Abstract
Dysphagia in children poses a significant health concern. Difficulties in swallowing can lead to an impairment in food intake and malnutrition, as well as a risk of aspiration and pneumonia. It is a life-threatening condition, especially for newborns and infants. Children with dysphagia and their parents are experiencing increased anxiety and stress. Traditional methods of dysphagia therapy involve manual exercises of the orofacial muscles and modifications of the diet to fit the child’s abilities. These methods often do not achieve the desired results, which prompts researchers to look for new solutions to increase the effectiveness of standard therapy. One promising approach is neuromuscular electrical stimulation (NMES) applied to muscles involved in the process of swallowing. The purpose of this paper is to highlight and discuss the feeding difficulties associated with pediatric dysphagia, as well as the possibility of NMES application in its treatment. It is anticipated that NMES, by enhancing muscles that regulate swallowing, may improve the nutritional status of children with dysphagia. More research is needed to show that NMES is effective in improving the feeding and nutritional status of children with dysphagia.
1. Introduction
Food intake depends on swallowing. It is a complex process that involves the actions of many muscles and nerves [1]. However, most children are born with this skill. Infants typically receive hydration and nutrition from the breast or bottle. Safe feeding requires precise coordination of swallowing and breathing [2,3]. Newborns normally master this coordination within 20–30 min of their first feed after birth [4]. Nonetheless, feeding disorder due to difficulty swallowing may arise in children born prematurely, with malformations, neurological issues, or medical complications [5,6,7].
The term ‘dysphagia’ refers to difficulty swallowing, which can be caused by various underlying conditions [8]. The exception is the occasional occurrence of difficulty in swallowing certain foods or liquids, such as when taking too large a bite of food. Dysphagia in pediatric patients is a kind of pediatric feeding disorder (PFD). According to the World Health Organization (WHO), PFD is defined as impaired oral intake that is not age-appropriate and is associated with medical, nutritional, feeding skill, and/or psychosocial dysfunction. Various causes of PFD are emphasized, such as medical, oromotor, or behavioral difficulties [9]. Any approach to feeding issues emphasizes that PFD or dysphagia is a complex and multifaceted issue that necessitates the involvement of numerous specialists from diverse fields [10,11,12].
Dysphagia concerns various activities associated with eating, including difficulties in oral intake of food, or liquids, forming a bolus (a bool-like food bite), or swallowing (Figure 1) [12,13]. It may manifest as a singular symptom or as one of a collection. Some kinds of feeding problems occur in about 25–45% of children, and in children with developmental delays, this percentage reaches as much as 80% [14,15]. This mainly affects children with neurological disorders of the functioning of the muscles responsible for swallowing. Dysphagia is also common in children with anatomical abnormalities, such as cleft palate, laryngeal deformities, or esophageal atresia, or genetic conditions such as Down syndrome [8,11,12,16]. Another case is that of neurological problems, e.g., disorders of the function of the orofacial muscles involved in, among others, the oral–facial complex taking part in feeding and its transfer to the esophagus, as well as cerebral palsy or brain injuries [7,17]. Dysphagia may also be attributed to disorders of the digestive system, such as gastroesophageal reflux, and may also be associated with posture and movement control issues [2,3,18]. Children with dysphagia resulting from neurological disorders exhibit an elevated frequency of upper respiratory tract infections, thereby increasing the likelihood of recurrent pneumonia [8,19].
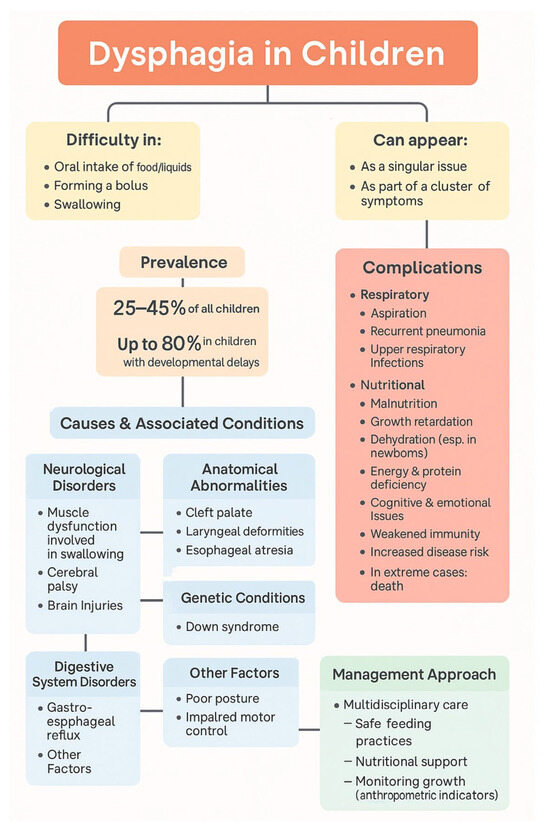
Figure 1.
Overview of dysphagia in children.
Malnutrition, growth retardation, cognitive impairment, emotional dysfunction, weakened immunity, and increased susceptibility to disease are some of the health consequences of chronic feeding difficulties caused by dysphagia [16,17,18]. Moreover, aspiration to the respiratory tract and pneumonia are common respiratory complications [2,16]. In extreme cases, they can even lead to death [16,20]. Malnutrition as a result of dysphagia is particularly dangerous in newborns [2,21]. In this situation, the child may lose weight quickly and develop energy and protein deficiencies, as well as dehydration. If this continues, it may lead to death [1]. To prevent the negative consequences of dysphagia, a multidisciplinary approach is necessary, including ensuring children’s safe feeding, and improving nutritional status, anthropometric indicators, and quality of life [8,22,23].
Dysphagia can occur in newborns, but a considerable number of infants overcome dysphagia within the initial few weeks of life. The age of 3–4 months is the next time when dysphagia can become apparent. At three months of age, the sucking reflex begins to diminish, and solid foods are introduced to the diet. Then, difficulties with swallowing and breathing coordination may become more apparent [1,8]. Dysphagia can also occur in older children who are more communicative, and signs of swallowing difficulties may be easier to spot. It may also develop after an illness or incident that causes swallowing difficulties [2,24]. It is important to note that dysphagia refers to impaired swallowing mechanics, but not to a sensory or psychological aversion to food [24].
Traditional treatments of dysphagia usually consist of physical exercises of the orofacial muscles and a diet modification to suit the child’s abilities [22,24]. These methods do not always bring the desired results, which prompts researchers to search for new therapeutic solutions. One of the promising methods supporting dysphagia therapy is neuromuscular electrical stimulation (NMES), which uses low-frequency currents for transcutaneous muscle stimulation [21,25]. In dysphagia therapy, this technique involves stimulating the muscles that control the swallowing process.
NMES has so far been used successfully in adults, e.g., in the treatment of dysphagia after myocardial infarction, after brain injury, or as an adjunct in the therapy of neck cancer or soft tissue and bone injuries, as well as in the rehabilitation of the muscles of the motor system in adults [26,27,28,29,30,31,32,33,34,35,36]. But the use of electrical stimulation in the treatment of dysphagia in children is relatively new and not standard practice [26].
A limited number of studies have demonstrated favorable outcomes of NMES in comparison to conventional therapy in children with dysphagia. In this paper, we describe the challenges of pediatric dysphagia and the possibility of using NMES for dysphagia therapy [20]. The purpose of this article is to highlight and discuss the difficulties associated with dysphagia in children, as well as the possibility of employing NMES in its treatment. It can be anticipated that NMES, by enhancing swallowing, may also improve the nutritional status of children with dysphagia.
2. The Swallowing Process
Swallowing is a complex process. It involves more than 30 muscles in the coordination of the lips, tongue, palate, pharynx, larynx, and esophagus [5,37]. The cranial nerves play an important role in swallowing, a complex cognitive and sensorimotor process that moves any bolus from the mouth to the stomach [38].
