Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Particle Size Composition
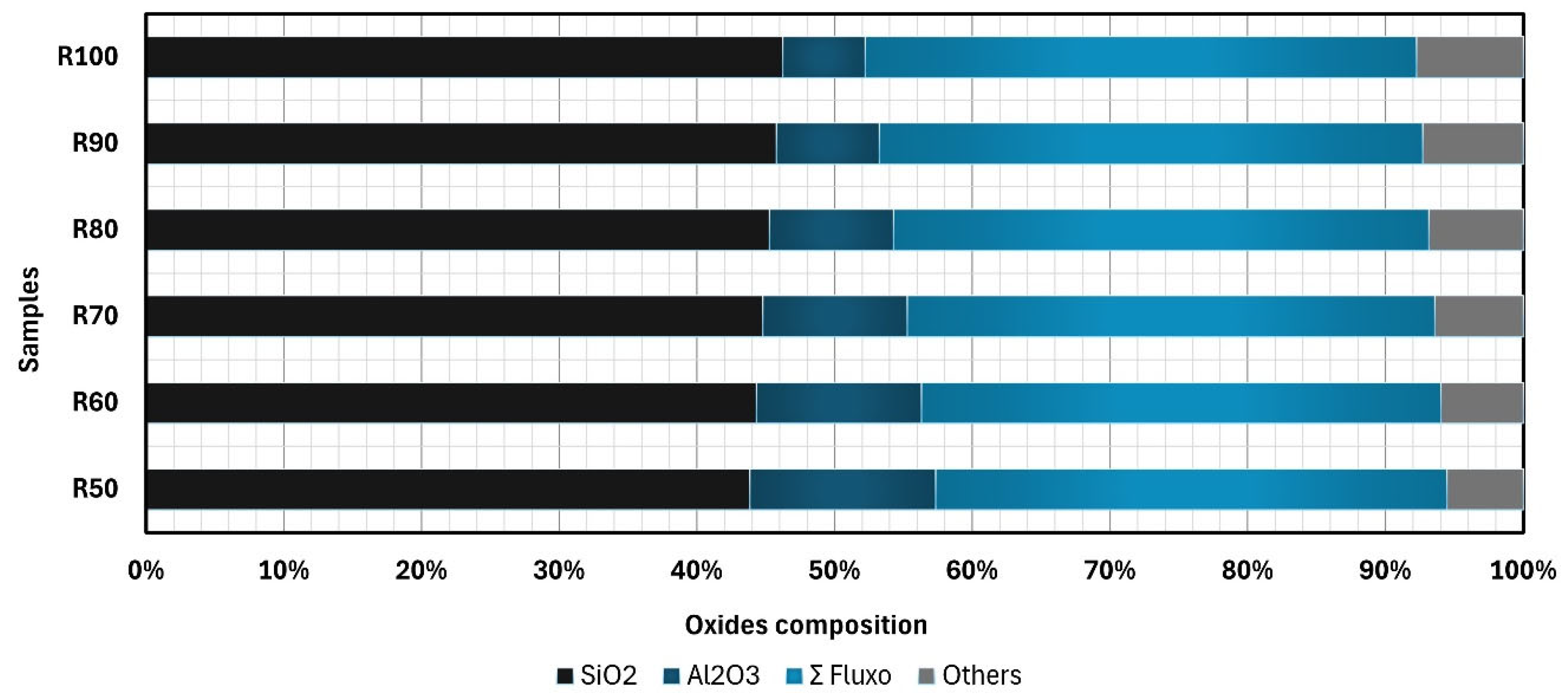
2.1.2. Chemical Composition
2.1.3. Mineralogical Composition of Raw Materials
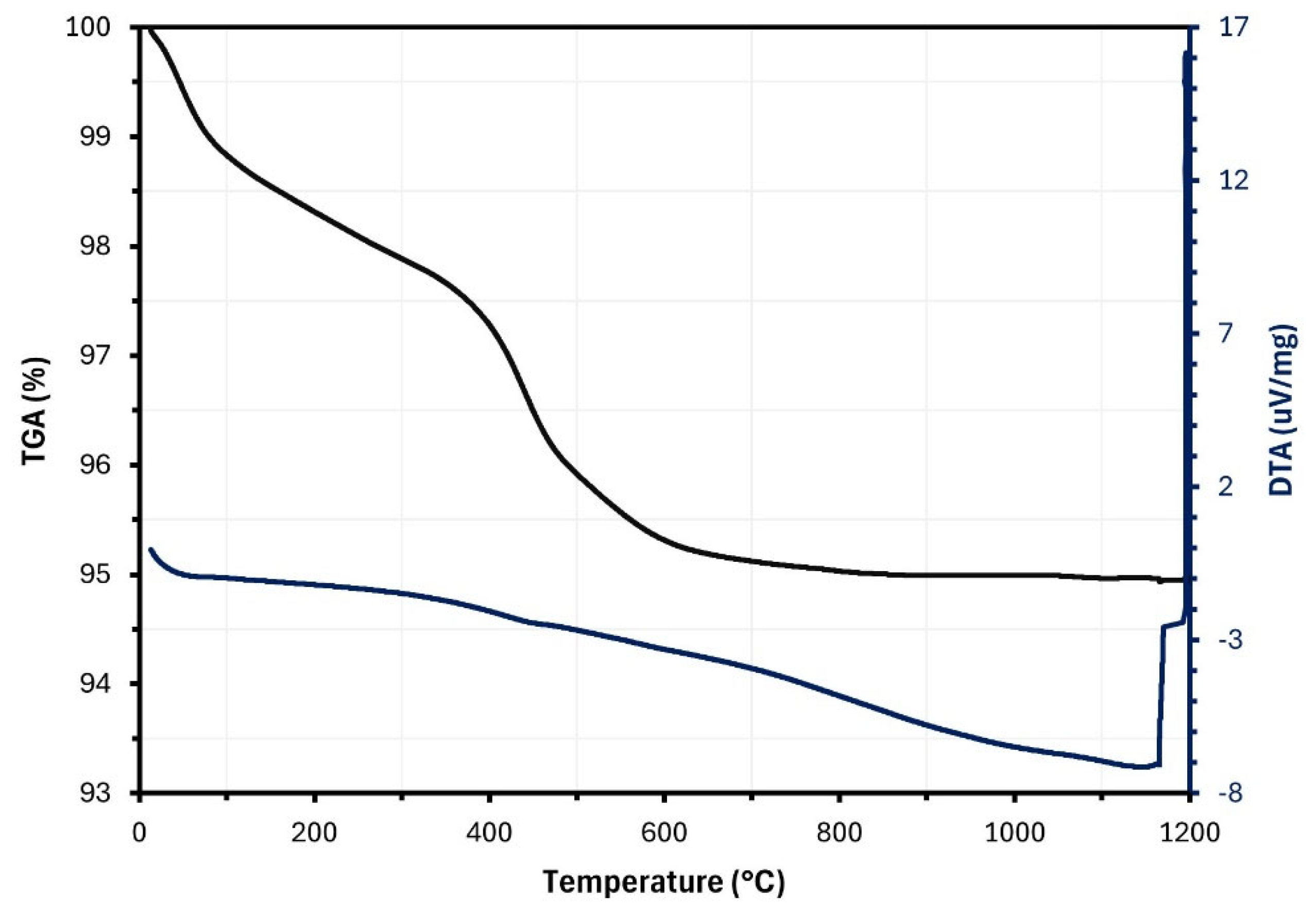
2.1.4. Loss of Mass
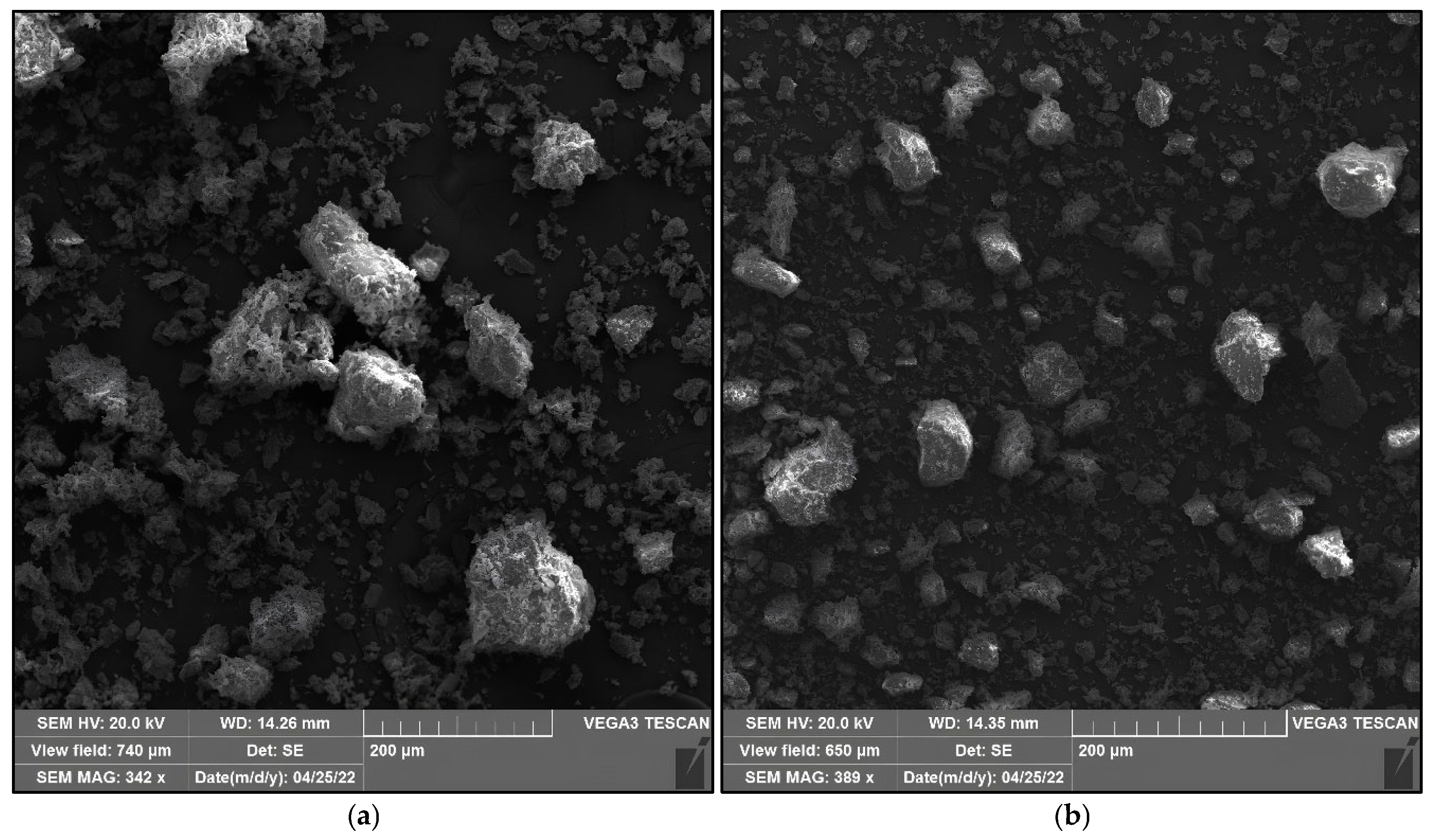
2.1.5. Microstructure of Raw Materials
2.2. Methods
2.2.1. Making the Mixtures
2.2.2. Manufacture of Aggregates
2.2.3. Characterisation of Lightweight Aggregates
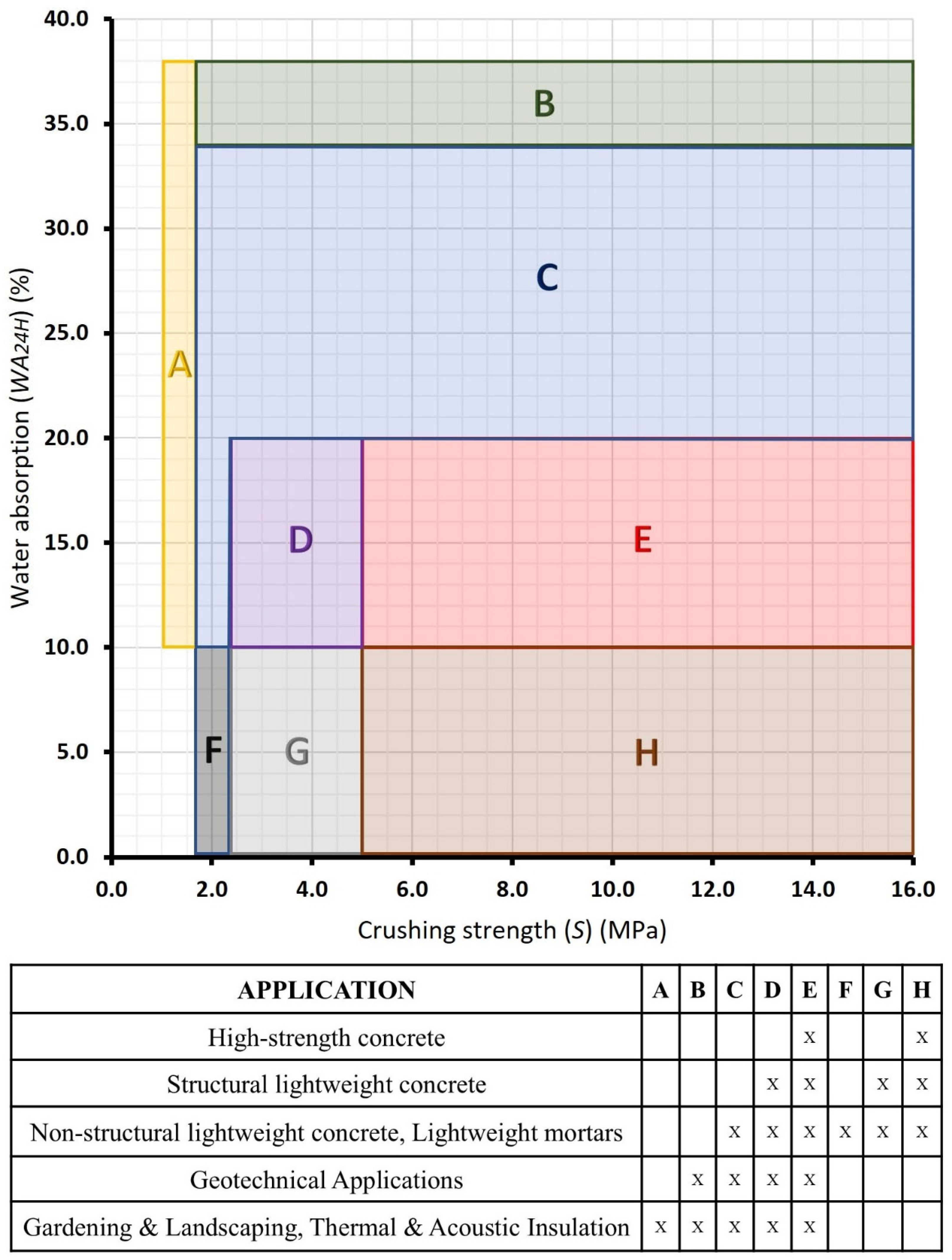
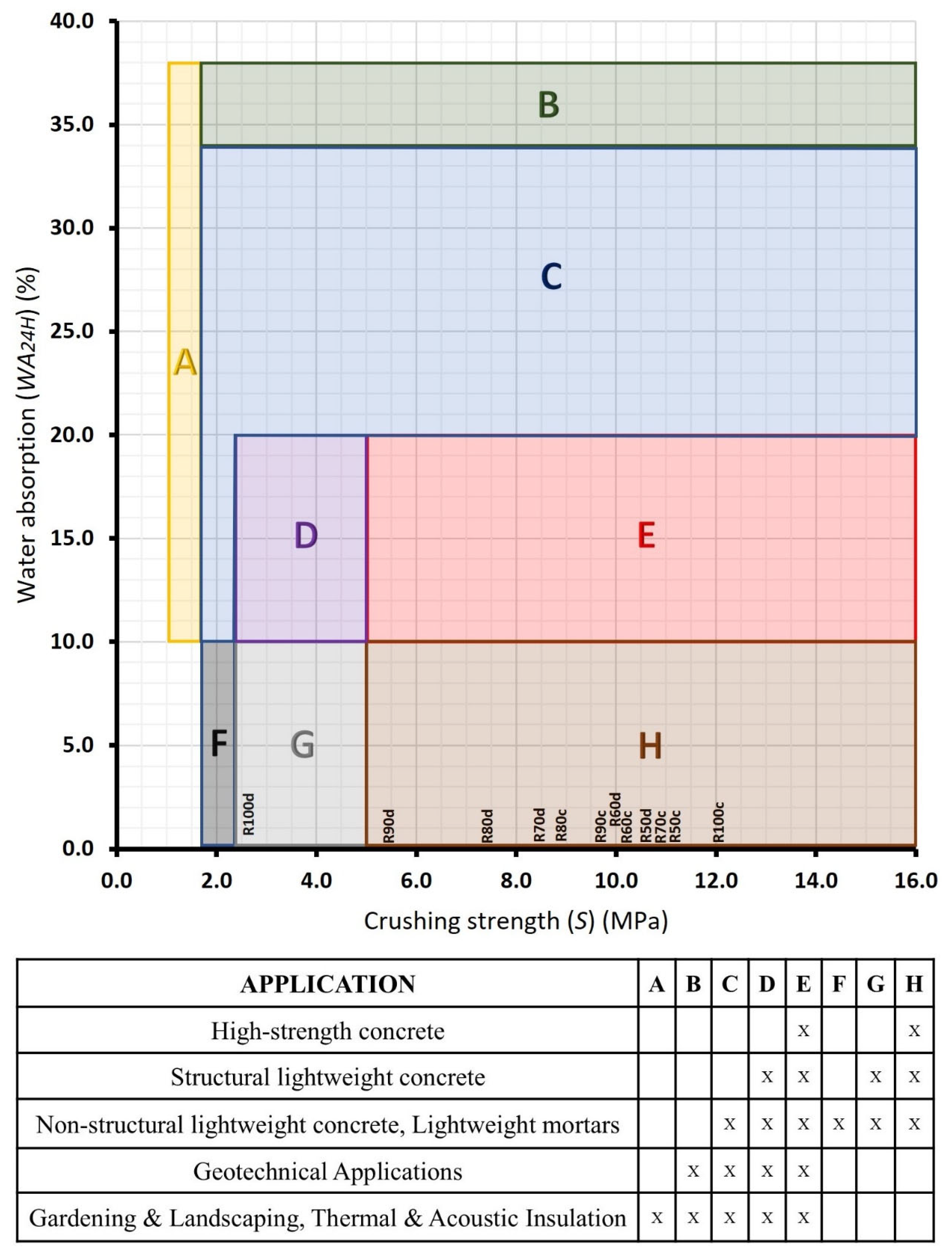
2.2.4. Sample Classification
3. Results and Discussion
3.1. Characterisation of Lightweight Aggregates
3.1.1. Bloating Index (BI)
3.1.2. Loss on Ignition (LOI)
3.1.3. Particle Density (ρd)
3.1.4. Closed Porosity (Pc)
3.1.5. Water Absorption (WA24H)
3.1.6. Crushing Strength (S)
3.1.7. Mineralogical Composition of LWA
3.1.8. Thermal Analysis
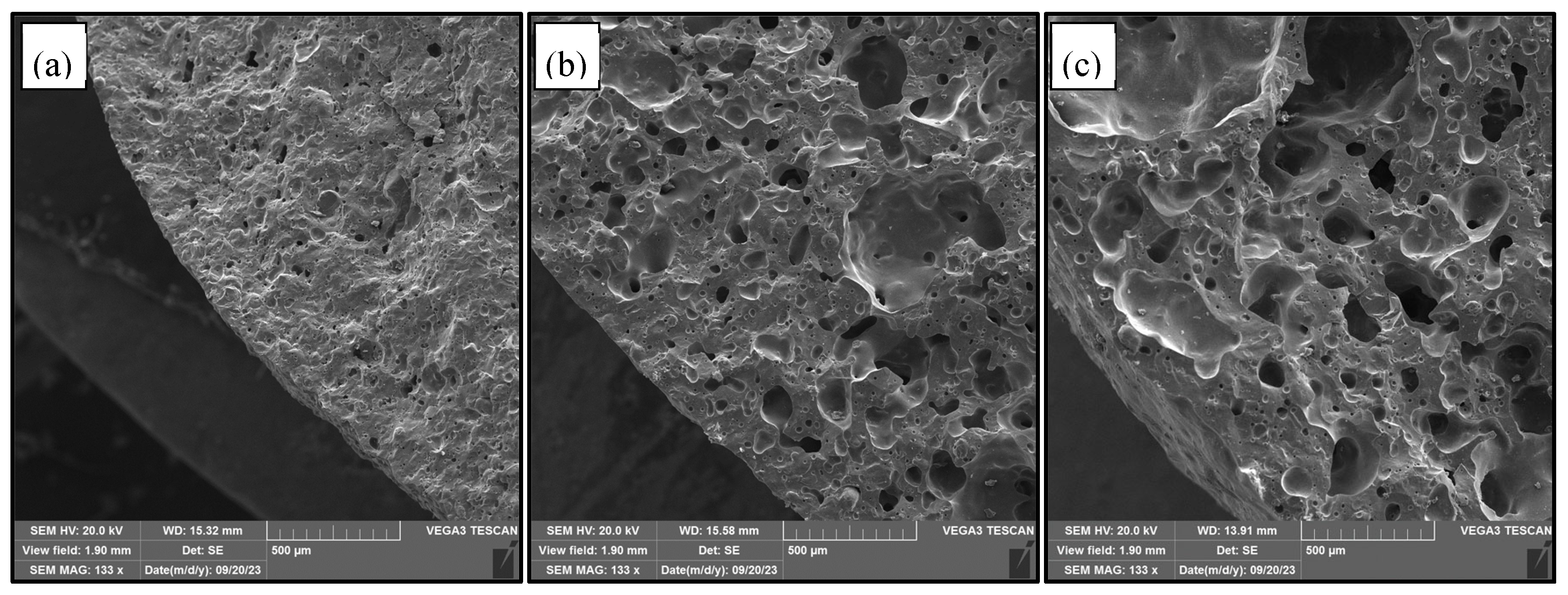
3.1.9. Microstructure of LWA
3.2. Commercial Potential of the Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Real Density (Dt) and Density of Dry Samples Excluding Permeable Pores (ρs) of the Mixtures
| Samples | Real Density (g/cm3) | Dry Density (Without Permeable Pores) (g/cm3) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 2.66 | 2.64 | 2.61 | 2.60 | 2.43 | 2.47 | 1.79 | 1.46 |
| R90 | 2.65 | 2.65 | 2.61 | 2.61 | 2.41 | 2.44 | 1.79 | 1.62 |
| R80 | 2.65 | 2.65 | 2.61 | 2.61 | 2.40 | 2.40 | 1.65 | 1.64 |
| R70 | 2.64 | 2.65 | 2.62 | 2.61 | 2.45 | 2.39 | 1.68 | 1.66 |
| R60 | 2.63 | 2.65 | 2.62 | 2.61 | 2.48 | 2.34 | 1.69 | 1.64 |
| R50 | 2.62 | 2.65 | 2.62 | 2.61 | 2.49 | 2.24 | 1.68 | 1.65 |
Appendix B. Total Porosity (PT) and Open Porosity (Po) of the Mixtures
| Samples | Total Porosity (%) | Open Porosity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 21.36 | 8.08 | 32.32 | 45.42 | 14.02 | 1.76 | 1.10 | 2.39 |
| R90 | 17.57 | 8.91 | 32.04 | 38.41 | 9.20 | 1.10 | 0.72 | 1.10 |
| R80 | 16.01 | 9.89 | 37.36 | 37.44 | 7.52 | 0.60 | 0.96 | 0.89 |
| R70 | 12.34 | 10.19 | 36.51 | 36.80 | 5.64 | 0.40 | 0.86 | 1.03 |
| R60 | 9.41 | 11.83 | 35.90 | 37.63 | 3.83 | 0.34 | 0.64 | 0.73 |
| R50 | 6.32 | 15.49 | 36.12 | 37.29 | 1.49 | 0.18 | 0.67 | 0.73 |
Appendix C. Chemical Composition of Mixtures
Appendix D. Comparative Table of Experimental Aggregates and a Commercial LWA Commonly Used in Brazil
| Samples | S (MPa) | WA24H (%) | ρd (g/cm3) |
|---|---|---|---|
| R100c | 12.01 | 0.62 | 1.77 |
| R90c | 9.68 | 0.40 | 1.78 |
| R80c | 8.81 | 0.58 | 1.64 |
| R70c | 10.77 | 0.52 | 1.66 |
| R60c | 9.76 | 0.38 | 1.68 |
| R50c | 11.06 | 0.40 | 1.67 |
| R100d | 2.64 | 1.68 | 1.42 |
| R90d | 5.41 | 0.68 | 1.60 |
