Abstract
Pressure injuries (PIs) are typically characterized by lesions of the dermis and subcutaneous tissue; they result from a downward force exerted on the area between a bony prominence and an external surface. Transcutaneous oxygen pressure (TcPO2) measurements are a standardized method for measuring tissue oxygen pressure. Standardized TcPO2 measurement data are lacking in regions susceptible to pressure injury. It is unclear whether the absence of data can be attributed to the unreliability and variability of the measurements obtained. This study aimed to assess the reliability and repeatability of TcPO2 measurements conducted using a fluorescence-sensitive photo-optical sensor at three sites on the foot where PIs can occur but for which no data are available. Thirty volunteers participated in this study. TcPO2 was measured in two sessions, one week apart, at the lateral of the fifth metatarsal head, the styloid process, and the lateral malleolus of both feet. TcPO2 (mmHg) exhibited moderate reliability for the left fifth metatarsal and right fifth metatarsal styloid process (ICC: 0.575 and 0.624, respectively). The right fifth metatarsal and the right and left lateral malleoli demonstrated good and excellent reliability of the styloid process of the left fifth metatarsal, with ICC values of 0.810, 0.816, 0.763, and 0.900, respectively. The implementation of a fluorescence-sensitive photo-optical sensor for the measurement of TcPO2 in the designated regions yielded reliable and reproducible measurements.
1. Introduction
Pressure ulcers, currently referred to as pressure injuries (PIs) according to the terminology adopted in 2016 by the National Pressure Ulcer Advisory Panel, encompass all forms of tissue damage and underlying soft tissue over a bony prominence caused by prolonged pressure and shear forces, including the stage prior to skin rupture [1,2].
It is important to note that such injuries are a frequent complication in cases of prolonged hospitalization, as well as day centers and geriatric hospitals or homes, particularly in patients with reduced mobility and among the elderly. While such injuries are not generally considered to be a cause of death during hospitalization, they are associated with a number of other complications during recovery, including an increased risk of infection, insufficient nutrition, prolonged hospitalization, and increased workload and medical expenses [3,4,5].
In Europe, the prevalence of PIs among hospitalized adults has been estimated to be approximately 12.8%, with an incidence of 5.4 per 10,000 patients [6].
In Spain, the Sixth National Study conducted by the National Group for the Study of and Advice on Pressure Ulcers and Chronic Wounds found a prevalence of hospital-acquired PIs of 7.7%, a figure that rises to 6.05% in nursing homes and social healthcare centers [7,8].
Prolonged pressure on the PI region is typically characterized by injury to the skin and subcutaneous tissue, resulting from the downward force exerted on the area between a bony prominence and an external surface, such as a mattress or wheelchair cushion. Sustained pressure exerted by medical devices has also been identified as a contributing factor. The prolonged application of pressure on the affected area can result in the occlusion of blood vessels, leading to tissue damage due to a decrease in the supply of oxygen and nutrients to the tissue [9].
The impact of pressure on anatomical sites that are prone to PIs, as well as its effect on tissue viability, has been the subject of extensive research. The effect on tissue varies according to anatomical location, with a more significant impact observed in the heel and ankle regions when a 50 mmHg increase in pressure is recorded, as opposed to the sacrum and trochanter, where an increase of 140 mmHg is required to elicit a comparable effect. This finding underscores the notion that an alteration in cutaneous blood flow, in conjunction with the tissue ischemia that it engenders due to a decline in tissue perfusion and oxygenation within a designated area, can be modulated by the quantity of tissue separating the surface from the bone, thereby facilitating the dissipation of the load [10].
Transcutaneous oxygen pressure (TcPO2) measurements are a standardized method of measuring oxygen pressure in tissues [11]. This technique has been demonstrated to be effective in assessing microcirculation, and, as such, it can be utilized as a predictive value for the wound healing process [12].
In cases of severe ischemia or diabetes, TcPO2 has been shown to be more sensitive than the ankle brachial index. This is due to its ability to detect changes in blood flow caused by both macrovascular and microvascular disease. The advantage of TcPO2 is that it is a direct indicator of microvascular function, as it measures the oxygen supply available to skin cells without the need to draw arterial blood [13].
The normal lower limb TcPO2 values are approximately 60 mmHg in healthy subjects and remain constant along the limb: 73.8 ± 10.9 mmHg at the thigh, 67 ± 10 mmHg at the dorsal skin of the leg, 70 ± 9 mmHg at the pretibial level, 70.0 ± 12.1 mmHg at the calf, and 69.8 ± 5.3 mmHg at the foot. However, in diseased individuals, a progressive decrease in TcPO2 is observed. The influence of TcPO2 is multifaceted, being subject to factors such as tissue temperature, alterations in the hemoglobin dissociation curve, the degree of tissue oxygen metabolism, the circulatory status, peripheral blood perfusion, local skin conditions, and anatomical factors [11,14].