Four stages of swallowing can be distinguished: oral preparatory, oral transit, pharyngeal, and esophageal [4,5,17]. During the oral preparatory stage, food is kept in the front part of the mouth, where it is moistened and mixed with saliva. The process involves various muscles of the orofacial complex, including the facial muscles, masticatory muscles, suprahyoid and infrahyoid muscles, tongue muscles, soft palate muscles, pharyngeal muscles, and their functions [39]. During the preparatory stage, the masticatory muscles play a key role in grinding and chewing food. The process involves the mixing of food with saliva to facilitate swallowing [40]. Next, as a result, a bolus is formed that is then placed on the tongue [4,5]. At this stage, temporomandibular muscles are responsible for chewing and jaw movements [18,41]. The lesser and greater pterygoid muscles allow forward and backward jaw movements. Cheek and tongue muscles aid in the formation of a food bolus and its transit to the throat [5,38,42].
Once a food bolus has been processed, the oral transit stage begins with the tongue raising up against the hard palate while the back of the tongue drops to allow the bolus to move into the back of the mouth (Figure 2). Simultaneously, the soft palate rises up against the back wall, closing off the passageway into the nose and preventing the regurgitation of any food or liquids into the nose [3,5,24]. In the transit stage, the bolus is moved to the pharyngeal isthmus. As a result, the soft palate begins to tense. This acts as a barrier, separating the nasal cavity from the oral cavity.4,40 The palatine arches move closer together, which stops food from going back into the mouth. The larynx moves up, which closes the epiglottis and protects the airway from food aspiration [38,40]. As a result, the only open path for the food bolus is the pharynx.
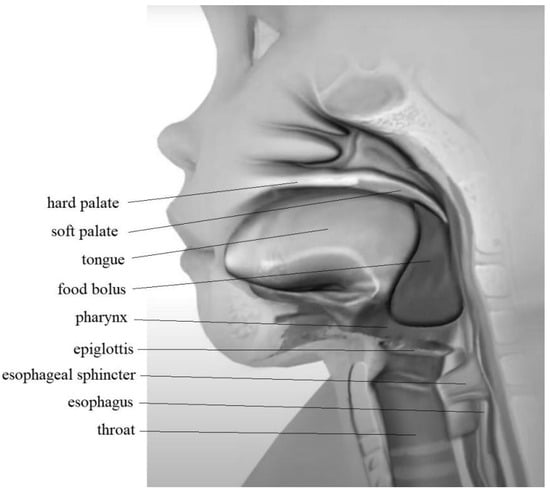
Figure 2.
Food bolus position in the transit stage of swallowing.
The pharyngeal stage is a rapid sequential activity during which the bolus is propelled into the esophagus. The larynx elevates while the epiglottis flips down, covers the trachea, and protects the airways from aspiration. Pharyngeal muscles contract, squeezing the bolus downwards. The upper esophageal sphincter (cricopharyngeal muscle) relaxes and opens to allow food and liquids to pass into the esophagus [4,40].
Once the bolus passes the esophageal sphincter, the esophageal stage begins. The sphincter closes back up to protect any regurgitation back up into the throat. The esophageal muscles continue to propel the bolus into the stomach via coordinated muscle contraction and relaxation, known as peristalsis. The mouth and throat reset to prepare for the next swallowing event [41,42].
3. Symptoms of Dysphagia
Oropharyngeal dysphagia occurs when it is difficult to move the food bolus from the oral cavity to the esophagus [22]. The symptoms pertain to difficulties encountered in initiating the transportation of a food bolus. Factors indicating dysphagia include the need to change feeding position or equipment, the use of food and fluid texture modifications, and/or the need to use modified feeding strategies and reliance on enteral feeding or oral supplements to maintain nutritional status and/or hydration [43]. Dysphagia in children manifests various diagnostic symptoms that may not always be discernible [22,44]. One typical symptom is the sensation that food or fluid is proving to be challenging to swallow, and that it is becoming entrapped in the esophagus or clinging to the stomach [8]. Another symptom is odynophagia, or pain during swallowing, which may occur in the throat or chest and may mimic heartburn or even cardiac symptoms [24,37].
Dysphagia may be accompanied by coughing during or immediately after swallowing and may be caused by food entering the throat too soon, food particles remaining in the throat after swallowing, or regurgitation of food [8,37,43]. Choking, which is a cough caused by food or fluid being stuck in the throat or esophagus or being aspirated into the airway, may also occur. If aspiration happens into the airways, it can lead to aspiration pneumonia [14,24]. Another symptom of dysphagia could be regurgitation [45]. This happens when food or liquid returns to the mouth. Unlike vomiting, regurgitation occurs without gag reflexes, nausea, or involvement of the stomach muscles. Nasal regurgitation occurs when food or fluid is swallowed and regurgitates into the nose. This occurs when the nasopharynx does not close properly and may indicate a problem with the nerves that regulate the muscles of the soft palate or throat [8,15,24,37].
Dysphagia may also cause difficulty breathing while feeding a child. Wet or congested sounds may also happen. If a child makes these sounds during feeding, it may indicate that food or liquid is entering the airway or remaining in the throat, increasing the risk of airway entry [19,46]. Coughing, gagging, or choking may also occur. Additionally, prolonged feeding time, unusually stressful mealtimes, and deteriorating anthropometric parameters are associated with dysphagia [1,37]. Oral motor dysfunction limits bolus control, manipulation, and/or passage of liquids and solids. These include ineffective ingestion, messy eating, poor control of fluids and foods, slow or ineffective bolus formation and movement, and gag reflex [37,40]. The symptoms may include repeated and difficult swallowing, throat clearing, nasal regurgitation, and poor airway protection [43,46,47,48].
Swallowing problems and potential respiratory complications can cause stress for both children and parents and caregivers [49]. Parents of children with dysphagia are more anxious and stressed during feeding than parents of children without dysphagia [7,50]. Sulivan et al. reported that parents of children with neurological problems spend an average of 3.5 h feeding, and nearly half of them describe mealtimes as stressful and not at all enjoyable [11]. Feeding stress usually involves the risk of choking, special preparation and serving of food, costs, and often additional assistance required for feeding [7,51]. As a result of prolonged and stressful feeding, a child with swallowing problems is exposed to a lack of adequate nutrition, which can lead to malnutrition and exacerbate developmental disorders [13]. Children with dysphagia often require an increased number of hospitalizations, longer hospital stays, and repeated emergency room visits. It makes it not only stressful but also results in high medical costs, including tube feeding [51,52]. This is a burden that adds stress to children with dysphagia and their parents [49].
4. Causes of Swallowing Disorder
There are several types of dysphagia, depending on which part of the swallowing process is challenging [8,43]. Oral dysphagia occurs when the problem involves the mouth, most often due to tongue movement disorders. It may also be due to anatomical defects, such as a cleft palate [53]. Pharyngeal or oropharyngeal dysphagia occurs when the issue concerns the passage of food through the throat. Esophageal dysphagia happens when food cannot move down the esophagus [1,2].
Problems in the oral phase may manifest themselves by avoiding eating, holding food in the mouth, and, secondarily, by fear of swallowing, among other things [39,54]. One of the causes of dysphagia in the oral phase is a dysfunction of the orofacial muscles involved in taking in and swallowing food [2,8,48]. Normally, coordinated muscle contractions move swallowed food toward the pharynx and esophagus and toward the stomach during the esophageal phase [17]. The upper and lower ends of the esophagus have muscle sphincters that allow food to pass from the pharynx to the esophagus and, at the lower end, from the esophagus to the stomach. If the muscles in the esophagus stop working together, or if one of them stops working at all, food may not pass into the stomach [5,13]. This can happen because of problems with the muscles or the nerves that regulate the function of these muscles [38].