| R80d | 7.30 | 0.55 | 1.63 |
| R70d | 8.54 | 0.63 | 1.65 |
| R60d | 9.68 | 0.45 | 1.63 |
| R50d | 10.59 | 0.45 | 1.64 |
| LWAB | 2.09 | 9.44 | 1.09 |
References
- Ren, Y.; Ren, Q.; Huo, Z.; Wu, X.; Zheng, J.; Hai, O. Preparation of Glass Shell Fly Ash-Clay Based Lightweight Aggregate with Low Water Absorption by Using Sodium Carbonate Solution as Binder. Mater. Chem. Phys. 2020, 256, 123606. [Google Scholar] [CrossRef]
- Jin, H.; Cheng, L.; Liu, J.; Chen, C.; Xing, F. Converting Municipal Solid Waste Incineration Bottom Ash into the Value-Added Artificial Lightweight Aggregates through Cold-Bonded Granulation Technology. Constr. Build. Mater. 2024, 438, 136930. [Google Scholar] [CrossRef]
- NBR 7211; Aggregates for Concrete—Specification. ABNT: Rio de Janeiro, Brazil, 2009.
- Chandra, S.; Berntsson, L. Lightweight Aggregate Concrete; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 081551820X. [Google Scholar]
- Rossignolo, J.A. Structural Lightweight Concrete: Production, Properties, Microstructure and Applications; PINI: São Paulo, Brazil, 2009; ISBN 9788572662208. [Google Scholar]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Production of Lightweight Aggregates from Mining and Industrial Wastes. J. Environ. Manag. 2009, 90, 2801–2812. [Google Scholar] [CrossRef]
- Lau, P.C.; Teo, D.C.L.; Mannan, M.A. Mechanical, Durability and Microstructure Properties of Lightweight Concrete Using Aggregate Made from Lime-Treated Sewage Sludge and Palm Oil Fuel Ash. Constr. Build. Mater. 2018, 176, 24–34. [Google Scholar] [CrossRef]
- de Souza, M.M. Development of Lightweight Aggregates from Scheelite Waste, Sewage Sludge and Rice Husk Ash. Master’s Thesis, Federal University of Rio Grande do Norte, Natal, Brazil, 2019. [Google Scholar]
- Chung, S.-Y.; Sikora, P.; Kim, D.J.; El Madawy, M.E.; Abd Elrahman, M. Effect of Different Expanded Aggregates on Durability-Related Characteristics of Lightweight Aggregate Concrete. Mater. Charact. 2021, 173, 110907. [Google Scholar] [CrossRef]
- Bernhardt, M.; Tellesbø, H.; Justnes, H.; Wiik, K. Mechanical Properties of Lightweight Aggregates. J. Eur. Ceram. Soc. 2013, 33, 2731–2743. [Google Scholar] [CrossRef]
- Ayati, B.; Ferrándiz-Mas, V.; Newport, D.; Cheeseman, C. Use of Clay in the Manufacture of Lightweight Aggregate. Constr. Build. Mater. 2018, 162, 124–131. [Google Scholar] [CrossRef]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight Aggregates from Waste Materials: Reappraisal of Expansion Behavior and Prediction Schemes for Bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- Quina, M.J.; Almeida, M.A.; Santos, R.; Bordado, J.M.; Quinta-Ferreira, R.M. Compatibility Analysis of Municipal Solid Waste Incineration Residues and Clay for Producing Lightweight Aggregates. Appl. Clay Sci. 2014, 102, 71–80. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K. Partial Replacement of Metakaolin with Red Ceramic Waste in Geopolymer. Ceram. Int. 2021, 47, 3473–3483. [Google Scholar] [CrossRef]
