago/Turabian StyleFerreira, Henrique S., Teresa Mouga, Sílvia Lourenço, Margarida H. Matias, Marta V. Freitas, and Clélia Neves Afonso. 2025. "Assessing High-Value Bioproducts from Seaweed Biomass: A Comparative Study of Wild, Cultivated and Residual Pulp Sources" Applied Sciences 15, no. 10: 5745. https://doi.org/10.3390/app15105745
APA StyleFerreira, H. S., Mouga, T., Lourenço, S., Matias, M. H., Freitas, M. V., & Afonso, C. N. (2025). Assessing High-Value Bioproducts from Seaweed Biomass: A Comparative Study of Wild, Cultivated and Residual Pulp Sources. Applied Sciences, 15(10), 5745. https://doi.org/10.3390/app15105745