A common esophageal motor problem is achalasia, which is a loss of function of the lower esophageal sphincter [44]. Another is a distal esophageal spasm that can make food go back into the throat [55,56]. The next one is known as a jackhammer esophagus, which refers to the extremely powerful muscle contractions of the esophagus that can lead to severe chest pain during swallowing [57]. It is also possible that the lower esophageal sphincter is either too tight or too loose. If the lower sphincter is too loose, stomach contents can flow back up into the esophagus, or reflux [37]. Additionally, previous esophageal surgery or the insertion of an endotracheal tube can often lead to difficulty swallowing [4,5]. Another factor contributing to swallowing difficulties during the esophageal phase is the presence of structural anomalies. The esophageal narrowing or the formation of pockets in it is an example [58]. An esophageal diverticulum, or pocket-like sac, can form in the lining of the esophagus and trap food that has been swallowed. Sometimes swallowing problems are caused by congenital anatomic defects of the esophagus. However, more often the cause is structural changes that develop over time. These include thickening of the esophageal walls, tumor growth, and other causes that cause the esophagus to narrow [24,58].
Another cause of dysphagia is neurological issues. Neurogenic dysphagia is related to the nerves that regulate the function of the muscles of the mouth, throat, and esophagus [5,54]. A patient experiencing nasal regurgitation or coughing when swallowing probably has some form of neurological disease [5,45].
Furthermore, swallowing difficulties may be associated with muscle weakness in conditions such as stroke, amyotrophic lateral sclerosis (ALS), traumatic or surgical damage to the nerves in the head and neck, myasthenia gravis, polymyositis, and Parkinson’s disease [24,49].
In some cases, swallowing disorders may also result from an allergic reaction, causing eosinophilic esophagitis [59]. In time, as a result of frequent inflammatory reactions, a ring-shaped, sometimes suppurating, thickening of the esophagus can form, making swallowing difficult. Similarly, any severe infection of the throat or esophagus can make swallowing difficult, but usually this is only temporary and recover after the infection is treated [24].
5. Treatment of Dysphagia
Dysphagia therapy for children is a complex process that requires the use of various strategies and interventions tailored to the individual condition and capabilities of the patient. Before starting them, it is important to diagnose the problem [2,8,18,23]. The diagnosis includes a medical interview and an analysis of the child’s health history, including previous swallowing problems, neurological diseases, anatomical congenital defects, and other risk factors.
Another diagnostic element is observation during eating, how the child eats food, paying attention to swallowing problems, coughing, hiccups, and other diagnostic elements. Furthermore, it is essential to investigate the function of the vocal organ and how it affects the capacity to swallow [21]. The treatment of dysphagia is based on an individual plan. The goal of the treatment is to ensure the safety, hydration, and nutrition of children and to remove the cause of dysphagia if possible. In some cases, it is possible to remove the cause of swallowing disorder, such as by relaxing the esophageal sphincter, which is too tight [24]. However, if the cause cannot be removed, dysphagia treatment may focus on making swallowing safer and relieving discomfort. Treatment can include drugs, swallowing therapy, dietary modification, surgery, and other treatments, depending on the cause [8,21].
Drugs may be effective in relieving some symptoms of dysphagia or some of its causes, such as infection [59]. However, dysphagia therapy usually focuses on strengthening the muscles to improve swallowing motor abilities and reduce the chance of choking or aspirating. Exercises are designed to develop or improve oral motor skills, such as chewing and swallowing. Physical motor techniques in dysphagia therapy include manual and mechanical motor exercises that strengthen the muscles involved in swallowing, including exercises for the tongue, lips, and pharyngeal muscles (in the area of a range of motion or strength) [8]. This therapy is usually provided by neuro speech therapists also known as a speech-language pathologist (SLP) or neurologopedist who specializes in treating communication and swallowing disorders caused by neurological conditions [2,8]. Swallowing exercises include, but are not limited to, tongue strengthening exercises, laryngeal exercises, and mouth closing exercises to increase lip strength and mouth closure. Swallowing training may be helpful for patients with dysphagia caused by neurologic disorders or for those who have had surgery on the pharyngeal muscles [2,13].
Some cases of dysphagia require surgical intervention [60]. In the case of achalasia, peroral endoscopic myotomy (POEM) is one of the surgical procedures for swallowing disorders [8,60]. This involves cutting through the tight ring of muscle to make it loosen. A diverticulectomy may be needed to remove a diverticulum in the esophagus. Another procedure entails dilation of the esophagus to gently expand the narrowed regions. Sometimes, an esophageal stent is needed to keep the narrowed esophagus open. If there is a tumor or other obstructing factor, surgery is needed to remove it [8]. For anatomical defects, such as a cleft palate, surgical repair can also eliminate the cause of the swallowing problem [53].
Dietary modification can help make swallowing safer and more comfortable if the direct cause of dysphagia cannot be eliminated [61,62]. The main methods used in this regard are the elimination of foods that cause difficulty swallowing or the adjustment of their consistency and portion sizes to ensure the safe feeding of the child [2,8,18]. A convenient consistency of the diet should be established, such as purée, mechanically modified foods, solid foods, or only semi-solid foods [1]. The use of thickeners in liquid diets is also considered to minimize the risk of inhaling food into the lungs and choking [16,63]. Additionally, changes in posture or head position during swallowing may bring improvement, as may the use of special techniques to keep food moving if there are difficulties in its passage to the stomach.
Simultaneously, nutritional assistance is provided, which entails ensuring the child’s adequate nutrition through the implementation of optimal feeding techniques and the consideration of alternative feeding methods [1,16]. Specialist equipment, such as specialist bottles and teats, is used to better control feeding for children with dysphagia. An example is the specialist teats for stones in children with cleft palate [8,21]. Furthermore, specialized feeding devices are employed to facilitate nutrient absorption, such as by feeding children through a gastric tube (tube feeding) [64]. Psychosocial support is especially important when dysphagia causes anxiety related to eating and has an impact on family relationships. Despite this, new techniques are being explored to complement dysphagia therapy. It is also helpful to implement techniques that support meal routines, involvement, and enjoyment of feeding, with particular emphasis on education and support for the family.
In particularly challenging states, temporary enteral feeding with a feeding tube is utilized [21]. However, for some children, despite compensatory strategies and therapeutic interventions, a permanent tube feeding through a gastrostomy may be required to provide nutrition safely, especially for children with neurological disabilities [1]. The use of a tube can ensure that nutrition evades the problem area, minimizing the danger of coughing, choking, and aspiration of food into the lungs [64]. This method of feeding is initiated when the patient is losing weight rapidly and is at risk of malnutrition or if he has already developed aspiration pneumonia. Once the patient’s swallowing ability and nutritional status improve, the tube can be removed. Breathing techniques to coordinate swallowing and breathing are also important. The pace of eating is also modified, with appropriate pauses for breathing. This can reduce the risk of aspiration of food into the trachea and lungs [21].
Many children with dysphagia experience improvements in swallowing with appropriate intervention and therapy [24,37]. The degree of improvement depends on the cause of the problem and the child’s condition. Early intervention and preventive strategies can significantly reduce the severity of the disease [61]. There is ongoing research into precise diagnostic tools and effective therapies for dysphagia in children. A new technique of potential therapeutic benefits in dysphagia is the use of NMES [19,20].
6. Electrical Stimulation
The usual methods of dysphagia therapy, based on exercises and dietary modifications, do not always achieve the desired results, which indicates the need for new therapeutic solutions [13,22]. One of the promising approaches to aid dysphagia therapy is NMES, which employs low-frequency current for stimulation of the muscles involved in the swallowing process [65]. NMES is a modality that sends electrical impulses to the nerves, which causes the muscles to contract, mimicking the action potential coming from the central nervous system. In this manner, it is possible to restore proper muscle contractility [6].