- Pitarch, A.M.; Reig, L.; Tomás, A.E.; Forcada, G.; Soriano, L.; Borrachero, M.V.; Payá, J.; Monzó, J.M. Pozzolanic Activity of Tiles, Bricks and Ceramic Sanitary-Ware in Eco-Friendly Portland Blended Cements. J. Clean. Prod. 2021, 279, 123713. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. The Potential of Raw Sewage Sludge in Construction Industry—A Review. J. Clean. Prod. 2018, 200, 342–356. [Google Scholar] [CrossRef]
- Aguiar, J.G.C.; de Souza, M.M.; de Farias, E.C. Development of Lightweight Aggregates from Coffee Grounds and Rice Husk Ash. Matéria 2024, 29, e20230313. [Google Scholar] [CrossRef]
- Souza, M.M.; Barbosa, N.P.; Anjos, M.A.S. Characteristics and Utilization Prospects of Red Ceramic Waste in Lightweight Aggregates: A Systematic Review. Cerâmica 2022, 68, 402–408. [Google Scholar] [CrossRef]
- Ray, S.; Haque, M.; Sakib, M.N.; Mita, A.F.; Rahman, M.D.M.; Tanmoy, B.B. Use of Ceramic Wastes as Aggregates in Concrete Production: A Review. J. Build. Eng. 2021, 43, 102567. [Google Scholar] [CrossRef]
- Linard, Z.Ú.S.d.A.; Khan, A.S.; Lima, P.V.P.-S. Perceptions of Environmental Impacts of Ceramic Industry in the Municipality of State Crato Ceará, Brazil. Econ. Soc. Territ. 2015, 15, 397–423. [Google Scholar]
- Geraldo, R.H.; Souza, J.D.; Campos, S.C.; Fernandes, L.F.R.; Camarini, G. Pressured Recycled Gypsum Plaster and Wastes: Characteristics of Eco-Friendly Building Components. Constr. Build. Mater. 2018, 191, 136–144. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Yehualaw, M.D.; Vo, D.-H.; Huynh, T.-P.; Largo, A. Performance Evaluation of Alkali Activated Mortar Containing High Volume of Waste Brick Powder Blended with Ground Granulated Blast Furnace Slag Cured at Ambient Temperature. Constr. Build. Mater. 2019, 223, 657–667. [Google Scholar] [CrossRef]
- Fiala, L.; Konrád, P.; Fořt, J.; Keppert, M.; Černý, R. Application of Ceramic Waste in Brick Blocks with Enhanced Acoustic Properties. J. Clean. Prod. 2020, 261, 121185. [Google Scholar] [CrossRef]
- Carrillo-Beltran, R.; Corpas-Iglesias, F.A.; Terrones-Saeta, J.M.; Bertoya-Sol, M. New Geopolymers from Industrial By-Products: Olive Biomass Fly Ash and Chamotte as Raw Materials. Constr. Build. Mater. 2021, 272, 121924. [Google Scholar] [CrossRef]