Electrical stimulation, through the use of direct, alternating, and pulsed current, is used mainly in the treatment of pain, improving circulation, and strengthening muscles. In adults, NMES has been successfully applied, for example, in the rehabilitation of the muscles of the musculoskeletal system, in the rehabilitation after myocardial infarction, after brain injury, or as an adjunct in the treatment of neck cancer or soft tissue and bone injuries [26,27,28,29,30,31,32,33,34,35,36].
The available literature indicates the possibility of using different types of electrical stimulation in the therapy of the orofacial area. NMES therapy in the head and neck area has been successfully used for swallowing rehabilitation in adult patients with acquired dysphagia [66,67]. Direct, alternating, and pulsed current are used in the treatment of pain, improving circulation, and strengthening muscles. NMES has been successfully used as an adjunctive therapy in numerous applications, including spinal cord injuries, muscle spasms and atrophy, urinary incontinence, gastroparesis, and temporomandibular joint dysfunction [68].
NMES contributing to the swallowing apparatus has been successfully used as a feeding therapy in adults after myocardial infarction and acute brain injury and after treatment for head and neck cancer [28,29,69,70]. Despite dysphagia, NMES of the orofacial complex muscles may be used in practice to treat temporomandibular joint dysfunction, rehabilitation after facial paralysis, improvement of facial aesthetics, treatment of sleep disorders such as snoring and sleep apnea, regeneration and improvement of muscle function after surgical procedures in the facial area, strengthening muscles in cases of facial hypotonia or hypertonia, and inhibition of skin aging [71]. It was also used in the treatment of sarcopenic dysphagia in elderly patients [72].
NMES involves the application of electrical current to muscles transcutaneously using surface electrodes [21,73]. The therapists combine transcutaneous stimulation with conventional swallowing therapy [47,73,74,75]. An example of electrode placement during therapy and example of NMES device are given in Figure 3. It was suggested that NMES improves dysphagia by strengthening the muscles involved in swallowing and by enhancing the sensory signals of the swallowing response [73]. Typically, NMES is performed repeatedly over a period of time. For example, some protocols use NMES up to five days per week for four weeks, but there are variations [72,74]. In adult patients, it was demonstrated that conventional dysphagia therapy is less effective than therapy, including NMES [20,70,76,77,78].
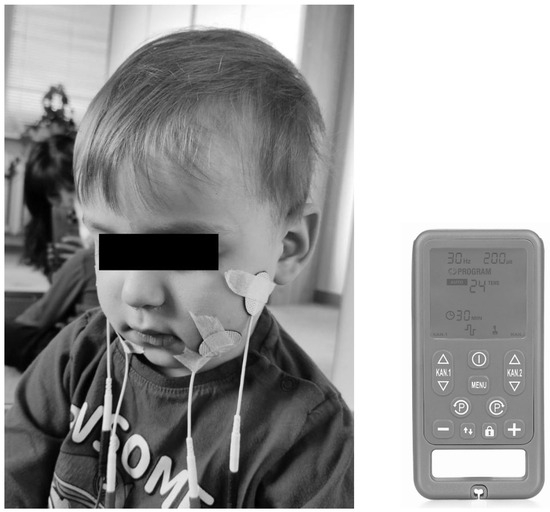
Figure 3.
An example of electrode placement during therapy using NMES device (Ecostim).
In the treatment of dysphagia in children, NMES is a novel therapeutic method that is fundamentally different from traditional methods. These methods include compensatory techniques (e.g., changes in head position) and swallowing training using foods of varying consistencies, as well as manual massages and exercises for the muscles of the throat and larynx. Table 1 presents a comparison of NMES with traditional dysphagia therapy methods.

Table 1.
Differences between NMES and traditional dysphagia therapy.
7. NMES in Children with Dysphagia
Findings from studies in adults should not be extrapolated to children, given that the etiology of dysphagia in adults is very different (e.g., pharyngeal tumors, stroke, dementia), as well as the fact that the goal of rehabilitation in adults is different. Adult patients undergo rehabilitation due to swallowing loss, whereas children typically undergo rehabilitation to develop and enhance their swallowing ability [20]. Moreover, neuroplasticity changes with age.
There are limited data on the effectiveness of NMES in children with dysphagia. These studies indicated that using NMES to strengthen the muscles of the orofacial complex may help treat dysphagia in children that is caused by dysfunctions of these muscle [6,65,79]. Other studies showed the advantages of NMES in enhancing speech function and reducing food aspiration into the respiratory tract, as observed in more than half of patients treated with the conventional method [25]. NMES combined with conventional therapy provided better results than conventional therapy alone. NMES has the potential to enhance swallowing biomechanics, including laryngeal and hyoid movements, thereby reducing the risk of aspiration and facilitating the passage of food through the pharyngeal passage [19,20]. In a few studies, it was suggested that NMES combined with nutritional therapy could improve nutritional status and reduce the risk of aspiration in children with neurological problems [19,80,81].
In almost all studies available in the literature, the swallowing function and feeding ability of children improved during NMES treatment. The children increased the ability to swallow different consistencies [19,80,82,83,84]. Improvement was also noted in patients who were dependent on gastrostomy [19]. Only one study by Christiaanse et al. found that the NMES intervention was not associated with an improvement in feeding ability compared with the control group [21]. These are only a few of the available studies that assessed the nutritional status of children during NMES treatment [19,80,81]. Studies on NMES therapy in children with dysphagia are presented in Table 2.

Table 2.
Studies on NMES therapy in children with dysphagia.
The NMES therapy was considered to be safe, but not without potential adverse events. Many authors indicated advantages of NMES in dysphagia therapy. First of all, NMES increases muscle strength and activity. Direct stimulation of facial, pharyngeal, or laryngeal muscles can support their function, even in patients with very limited voluntary movement or low behavioral cooperation [92]. It can be used in patients with severe dysphagia. NMES offers a therapeutic option even when manual massage or exercises are not possible [65,93]. Next is neuroplasticity. It was shown that repetitive stimulation may induce neuronal reorganization and support the restoration of functions such as swallowing [94]. NMES can be integrated with other methods. It can be effectively combined with functional exercises, enhancing overall therapy effectiveness [92].
This method, however, is not free from limitations. One of these is a lack of conclusive clinical evidence. There are conflicting study results regarding the effectiveness of NMES in dysphagia therapy; many studies have methodological limitations (e.g., small sample sizes, lack of randomization) [90]. There is also a risk of incorrect application. Improper electrode placement may lead to ineffective stimulation or undesirable reactions such as unintended neck muscle contractions or may cause mild skin irritation [95,96]. Next is cost and limited accessibility. The therapy requires specialized equipment and trained personnel, which may limit its availability in certain facilities [97]. Discomfort or limited cooperation during the NMES session is also indicated. Some patients, especially children, may poorly tolerate the sensation of electric stimulation or show limited cooperation and may exhibit protest behaviors [93]. Advantages and disadvantages are summarized in Table 3.

Table 3.
Advantages and disadvantages of NMES in dysphagia therapy in children.
8. Technical Parameters of NMES
NMES uses electrical impulses to elicit muscle contractions. Its effectiveness depends on key biophysical parameters such as waveform type, pulse duration, frequency, current intensity, and electrode placement. These factors influence the depth, comfort, and efficiency of stimulation. Tissue resistance also plays a significant role and is influenced by factors such as skin type, hydration level, and temperature. The key technical aspects of NMES based on the information include impulse parameters, current settings, electrode placement, and factors affecting tissue resistance [98]. Commonly used wave forms are symmetrical biphasic rectangular pulses and asymmetrical biphasic pulses. The first one is preferred for large muscle groups, as they ensure balanced current flow and reduce the risk of net charge buildup in tissues. The second one is used for smaller muscles or more targeted stimulation. Typical impulse parameters include the following: pulse duration: 200–400 µs; frequency: 20–50 Hz to induce tetanic contraction; and duty cycle (stimulation/rest time): e.g., 10 sec. on and 30 sec. off to minimize muscle fatigue [98,99,100]. Current intensity is individually adjusted, typically up to 100 mA (up to 120 mA in some devices), depending on patient’s tolerance and application site. Peak output voltage may reach 100–150 V per impulse, depending on skin impedance [100].