- Mendes, J.P.; Elyseu, F.; Nieves, L.J.J.; Zaccaron, A.; Bernardin, A.M.; Angioletto, E. Synthesis and Characterization of Geopolymers Using Clay Ceramic Waste as Source of Aluminosilicate. Sustain. Mater. Technol. 2021, 28, e00264. [Google Scholar] [CrossRef]
- Shao, J.; Gao, J.; Zhao, Y.; Chen, X. Study on the Pozzolanic Reaction of Clay Brick Powder in Blended Cement Pastes. Constr. Build. Mater. 2019, 213, 209–215. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; Mejía de Gutiérrez, R. Alternative Cements Based on Alkali-Activated Red Clay Brick Waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Santos, F.D.; da Conceição, L.R.V.; Ceron, A.; de Castro, H.F. Chamotte Clay as Potential Low Cost Adsorbent to Be Used in the Palm Kernel Biodiesel Purification. Appl. Clay Sci. 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Akinmusuru, J.O.; Dawson, A.R.; Ndambuki, J.M.; Thom, N.H. Microstructural Characteristics, Porosity and Strength Development in Ceramic-Laterized Concrete. Cem. Concr. Compos. 2018, 86, 224–237. [Google Scholar] [CrossRef]
- Cinexpan, E.C. Technical Data Sheet Type 1506. Available online: https://www.cinexpan.com.br/pdf/ficha-tecnica-tipo-1506.pdf (accessed on 24 June 2024).
- De Carvalho, O.O.; Leite, J.Y.P. Analysing the Production Process of Gato-Itajá/RN Ceramics. In Proceedings of the 43rd Brazilian Ceramics Congress, Florianópolis, Brazil, 2–5 June 1999; pp. 10201–10212. [Google Scholar]
- Souza, M.M.; Anjos, M.A.S.; Sá, M.V.V.A.; Souza, N.S.L. Developing and Classifying Lightweight Aggregates from Sewage Sludge and Rice Husk Ash. Case Stud. Constr. Mater. 2020, 12, e00340. [Google Scholar] [CrossRef]
- Cougny, G. Spécifications Sur Les Matières Premières Argileuses Pour La Fabrication de Granulats Légers Expanses. Bull. Int. Assoc. Eng. Geol. 1990, 41, 47–55. [Google Scholar] [CrossRef]
- Mun, K.J. Development and Tests of Lightweight Aggregate Using Sewage Sludge for Nonstructural Concrete. Constr. Build. Mater. 2007, 21, 1583–1588. [Google Scholar] [CrossRef]
- Dutra, R.P.S.; Varela, M.L.; do Nascimento, R.M.; Gomes, U.U.; Paskocimas, C.A.; de Melo, P.T. Evaluation of the Potential of Clays of Rio Grande Do Norte-Brazil. Ind. Ceram. 2006, 11, 42–46. [Google Scholar]
- Jerônimo, V.L.; Meira, G.R.; da Silva Filho, L.C.P. Performance of Self-Compacting Concretes with Wastes from Heavy Ceramic Industry against Corrosion by Chlorides. Constr. Build. Mater. 2018, 169, 900–910. [Google Scholar] [CrossRef]
- Martín, D.; Aparicio, P.; Galán, E. Mineral Carbonation of Ceramic Brick at Low Pressure and Room Temperature. A Simulation Study for a Superficial CO2 Store Using a Common Clay as Sealing Material. Appl. Clay Sci. 2018, 161, 119–126. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wang, W.; Guo, J.; Zhang, L.; Zhang, H. Effect of SiO2 and Al2O3 on Characteristics of Lightweight Aggregate Made from Sewage Sludge and River Sediment. Ceram. Int. 2018, 44, 4313–4319. [Google Scholar] [CrossRef]