In NMES, electrodes should be placed to cover the innervation zone of the target muscle: active electrode—over the motor point (where the nerve enters the muscle); dispersive electrode—along the muscle or nerve pathway. The distance between electrodes affects stimulation depth and spread: the greater the distance, the deeper the stimulation but with reduced selectivity [100]. Tissue resistance (impedance) affects stimulation efficiency. It depends on the application site (dry or keratinized skin has higher resistance), skin hydration, amount of subcutaneous fat, body temperature, and environmental humidity [99]. With longer application time and higher humidity, resistance tends to decrease [101,102]. Key NMES parameters and their clinical significance are presented in Table 4.

Table 4.
NMES technical parameters in therapy.
There are many producers of NMES devices. However, they all work on the same principles and with similar parameters. Table 5 shows three examples of NMES devices: VitalStim Plus (Chattanooga, Dallas, TX, USA), Ecostim (Novartag GmbH, Berlin, Germany), and Vitalstim (Enovis, Surrey, UK). NMES effectiveness depends on key biophysical parameters such as waveform type, pulse duration, frequency, electrode placement, and tissue resistance. Proper adjustment of these factors ensures safe and efficient electrical stimulation tailored to clinical needs.

Table 5.
Examples of NMES devices, based on producers’ information.
In addition to the widely used neuromuscular electrical stimulation (NMES) in the treatment of dysphagia, sensory stimulation using interferential current (IFC) is also applied in clinical practice. Its mechanism of action is primarily based on afferent modulation and sensitization of structures involved in the initiation of the swallowing reflex, rather than on direct muscle strengthening. IFC appears to be potentially beneficial, particularly in the pharyngeal phase of swallowing, where sensory stimulation may enhance reflex initiation through facilitation of neural centers [110]. It was shown that interferential electric stimulation applied to the neck increases swallowing frequency [111]. However, there is a lack of reliable clinical data supporting the effectiveness of this method in the oral phase of dysphagia. Unlike NMES, which was confirmed in numerous studies as an effective tool for improving the function of muscles responsible for bolus transport and airway protection, sensory stimulation remains supplementary and experimental in nature [112].
9. Future Perspectives of NMES in Dysphagia Treatment
The development of NMES in dysphagia therapy encompasses several important directions [54,113,114]. Increasing emphasis is being placed on personalized therapy, allowing stimulation parameters to be tailored to the individual needs of each patient. Modern devices offer more advanced configuration options, enabling more precise and effective treatment. NMES is also being increasingly applied in pediatric populations, where traditional therapeutic approaches are often insufficient or difficult to implement [54,93]. Currently, numerous randomized clinical trials are being conducted to evaluate the efficacy and safety of NMES in different patient groups. The results of these studies may lead to the recognition of NMES as a standard method for dysphagia treatment in the future [85,87].
There was another case of pharmacological treatment of dysphagia. It was shown that dopaminergic drugs have some potential to improve dysphagia in patients with Parkinson’s disease [115]. However, such pharmacological therapy is used in children. Morgan et al. suggested that interventions such as intrathecal baclofen or Botox to increase or decrease tone may enable more functional oropharyngeal swallowing physiology [116]. However, studies with high-quality evidence are lacking.
10. Conclusions
NMES of the orofacial muscles has great potential for the treatment of swallowing disorders in children with neurological and developmental problems and offers effective support to traditional therapeutic methods. It can be considered a supplementary therapy for dysphagia in children. NMES also has the potential to play an important role in improving the nutritional status and quality of life of children with dysphagia. However, the variety of NMES protocols and electrode locations indicates the need for further research to determine its optimal parameters for use in the treatment of dysphagia. The outcomes of the studies to date are encouraging, but there remain numerous unexplored aspects regarding the long-term effectiveness of this therapeutic approach. More research is needed to show that NMES is effective in improving the feeding and nutritional status of children with dysphagia.
Author Contributions
Conceptualization, M.B. and W.K.; methodology, M.B and W.K.; software, M.B. and W.K.; validation, M.B. and W.K.; writing—original draft preparation, M.B.; writing—review and editing, W.K.; visualization, M.B.; supervision, W.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting reported results are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NMES | Neuromuscular Electric Stimulation |
| PFD | Pediatric Feeding Disorders |
| WHO | World Health Organization |
References
- Prasse, J.E.; Kikano, G.E. Clinical pediatrics an overview of pediatric dysphagia. Clin. Pediatr. 2009, 48, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Dodrill, P.; Gosa, M.M. Pediatric dysphagia: Physiology, assessment, and management. Ann. Nutr. Metab. 2015, 66, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, D. Dysfunctional swallowing in the pediatric patient: Clinical considerations. Dysphagia 1998, 23, 203–208. [Google Scholar] [CrossRef]
- Matsuo, K.; Palmer, J.F. Anatomy and Physiology of Feeding and Swallowing—Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef]
- Ertekin, C.; Aydogdu, I. Neurophysiology of swallowing. Clin. Neurophysiol. 2003, 114, 2226–2244. [Google Scholar] [CrossRef]
- Miller, S.; Jungheim, M.; Kühn, D.; Ptok, M. Electrical stimulation in treatment of pharyngolaryngeal dysfunctions. Folia Phoniatr. Logop. 2013, 65, 154–168. [Google Scholar] [CrossRef]
- Jaafar, N.H.; Othman, A.; Majid, N.A.; Harith, S.; Zabidi-Hussin, Z. Parent-report Instruments for assessing feeding difficulties in children with neurological impairments: A systematic review. Dev. Med. Child. Neurol. 2019, 61, 135–144. [Google Scholar] [CrossRef]
- Lawlor, C.M.; Choi, S. Diagnosis and Management of Pediatric Dysphagia: A Review. JAMA Pediatr. 2019, 173, 91–98. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Barikroo, A. Transcutaneous electrical stimulation and dysphagia rehabilitation: A narrative review. Rehabil. Res. Pract. 2020, 2020, 4865614. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Lambert, B.; Rose, M.; Ford-Adams, M.; Johnson, A.; Griffiths, P. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev. Med. Child. Neurol. 2000, 42, 674–680. [Google Scholar] [CrossRef]