- Lau, P.C.; Teo, D.C.L.; Mannan, M.A. Characteristics of Lightweight Aggregate Produced from Lime-Treated Sewage Sludge and Palm Oil Fuel Ash. Constr. Build. Mater. 2017, 152, 558–567. [Google Scholar] [CrossRef]
- da Silva Neto, J.A.; dos Anjos, M.A.S.; Dutra, R.P.S.; Mendonça de Souza, M.; Pederneiras, C.M. Development of Sustainable Artificial Lightweight Aggregates with Binary Mixtures of Waste Rich in Aluminosilicate and Carbonate in Kaolinitic Clay. Sustainability 2025, 17, 2017. [Google Scholar] [CrossRef]
- Tremiño, R.M.; Real-Herraiz, T.; Letelier, V.; Ortega, J.M. Four-Years Influence of Waste Brick Powder Addition in the Pore Structure and Several Durability-Related Parameters of Cement-Based Mortars. Constr. Build. Mater. 2021, 306, 124839. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Z.; Yang, H.; Ji, Y.; Yan, Z. Enhancement of Triisopropanolamine on the Compressive Strength Development of Cement Paste Incorporated with High Content of Wasted Clay Brick Powder and Its Working Mechanism. Constr. Build. Mater. 2021, 302, 124052. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Burns, S.; Simmons, D.; Francis, E.; Chai, H.K.; Koutsos, V.; Huang, Y. Chemical, Microstructural and Mechanical Properties of Ceramic Waste Blended Cementitious Systems. J. Clean. Prod. 2019, 211, 1228–1238. [Google Scholar] [CrossRef]
- RILEY, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Dang, J.; Zhao, J.; Hu, W.; Du, Z.; Gao, D. Properties of Mortar with Waste Clay Bricks as Fine Aggregate. Constr. Build. Mater. 2018, 166, 898–907. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage Sludge Ash Characteristics and Potential for Use in Concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef]
- Singh, N.; Raza, J.; Colangelo, F.; Farina, I. Advancements in Lightweight Artificial Aggregates: Typologies, Compositions, Applications, and Prospects for the Future. Sustainability 2024, 16, 9329. [Google Scholar] [CrossRef]
- Souza, M.M.; Anjos, M.A.S.; Sá, M.V.V.A. Using Scheelite Residue and Rice Husk Ash to Manufacture Lightweight Aggregates. Constr. Build. Mater. 2021, 270, 121845. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Virdi, G.S. Properties and Microstructure of Lightweight Aggregate Produced from Sintered Sewage Sludge Ash. Resour. Conserv. Recycl. 2005, 45, 18–30. [Google Scholar] [CrossRef]
- NBR 16917; Coarse Aggregate—Determination of Density and Water Absorption. ABNT: Rio de Janeiro, Brazil, 2021.