- Manikam, R.; Perman, J.A. Pediatric feeding disorders. J. Clin. Gastroenterol. 2000, 30, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Motion, S.; Northstone, K.; Emond, A.; Stucke, S.; Golding, J. Early feeding problems in children with cerebral palsy: Weight and neurodevelopmental outcomes. Dev. Med. Child Neurol. 2002, 44, 40–43. [Google Scholar] [PubMed]
- Sdravou, K.; Fotoulaki, M.; Emmanouilidou-Fotoulaki, E.; Andreoulakis, E.; Makris, G.; Sotiriadou, F.; Printza, A. Feeding Problems in Typically Developing Young Children, a Population-Based Study. Children 2021, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N. The prevalence of pediatric voice and swallowing problems in the United States. Laryngoscope 2015, 125, 746–750. [Google Scholar] [CrossRef]
- Tutor, J.D.; Gosa, M.M. Dysphagia and aspiration in children. Pediatr. Pulmonol. 2012, 47, 321–337. [Google Scholar] [CrossRef]
- Bass, N.H.; Morrell, R.M. The neurology of swallowing. In Dysphagia, Diagnosis and Management; Groher, M.E., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1992; pp. 1–29. [Google Scholar]
- Duffy, K.L. Dysphagia in Children. J. Pediatr. Health Care 2018, 32, 321–329. [Google Scholar] [CrossRef]
- Andreoli, S.M.; Wilson, B.L.; Swanson, C. Neuromuscular electrical stimulation improves feeding and aspiration status in medically complex children undergoing feeding therapy. Int. J. Pediatr. Otorhinolaryngol. 2019, 127, 109646. [Google Scholar] [CrossRef]
- Propp, R.; Gil, P.J.; Marcus, S.; Ren, L.; Cohen, E.; Friedman, J.; Mahant, S. Neuromuscular electrical stimulation for children with dysphagia: Asystematic review. J. Pediatr. Dysphagia Ther. 2024, 12, 45–56. [Google Scholar] [CrossRef]
- Christiaanse, M.E.; Mabe, B.; Russell, G.; Long Simeone, T.; Fortunato, J.; Rubin, B. Neuromuscular electrical stimulation is no more effective than usual care for the treatment of primary dysphagia in children. Pediatr. Pulmonol. 2011, 46, 559–565. [Google Scholar] [CrossRef]
- Phalen, J.A. Managing feeding problems and feeding disorders. Pediatrics Rev. 2013, 34, 549–557. [Google Scholar] [CrossRef]
- Printza, A.; Sdravou, K.; Triaridis, S. Dysphagia Management in Children: Implementation and Perspectives of Flexible Endoscopic Evaluation of Swallowing (FEES). Children 2022, 9, 1857. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A. Evaluation and Treatment of Swallowing Disorders; Pro-Ed, Inc.: Austin, TX, USA, 1998. [Google Scholar]
- Kubitscheck de Oliveira Santos, J.; Côrtes Gama, A.C.; Alves Silvério, K.C.; Cordeiro Diniz Oliveira, N.F. The use of electrical stimulation in speech therapy clinical: An integrative literature review. Braz. J. Speech Ther. 2015, 20, 201–209. [Google Scholar]
- Alamer, A.; Melese, H.; Nigussie, F. Effectiveness of neuromuscular electrical stimulation on post-stroke dysphagia: A systematic review of randomized controlled trials. Clin. Interv. Aging 2020, 15, 1521–1531. [Google Scholar] [CrossRef]
- Diéguez-Pérez, I.; Leirós-Rodríguez, R. Effectiveness of different application parameters of neuromuscular electrical stimulation for the treatment of dysphagia after a stroke: A systematic review. J. Clin. Med. 2020, 9, 2618. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chang, K.H.; Chen, H.C.; Liang, W.M.; Wang, Y.H.; Lin, Y.N. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: A systematic review and metaanalysis. Clin. Rehabil. 2016, 30, 24–35. [Google Scholar] [CrossRef]
- Bhatt, A.D.; Goodwin, N.; Cash, E. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head. Neck 2015, 37, 1051–1056. [Google Scholar] [CrossRef]
- Benfer, K.A.; Weir, K.A.; Boyd, R.N. Clinimetrics of measures of oropharyngeal dysphagia for preschool children with cerebral palsy and neurodevelopmental disabilities: A systematic review. Dev. Med. Child. Neurol. 2012, 54, 784–795. [Google Scholar] [CrossRef]
- Terré, R.; Mearin, F. A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. Eur. J. Neurol. 2015, 22, 687-e44. [Google Scholar] [CrossRef]
- Simonelli, M.; Ruoppolo, G.; Iosa, M.; Morone, G.; Fusco, A.; Grasso, M.G.; Gallo, A.; Paolucci, S. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: A pilot randomized controlled trial. NeuroRehabilitation 2019, 44, 103–110. [Google Scholar] [CrossRef]
- Kushner, D.S.; Peters, K.; Eroglu, S.T.; Perless-Carroll, M.; Johnson-Greene, D. Neuromuscular electrical stimulation efficacy in acute stroke feeding tube-dependent dysphagia during inpatient rehabilitation. Am. J. Phys. Med. Rehabil. 2013, 92, 486–495. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, B.H.; Park, Y.H. Analysis of Dysphagia Patterns Using a Modified Barium Swallowing Test Following Treatment of Head and Neck Cancer. Yonsei Med. J. 2015, 56, 1221–1226. [Google Scholar] [CrossRef]
- Cakmak, T.E.; Sen, E.I.; Doruk, C.; Sen., C.; Sezikli, S.; Yaliman, A. The Effects of Neuromuscular Electrical Stimulation on Swallowing Functions in Post-stroke Dysphagia: A Randomized Controlled Trial. Dysphagia 2023, 38, 874–885. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Qiao, J.; Song, G.; Xu, Y.; Zhang, Y.; Xu, D.; Gao, W.; Li, Y.; Xu, C. Effects of transcutaneous neuromuscular electrical stimulation on swallowing disorders: A systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 701–711. [Google Scholar] [CrossRef]
- Schwemmle, C.; Arens, C. Feeding, eating, and swallowing disorders in infants and children: An overview. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 151–158. [Google Scholar]
- Florie, M.G.M.H.; Pilz, W.; Dijkman, R.H.; Kremer, B.; Wiersma, A.; Winkens, B.; Baijens, L.W.J. The Effect of Cranial Nerve Stimulation on Swallowing: A Systematic Review. Dysphagia 2020, 36, 216–230. [Google Scholar] [CrossRef]
- Kilinc, D.D.; Mansiz, D. Myofunctional orofacial examination tests: A literature review. J. Orofacial Res. 2023, 12, 201–215. [Google Scholar] [CrossRef]
- Hennessy, M.; Goldenberg, D. Surgical anatomy and physiology of swallowing. Operative Tech. Otolaryngol. Head. Neck Surg. 2016, 27, 60–66. [Google Scholar] [CrossRef]
- Costa, M.M.B. Neural control of swallowing. Arq. Gastroenterol. 2018, 55, 61–75. [Google Scholar] [CrossRef]
- Feher, J. Mouth and Esophagus. In Quantitative Human Physiology, 2nd ed.; Feher, J., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 771–784. [Google Scholar]
- Goday, P.S.; Huh, S.Y.; Silverman, A.; Lukens, C.T.; Dodrill, P.; Cohen, S.C.; Delaney, A.L.; Feuling, M.B.; Noel, R.J.; Gisel, E.; et al. Pediatric Feeding Disorder: Consensus Definition and Conceptual Framework. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 124–129. [Google Scholar] [CrossRef]
- Mari, A.; Sweis, R. Assessment and management of dysphagia and achalasia. Clin. Med. 2021, 21, 119–123. [Google Scholar] [CrossRef]
- Russo, G.; Strisciugli, C. Dysphagia: A practical approach. Glob. Pediatr. 2024, 7, 100136. [Google Scholar] [CrossRef]
- Weir, K.; McMahon, S.; Barry, L. Clinical signs and symptoms of oropharyngeal aspiration and dysphagia in children. Eur. Res. J. 2009, 33, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.J.; Shin, J.H.; Park, E.S. Effects of Neuromuscular Electrical Stimulation on the Swallowing Function in a Child—A Case Report. J. Korean Dysphagia Soc. 2018, 2, 110–116. [Google Scholar] [CrossRef]