- ME 093; Soils—Determination of Real Density. DNER: Rio de Janeiro, Brazil, 1994.
- Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Uceda-Rodríguez, M.; Cotes-Palomino, T.; Martínez García, C.; Alonso-Azcárate, J. Unraveling the Expansion Mechanism in Lightweight Aggregates: Demonstrating That Bloating Barely Requires Gas. Constr. Build. Mater. 2020, 247, 118583. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, S.-Y. Application of Water Treatment Sludge in the Manufacturing of Lightweight Aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- Soltan, A.M.M.; Kahl, W.-A.; Abd EL-Raoof, F.; Abdel-Hamid El-Kaliouby, B.; Abdel-Kader Serry, M.; Abdel-Kader, N.A. Lightweight Aggregates from Mixtures of Granite Wastes with Clay. J. Clean. Prod. 2016, 117, 139–149. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M.; Barrenechea, J.F.; Luque, F.J. Microstructure and Mineralogy of Lightweight Aggregates Manufactured from Mining and Industrial Wastes. Constr. Build. Mater. 2011, 25, 3591–3602. [Google Scholar] [CrossRef]
- Li, B.; Ling, T.-C.; Qu, L.; Wang, Y. Effects of a Two-Step Heating Process on the Properties of Lightweight Aggregate Prepared with Sewage Sludge and Saline Clay. Constr. Build. Mater. 2016, 114, 119–126. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yu, G.; Liu, J.; Du, Y.; Li, Y.; Li, H. Controlled Synthesis of Zeolite Adsorbent from Low-Grade Diatomite: A Case Study of Self-Assembled Sodalite Microspheres. J. Environ. Sci. 2020, 91, 92–104. [Google Scholar] [CrossRef]
- Quina, M.J.; Almeida, M.A.; Santos, R.C.; Bordado, J.C.; Quinta-Ferreira, R.M. Prediction of Solid Waste Incineration Residues Quantity for Valorization in Lightweight Aggregates. Mater. Sci. Forum 2006, 514–516, 1731–1735. [Google Scholar] [CrossRef]
- Huang, S.-C.; Chang, F.-C.; Lo, S.-L.; Lee, M.-Y.; Wang, C.-F.; Lin, J.-D. Production of Lightweight Aggregates from Mining Residues, Heavy Metal Sludge, and Incinerator Fly Ash. J. Hazard. Mater. 2007, 144, 52–58. [Google Scholar] [CrossRef]
- EN, B. 13055-1; Lightweight Aggregates-Part 1: Lightweight Aggregates for Concrete, Mortar and Grout. British Standards Institution: London, UK, 2002; pp. 1–40.
- Fan, C.-S.; Huang, C.-Y.; Li, K.-C. Bloating Mechanism of the Mixture of Thin-Film Transistor Liquid-Crystal Display Waste Glass and Basic Oxygen Furnace Slag. Constr. Build. Mater. 2014, 66, 664–670. [Google Scholar] [CrossRef]
- Arriagada, C.; Navarrete, I.; Lopez, M. Understanding the Effect of Porosity on the Mechanical and Thermal Performance of Glass Foam Lightweight Aggregates and the Influence of Production Factors. Constr. Build. Mater. 2019, 228, 116746. [Google Scholar] [CrossRef]
- Tuan, B.L.A.; Hwang, C.-L.; Lin, K.-L.; Chen, Y.-Y.; Young, M.-P. Development of Lightweight Aggregate from Sewage Sludge and Waste Glass Powder for Concrete. Constr. Build. Mater. 2013, 47, 334–339. [Google Scholar] [CrossRef]
- Bernhardt, M.; Justnes, H.; Tellesbø, H.; Wiik, K. The Effect of Additives on the Properties of Lightweight Aggregates Produced from Clay. Cem. Concr. Compos. 2014, 53, 233–238. [Google Scholar] [CrossRef]
- Fragoulis, D.; Stamatakis, M.G.; Chaniotakis, E.; Columbus, G. Characterization of Lightweight Aggregates Produced with Clayey Diatomite Rocks Originating from Greece. Mater. Charact. 2004, 53, 307–316. [Google Scholar] [CrossRef]