- Winnicka, E. Therapy of dysphagia using electrostimulation: Experience in pediatric population. Gastroenterol. Klin. 2019, 15, 85–92. [Google Scholar]
- Hewetson, R.; Singh, S. The lived experience of mothers of children with chronic feeding and/or swallowing difficulties. Dysphagia 2009, 24, 322–332. [Google Scholar] [CrossRef]
- Arslan, S.S.; Soyer, T.; Demir, N.; Yalcın, S.; Karaduman, A.; Karnak, I.; Tanyel, F.C. Effect of swallowing rehabilitation protocol on swallowing function in patients with esophageal atresia and/or tracheoesophageal fistula. Eur. J. Pediatr. Surg. 2017, 27, 526–532. [Google Scholar] [CrossRef]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef]
- Dziewas, R.; Beck, A.M.; Clave, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.E.; Leischker, A.; Martino, R.; Pluschinski, P.; Roester, A.; et al. Recognizing the importance of dysphagia: Stumbling blocks and stepping stones in the twenty-first century. Dysphagia 2017, 32, 78–82. [Google Scholar] [CrossRef]
- Todorović, J.; Zelić, M.; Jerkić, L. Eating and Swallowing Disorders in Children with Cleft Lip and/or Palate. Acta Fac. Med. Naiss. 2022, 39, 5–13. [Google Scholar] [CrossRef]
- Dharmaraj, R.; Elmaoued, R.; Alkhouri, R.; Vohra, P.; Castillo, R.O. Evaluation and Management of Pediatric Feeding Disorder. Gastrointest. Disord. 2023, 5, 75–86. [Google Scholar] [CrossRef]
- Lanzoni, G.; Sembenini, C.; Gastaldo, S.; Leonardi, L.; Bentivoglio, V.P.; Faggian, G.; Bosa, L.; Gaio, P.; Cananzi, M. Esophageal Dysphagia in Children: State of the Art and Proposal for a Symptom-Based Diagnostic Approach. Front. Pediatr. 2022, 10, 885308. [Google Scholar] [CrossRef] [PubMed]
- Zaher, E.A.; Patel, P.; Atia, G.; Sigdel, S. Distal Esophageal Spasm: An Updated Review. Cureus 2023, 15, e41504. [Google Scholar] [CrossRef] [PubMed]
- Sirinawasatien, A.; Sakulthongthawin, P. Manometrically jackhammer esophagus with fluoroscopically/endoscopically distal esophageal spasm: A case report. BMC Gastroenterol. 2021, 21, 222. [Google Scholar] [CrossRef]
- Garcia, D.J.; Nashwan, A.J.; Al-Ansari, A.N. Congenital and Iatrogenic Esophageal Diverticula in Infants and Children: A Case Series of Four Patients. Cureus 2024, 16, e68806. [Google Scholar] [CrossRef]
- Young, E.; Philpott, H. Pathophysiology of Dysphagia in Eosinophilic Esophagitis: Causes, Consequences, and Management. Dig. Dis. Sci. 2022, 67, 1101–1115. [Google Scholar] [CrossRef]
- Peng, D.; Tan, Y.; Li, C.; Lv, L.; Zhu, H.; Liang, C.; Li, R.; Liu, D. Peroral Endoscopic Myotomy for Pediatric Achalasia: A Retrospective Analysis of 21 Cases With a Minimum Follow-Up of 5 Years. Front. Pediatr. 2022, 10, 845103. [Google Scholar] [CrossRef]
- Ortiz Pérez, P.; Valero-Arredondo, I.; Torcuato-Rubio, E.; Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. Nutritional Issues in Children with Dysphagia. Nutrients 2024, 16, 1590. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Cherubini, A.; Keller, H.; Lam, P.; Rolland, Y.; Simmons, S.F. Texture-Modified Diet for Improving the Management of Oropharyngeal Dysphagia in Nursing Home Residents: An Expert Review. J. Nutr. Health Age 2020, 24, 576–581. [Google Scholar] [CrossRef]
- Lazarus, C.L. History of the use and impact of compensatory strategies in management of swallowing disorders. Dysphagia 2017, 32, 3–10. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cucinotta, U.; Alibrandi, A.; Laganà, F.; Ramistella, V.; Romano, C. Early Tube Feeding Improves Nutritional Outcomes in Children with Neurological Disabilities: A Retrospective Cohort Study. Nutrients 2023, 15, 2875. [Google Scholar] [CrossRef]
- Miller, S.; Peters, K.; Ptok, M. Review of the effectiveness of neuromuscular electrical stimulation in the treatment of dysphagia—An update. J. Clin. Rehabil. 2022, 36, 455–466. [Google Scholar]
- Frost, J.; Robinson, H.F.; Hibberd, J. A comparison of neuromuscular electrical stimulation and traditional therapy, versus traditional therapy in patients with longstanding dysphagia. Curr. Oppin. Otolaryngol. Head Neck Surg. 2018, 26, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Jang, J.; Jang, E.G.; Park, Y.; Lee, S.Y.; Kim, B.R.; Park, D.; Park, S.; Hwang, H.; Kim, N.H.; et al. Clinical effectiveness of the sequential 4-channel NMES compared with that of the conventional 2-channel NMES for the treatment of dysphagia in a prospective double-blind randomized controlled study. J. Neuroeng. Rehabil. 2021, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Pechkham, P. Principles of electrical stimulation, Top. Spinal Cord. Inj. Rehabil. 1999, 5, 1–5. [Google Scholar] [CrossRef]
- Ryu, J.S.; Kang, J.Y.; Park, J.Y.; Nam, S.Y.; Choi, S.H.; Roh, J.L.; Kim, S.Y.; Choi, K.H. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral. Oncol. 2009, 45, 665–668. [Google Scholar] [CrossRef]
- Bengisu, S.; Demir, N.; Krespi, Y. Effectiveness of Conventional Dysphagia Therapy (CDT), Neuromuscular Electrical Stimulation (NMES), and Transcranial Direct Current Stimulation (tDCS) in Acute Post-Stroke Dysphagia: A Comparative Evaluation. Dysphagia 2024, 39, 77–91. [Google Scholar] [CrossRef]
- Guimarães, B.T.L.; Lepri, J.R. Możliwości zastosowania elektrostymulacji w motoryce twarzowo-ustnej. J. Orofac. Ther. 2024, 18, 45–53. [Google Scholar]
- Clark, H.; Lazarus, C.; Arvedson, J.; Schooling, T.; Frymark, T. Evidence-based systematic review: Effects of neuromuscular electrical stimulation on swallowing and neural activation. Am. J. Speech Lang. Pathol. 2009, 18, 361–375. [Google Scholar] [CrossRef]
- Eimoto, K.; Nagai, K.; Nakao, Y.; Uchiyama, Y.; Domen, K. Swallowing Rehabilitation With Neuromuscular Electrical Stimulation for Sarcopenic Dysphagia:A Case Report. Cureus 2024, 16, e59256. [Google Scholar]
- Elluru, R. Contemporary Trends in the Diagnosis and Management of Pediatric Dysphagia. Curr. Treat. Opt. Pediatr. 2024, 10, 295–300. [Google Scholar] [CrossRef]
- Carnaby-Mann, G.D.; Crary, M.A. Examining the evidence on neuromuscular electrical stimulation for swallowing. Arch. Otolaryngol. Head. Neck Surg. 2007, 133, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Liu, T.Y.; Huang, Y.C.; Leong, C.P. Functional outcome in acute stroke patients with oropharyngeal dysphagia after swallowing therapy. J. Stroke Cerebrovasc. Dis. 2014, 23, 2547–2553. [Google Scholar] [CrossRef]
- Permsirivanich, W.; Tipchatyotin, S.; Wongchai, M. Comparing the effect of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: A randomized controlled study. J. Med. Ass Thail. 2009, 92, 265. [Google Scholar]
- Blumenfeld, L.; Hahn, Y.; Lepage, A.; Leonard, R. Transcutaneous electrical stimulation versus traditional dysphagia therapy: A nonconcurrent cohort study. Otolaryngol. Head Neck Surg. 2006, 135, 754–757. [Google Scholar] [CrossRef]
- Pownall, S.; Enderby, P.; Sproson, L. Electrical stimulation for the treatment of dysphagia. In Electroceuts; Springer: Cham, Switzerland, 2017; pp. 137–156. [Google Scholar]
- Lv, J.; Zhu, M.; Zhang, Y. Effects of different intensity neuromuscular electrical stimulation on dysphagia in children with cerebral palsy. Chin. J. Rehabil. Med. 2019, 34, 159–164. [Google Scholar]
- El-Sheikh, A.; El-Tohamy, A.; Abd El-Aziz, B. Neuromuscular electrical stimulation therapy on controlling dysphagia in spastic cerebral palsy: A randomized controlled clinical trial. Polish J. Physiother. 2020, 20, 194–198. [Google Scholar]
- Ma, S.R.; Choi, J.B. Effect of electrical stimulation on aspiration in children with cerebral palsy and dysphagia. J. Phys. Ther. Sci. 2019, 31, 93–94. [Google Scholar] [CrossRef]
- Gao, S.; Gao, D.; Su, N.; Ge, K.J.; Liu, Y.M. Effect of acupuncture at proximal and distal acupoints combined with neuromuscular electrical stimulation on children with cerebral palsy salivation. Chin. Acupunct. Moxibustion 2018, 38, 825–830. [Google Scholar]
- Rice, K.L. Neuromuscular electrical stimulation in the early intervention population: A series of five case studies. Int. J. Allied Heal. Sci. Pract. 2012, 10, 9. [Google Scholar] [CrossRef]
- Propp, K.; Jiang, H.; Thoyre, S. Neuromuscular electrical stimulation for children with dysphagia: A systematic review. Phys. Occup. Ther. Pediatr. 2022, 42, 123–140. [Google Scholar] [CrossRef]
- Marcus, S.; Friedman, J.N.; Lacombe-Duncan, A.; Mahant, S. Neuromuscular electrical stimulation to treat dysphagia in infants and young children with neurological impairment. Clin. Pediatr. 2019, 58, 150–156. [Google Scholar]
- Andreoli, M.T.; Langmore, S.E.; Sohn, H. Electrical stimulation therapy for severe dysphagia in medically complex children: A case series. Int. J. Pediatr. Otorhinolaryngol. 2019, 124, 117–122. [Google Scholar]
- Winnicka, K.; Kowalska, K.; Szczepańska, J.; Mańczak, M. Improvement of swallowing function due to neuromuscular electrical stimulation in children with primary dysphagia. Polish J. Pediatr. 2024, 99, 54–61. [Google Scholar] [CrossRef]
- Christiaanse, M.E.; Mabe, B.; Russell, G. Evaluation of the use of neuromuscular electrical stimulation in children with dysphagia. Pediatr. Pulmonol. 2011, 46, 1031–1035. [Google Scholar] [CrossRef]
- Ma, L.; Choi, M. Neuromuscular electrical stimulation for children with dysphagia: A case study. Korean J. Pediatr. 2019, 62, 377–381. [Google Scholar]
- Song, W.J.; Park, J.H.; Lee, J.H.; Kim, M.Y. The effect of neuromuscular electrical stimulation on swallowing function in children with cerebral palsy: A randomized controlled trial. Hong Kong J. Occup. Therap 2015, 25, 30–35. [Google Scholar] [CrossRef]
- Freed, M.L.; Freed, L.; Chatburn, R.L.; Christian, M. Electrical stimulation for swallowing disorders caused by stroke. Dysphagia 2001, 16, 171–177. [Google Scholar]
- Ekberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Humbert, I.; Saxon, K.; Poletto, C.; Sonies, B.; Crujido, L. Effects of surface electrical stimulation during swallowing in chronic pharyngeal dysphagia. Dysphagia 2007, 22, 1–10. [Google Scholar] [CrossRef]
- Blumenfeld, L.; Hahn, Y.; Lepage, A.; Leonard, R. Transcutaneous neuromuscular electrical stimulation for dysphagia: A review of the literature. Otolaryngol. Head Neck Surg. 2006, 135, 754–757. [Google Scholar] [CrossRef]
- Guo, X.; Mu, H.; Sun, Y.; Wang, J.; Wei, J. Analysis of the Improvement Effect of Combined Application of Oral Rehabilitation Training and Neuromuscular Electrical Stimulation on Pediatric Swallowing Disorders. Int. J. Neurosci. 2024, 1–7, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, K.; Rycyk, A.; Lebensztejn, D.M.; Daniluk, U. Dysphagia Among Children—A Single-Center Experience. J. Clin. Med. 2025, 14, 2906. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.L.; Parker, K. Neuromuscular Electrical Stimulation—A Practical Guide; Los Amigos Research and Education Institute: Downey, CA, USA, 2006. [Google Scholar]
- Petrofsky, J.S. Electrical stimulation: Neurophysiological basis and application. Crit. Rev. Biomed. Eng. 2001, 29, 59–124. [Google Scholar]
- Chow, T.; Boutet, A.; Loh, A.; Germann, J.; Elias, G.J.B.; Hutchison, W.D.; Lozano, A.M. Kilohertz-frequency stimulation of the nervous system: A review of underlying mechanisms. Brain Funct. 2021, 14, 513–530. [Google Scholar]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Robinson, A.J.; Snyder-Mackler, L. Clinical Electrophysiology: Electrotherapy and Electrophysiologic Testing, 3rd ed.; Lippincott Williams & Wilkins: Baltimore, PA, USA, 2008. [Google Scholar]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef]
- Sillen, M.J.H.; Franssen, F.M.E.; Gosker, H.R.; Wouters, E.F.M.; Spruit, M.A. Metabolic and Structural Changes in Lower-Limb Skeletal Muscle Following Neuromuscular Electrical Stimulation: A Systematic Review. PLoS ONE 2013, 9, e69391. [Google Scholar] [CrossRef]
- Lake, D.A. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med. 1992, 13, 320–336. [Google Scholar] [CrossRef]
- Ward, A.R. Electrical stimulation using kilohertz-frequency alternating current. Phys. Ther. Rev. 2009, 89, 181–190. [Google Scholar] [CrossRef]
- Bax, L.; Staes, F.; Verhagen, A. Does Neuromuscular Electrical Stimulation Strengthen the Quadriceps Femoris? Sports Med. 2005, 35, 191–212. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Gondin, J.; Place, N.; Stevens-Lapsley, J.; Vivodtzev, I.; Minetto, M.A. Clinical Use of Neuromuscular Electrical Stimulation for Neuromuscular Rehabilitation: What Are We Overlooking? Arch. Phys. Med. Rehabil. 2018, 99, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.R.; Shkuratova, N. Russian electrical stimulation: The early experiments. Phys. Ther. 2002, 82, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Oh, D.H.; Chang, M.Y. Effects of sensory stimulation using interferential current on swallowing function in children with cerebral palsy: A pilot study. Ann. Rehabil. Med. 2017, 41, 828–835. [Google Scholar]
- Furuta, T.; Takemura, M.; Tsujita, J.; Oku, Y. Interferential electric stimulation applied to the neck increases swallowing frequency. Dysphagia 2012, 27, 94–100. [Google Scholar] [CrossRef]
- Assoratgoon, I.; Shiraishi, N.; Tagaino, R.; Ogawa, T.; Sasaki, K. Sensory neuromuscular electrical stimulation for dysphagia rehabilitation: A literature review. J. Oral Rehabil. 2022, 50, 157–164. [Google Scholar] [CrossRef]
- Ebdah, S. Neuromuscular Electrical Stimulation for Dysphagia Treatment: Adoption, Perceived Barriers, and Clinical Practices. Am. J. Speech Lang. Pathol. 2024, 33, 2839–2854. [Google Scholar] [CrossRef]
- Shune, S.; Moon, J.B. Neuromuscular Electrical Stimulation in Dysphagia Management: Clinician Use and Perceived Barriers. Contemp. Issues Commun. Sci. Disord. 2012, 39, 55–68. [Google Scholar] [CrossRef]
- Chang, M.C.; Park, J.S.; Lee, B.J.; Park, D. Effectiveness of pharmacologic treatment for dysphagia in Parkinson’s disease: A narrative review. Neurol. Sci. 2020, 42, 513–519. [Google Scholar] [CrossRef]
- Morgan, T.A.; Dodrill, P.; Ward, E.C. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst. Rev. 2012, 10, CD009456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).