- Roufael, G.; Beaucour, A.-L.; Eslami, J.; Hoxha, D.; Noumowé, A. Influence of Lightweight Aggregates on the Physical and Mechanical Residual Properties of Concrete Subjected to High Temperatures. Constr. Build. Mater. 2021, 268, 121221. [Google Scholar] [CrossRef]
- Shuguang, H.; Tingting, Y.; Fazhou, W. Influence of Mineralogical Composition on the Properties of Lightweight Aggregate. Cem. Concr. Compos. 2010, 32, 15–18. [Google Scholar] [CrossRef]
- Piszcz-Karaś, K.; Klein, M.; Hupka, J.; Łuczak, J. Utilization of Shale Cuttings in Production of Lightweight Aggregates. J. Environ. Manag. 2019, 231, 232–240. [Google Scholar] [CrossRef]
- Gao, W.; Jian, S.; Li, X.; Tan, H.; Li, B.; Lv, Y.; Huang, J. Efficient Utilization of Multi-Source Solid Waste to Prepare the Novel Core-Shell Structured Lightweight Aggregates and Its Immobilization for Volatile Heavy Metals. J. Build. Eng. 2023, 71, 106549. [Google Scholar] [CrossRef]
- Bernhardt, M.; Tellesbø, H.; Justnes, H.; Wiik, K. Fibre Reinforced Lightweight Aggregates. J. Eur. Ceram. Soc. 2014, 34, 1341–1351. [Google Scholar] [CrossRef]
- Franus, M.; Panek, R.; Madej, J.; Franus, W. The Properties of Fly Ash Derived Lightweight Aggregates Obtained Using Microwave Radiation. Constr. Build. Mater. 2019, 227, 116677. [Google Scholar] [CrossRef]
- Koroneos, C.; Dompros, A. Environmental Assessment of Brick Production in Greece. Build. Environ. 2007, 42, 2114–2123. [Google Scholar] [CrossRef]
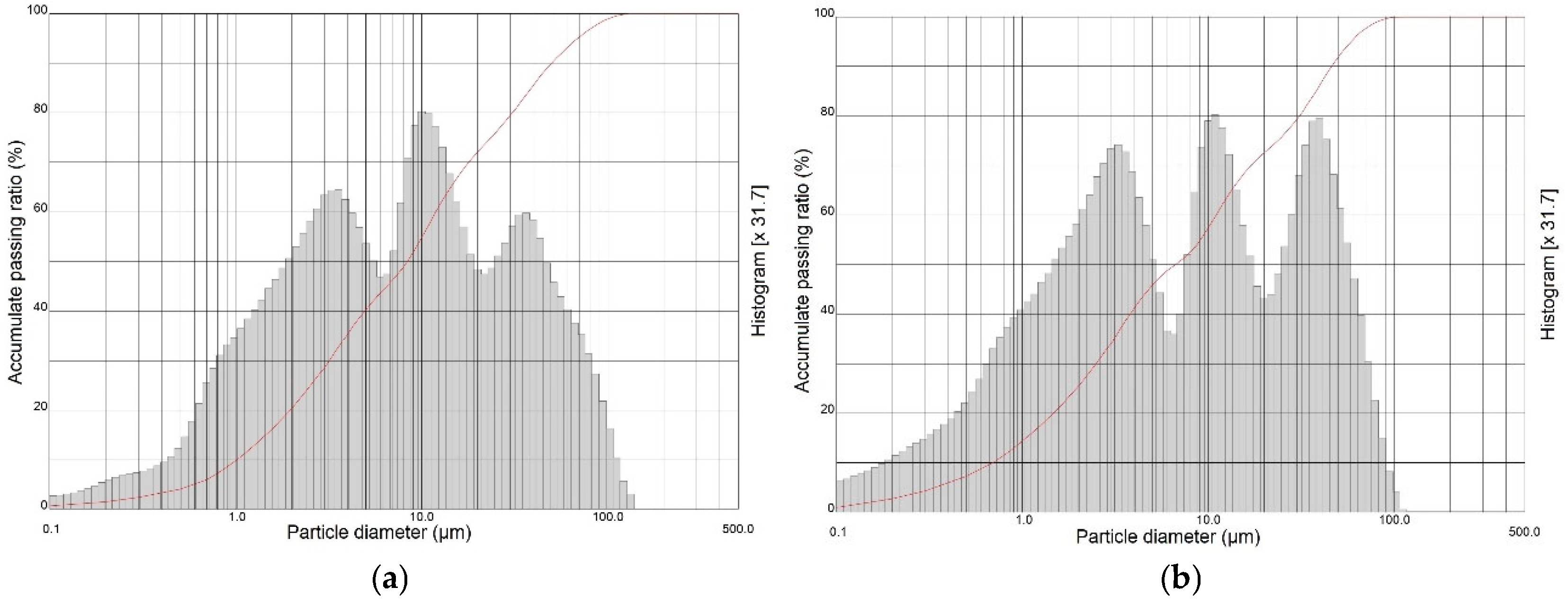
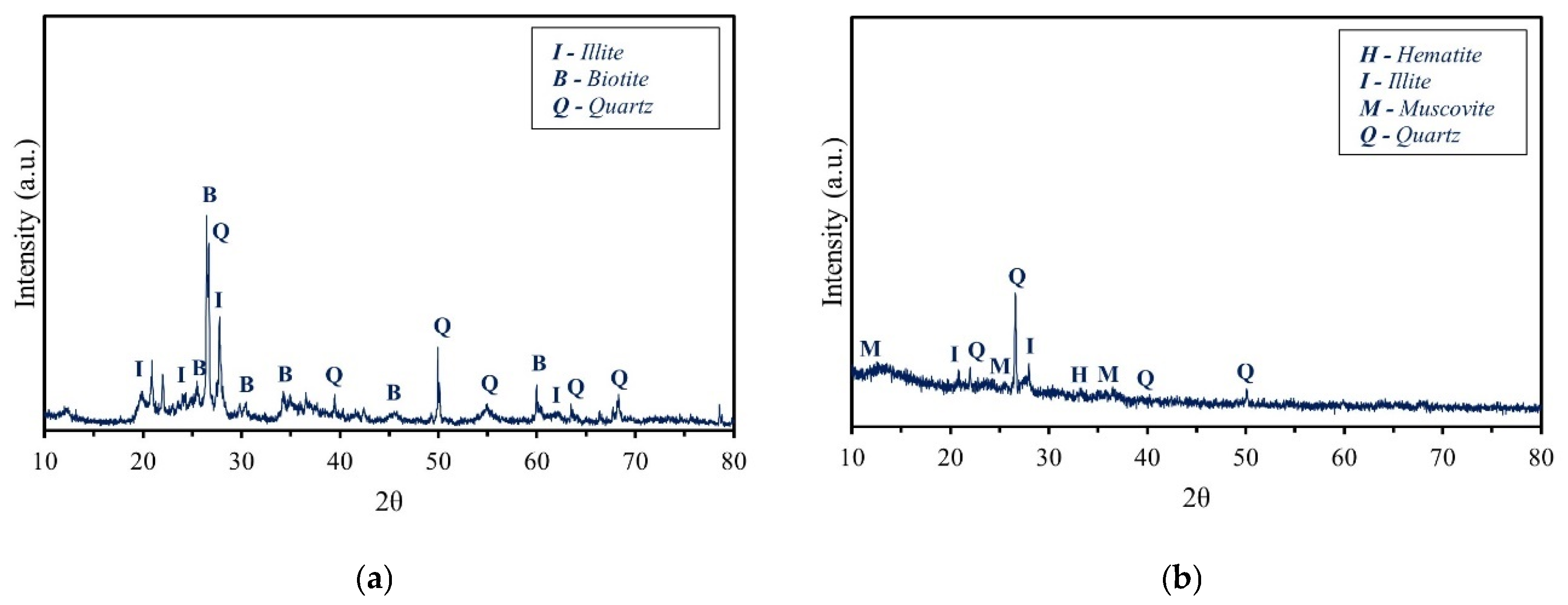
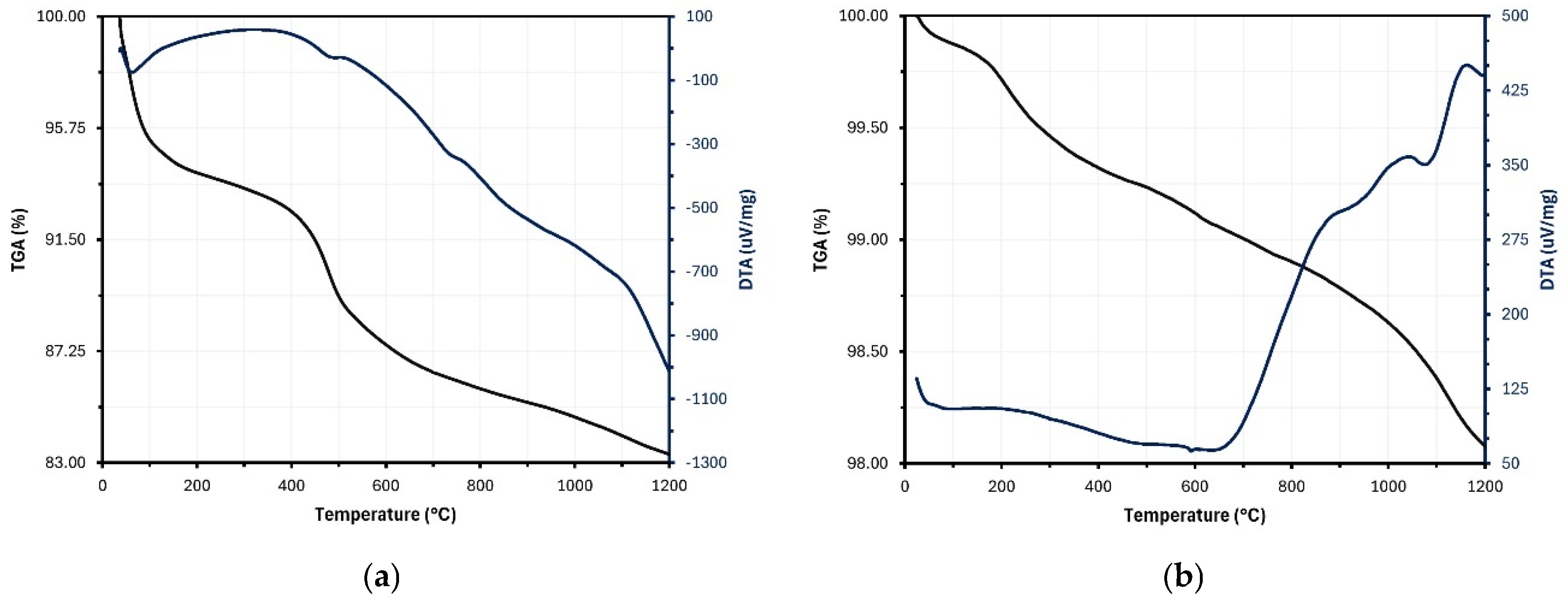



| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Others | LOI (%) |
|---|---|---|---|---|---|---|---|---|---|
| RC | 41.45 | 21.02 | 27.99 | 1.17 | 2.09 | 0.00 | 2.96 | 3.32 | 8.6% |
| RCW | 46.23 | 5.98 | 26.31 | 5.19 | 0.00 | 0.00 | 8.56 | 7.73 | 1.09% |
| Mixtures | R100 | R90 | R80 | R70 | R60 | R50 |
|---|---|---|---|---|---|---|
| RCW | 100.0 | 90.0 | 80.0 | 70.0 | 60.0 | 50.0 |
| RC | 0.0 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 |
| WATER | 40.0 | 35.0 | 31.0 | 30.0 | 30.0 | 30.0 |
| Determination | Method | Equation or Parameters | References |
|---|---|---|---|
| Water absorption (WA24H) | Hydrostatic weighing. Sample mass: dry (mA), saturated surface dry (mB) and submerged in water (mC). | WA24H = 100 (mB − mA)/mA (%) | [50] |
| Particle density (ρd) | ρd = mA/(mB − mC) (g/cm3) | [50] | |
| Density of dry samples, excluding permeable pores (ρs) | ρs = mA/(mA − mC) (g/cm3) | [50] | |
| Real density (Dt) | Water pycnometry. Pycnometer mass: empty (P1), plus sample (P2), plus sample plus water (P3), plus water (P4). | Dt = (P2 − P1)/[(P4 − P1) − (P3 − P2)] (g/cm3) | [51] |
| Total porosity (PT) | Relationships between Particle density (ρd), Density of dry samples excluding permeable pores (ρs) and Actual density (Dt). | PT = 100 [1 − (ρd/Dt)] (%) | [52] |
| Open porosity (PO) | PO = 100 [1 − (ρd/ρs)] (%) | [52] | |
| Closed porosity (PC) | Difference between Total Porosity (PT) and Open Porosity (PO). | PC = PT − PO (%) | [52] |
| Bloating Index (BI) | Variation in the average volume of the particles, from the initial state (V1) to the final state after sintering (V2). | BI = 100 (V2 − V1)/V1 (%) | [53] |
| Microstructure (SEM) | Scanning electron microscopy. Vega-3 LMU TESCAN microscope. | Acceleration voltage of 20 kV. | [54] |
| Mineralogy (XRD) | X-ray diffractometry. Shimadzu XRD-7000 diffractometer. | Cu Kα radiation at 40 Ma; 40 kV; 10 to 80° (2θ); 0.02° pitch and 1.20°/min speed. | [55] |
| Thermal analysis (TG- DTA) | Simultaneous thermogravimetric analysis. Shimadzu DTG-60 analyser. | 30 °C to 1200 °C, rate of 8 °C/min, nitrogen gas, isotherm for 15 min. | [13] |
| Loss on ignition (LOI) | Difference between the mass of the samples before and after sintering. | LOI = 100 (Mi − Mf)/Mi (%) | [56] |
| Crushing resistance (S) | Average of the individual strength of the granules. Where, load at failure (Pc) and particle diameter (x). | S = 2.8Pc/πx2 (MPa) | [57] |
| Samples | Bloating Index (BI) (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | −22.5 | −43.5 | 3.4 | 29.4 |
| R90 | −29.5 | −23.2 | −6.7 | 19.5 |
| R80 | −16.0 | −24.8 | 7.9 | 10.7 |
| R70 | −25.4 | −27.6 | 7.8 | 15.4 |
| R60 | −22.7 | −22.2 | 17.0 | 23.4 |
| R50 | −20.8 | −13.4 | 15.7 | 16.0 |
| Samples | Particle Density (ρd) (g/cm3) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 2.09 | 2.43 | 1.77 | 1.42 |
| R90 | 2.19 | 2.41 | 1.78 | 1.60 |
| R80 | 2.22 | 2.38 | 1.64 | 1.63 |
| R70 | 2.31 | 2.38 | 1.66 | 1.65 |
| R60 | 2.38 | 2.33 | 1.68 | 1.63 |
| R50 | 2.46 | 2.24 | 1.67 | 1.64 |
| Samples | Closed Porosity (Pc) (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 7.3 | 6.3 | 31.2 | 43.0 |
| R90 | 8.4 | 7.8 | 31.3 | 37.3 |
| R80 | 8.5 | 9.3 | 36.4 | 36.6 |
| R70 | 6.7 | 9.8 | 35.6 | 35.8 |
| R60 | 5.6 | 11.5 | 35.3 | 36.9 |
| R50 | 4.8 | 15.3 | 35.5 | 36.6 |
| Samples | Water Absorption (WA24H) (%) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 6.7 | 0.7 | 0.6 | 1.7 |
| R90 | 4.2 | 0.5 | 0.4 | 0.7 |
| R80 | 3.4 | 0.3 | 0.6 | 0.5 |
| R70 | 2.4 | 0.2 | 0.5 | 0.6 |
| R60 | 1.6 | 0.1 | 0.4 | 0.5 |
| R50 | 0.6 | 0.1 | 0.4 | 0.4 |
| Samples | Crushing Strength (S) (MPa) | |||
|---|---|---|---|---|
| 1100 °C | 1150 °C | 1200 °C | 1250 °C | |
| R100 | 10.29 | 29.07 | 12.01 | 2.64 |
| R90 | 17.66 | 31.92 | 9.68 | 5.41 |
| R80 | 19.42 | 31.77 | 8.81 | 7.30 |
| R70 | 25.65 | 40.52 | 10.77 | 8.54 |
| R60 | 32.78 | 36.76 | 9.76 | 9.68 |
| R50 | 31.72 | 32.51 | 11.06 | 10.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça de Souza, M.; Barbosa, N.P.; dos Anjos, M.A.S.; Aguiar, J.G.C.; da Silva Neto, J.A.; Pederneiras, C.M. Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates. Appl. Sci. 2025, 15, 5729. https://doi.org/10.3390/app15105729
Mendonça de Souza M, Barbosa NP, dos Anjos MAS, Aguiar JGC, da Silva Neto JA, Pederneiras CM. Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates. Applied Sciences. 2025; 15(10):5729. https://doi.org/10.3390/app15105729
Chicago/Turabian StyleMendonça de Souza, Maelson, Normando Perazzo Barbosa, Marcos Alyssandro Soares dos Anjos, João Gabriel Cruz Aguiar, José Anselmo da Silva Neto, and Cinthia Maia Pederneiras. 2025. "Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates" Applied Sciences 15, no. 10: 5729. https://doi.org/10.3390/app15105729
APA StyleMendonça de Souza, M., Barbosa, N. P., dos Anjos, M. A. S., Aguiar, J. G. C., da Silva Neto, J. A., & Pederneiras, C. M. (2025). Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates. Applied Sciences, 15(10), 5729. https://doi.org/10.3390/app15105729