Electrochemical sensors have been utilized for the measurement of TcPO2 for several decades; however, recent advancements have led to the development of a fluorescence-sensitive photo-optical sensor, which capitalizes on the interactions between light and matter. In comparison with electrochemical TcPO2, this method has the advantages of not requiring oxygen consumption or extraction, and of being less time-consuming for probe preparation, with no need for calibration prior to each measurement [15,16].
While the advantages of TcPO2 measurement in diabetic foot syndrome are well-documented [17], it is noteworthy that the application of the same technique for the study of pressure injuries is typically limited to the sacrum, trochanter, and the lateral side of the calcaneus [10,11]. Conversely, in the context of TcPO2 measurements in the context of diabetic foot syndrome, the sensor is specifically positioned on the dorsal aspect of the foot, between the navicular bone and the medial malleolus [17]. While the lateral aspect of calcaneus has been shown to coincide with regions of pressure injury occurrence, no comparable data are available from other areas of comparable frequency: styloid process and the fifth metatarsal head and the lateral malleolus [18,19]. It is not certain whether data are lacking in these areas because the application of fluorescence-sensitive photo-optical sensors in the described anatomical locations of the foot does not provide reliable and repeatable data.
In view of the aforementioned points, the primary objective of this study was to assess the reliability and repeatability of TcPO2 measurements using a fluorescence-sensitive photo-optical sensor at three sites that are susceptible to foot PIs for which no data were available: the styloid process and head of the fifth metatarsal and the lateral malleolus.
2. Materials and Methods
2.1. Study Design
The present study employed a measurement design to ascertain the reliability of TcPO2 measurements using a fluorescence-sensitive photo-optical sensor. We estimated this variable at three specific anatomical sites: the styloid process, the head of the fifth metatarsal, and the lateral malleolus.
The participants were present for two days while undergoing the measurements. On the first day, the anthropometric measurements were recorded, and the initial measurement was conducted. In the absence of analogous studies, and given the substantial variability found in the test-retest studies consulted [20,21,22,23,24,25,26], a period of one week between each measurement was selected. This period corresponds to the time that should be established in future fieldwork with patients at risk of developing pressure injuries. These evaluations were conducted in a designated practice room at the University of Valencia. The temperature of the room was recorded at 24 °C ± 3 °C.
2.2. Participants
The sample calculation for obtaining the interclass correlation coefficient (ICC) was conducted with the following characteristics being taken into account: a power of 0.90, a minimum acceptable reliability of 0.07, and an expected reliability of 0.90 (excellent reliability) [27]. The alpha value was set at 0.05. The total required number of participants was 25 [28]. However, a sample of 30 volunteers was utilized, which aligns with the typical sample size in other test–retest studies that measure TcPO2 [20,21,22,23,24,25,26].
A total of 30 volunteers participated in this study, during which basic anthropometric data were collected for the purpose of describing the sample. The specific data collected included sex, age, weight in kilograms (kg), height in centimeters (cm), and body mass index (BMI). These measurements are given in Table 1.

Table 1.
Sample description (mean and standard deviation).
All subjects met the established inclusion criteria: they were over 40 years of age, their BMI was within a healthy range or slightly overweight, they had no history of cardiovascular disease, no ulcers, no foot surgery in the six months prior to the study, and were taking no medication that affects peripheral circulation. The objectives and characteristics of this study were explained to all participants, who provided their informed consent in writing. This study was approved by the Ethics Committee of the University of Valencia (2024-ENFPOD-3198972).
2.3. Materials
The device utilized for this study was the MediCap© Précise 8001® (Ulrichstein, Germany). This compact, portable device is intended for ambulatory use and is designed to measure TcPO2 in the skin. This monitor is based on the principle of optical fluorescence and consists of a sensor that produces local hyperemia (between 43 °C and 44 °C) to generate vasodilation in the area, thereby facilitating greater oxygen diffusion. This sensor does not require recalibration prior to each utilization. It is capable of recording values ranging from 0 to 2000 mmHg with a margin of error of ±10%, providing valid results at ambient temperatures ranging from 15 to 35 °C and at relative air humidity levels ranging from 10 to 95%. A noteworthy advantage of the device lies in its capacity to adjust the sensor temperature between 44 and 40 °C [29]. Figure 1 and Figure 2 illustrate the device and offer a detailed view of its sensor, respectively.
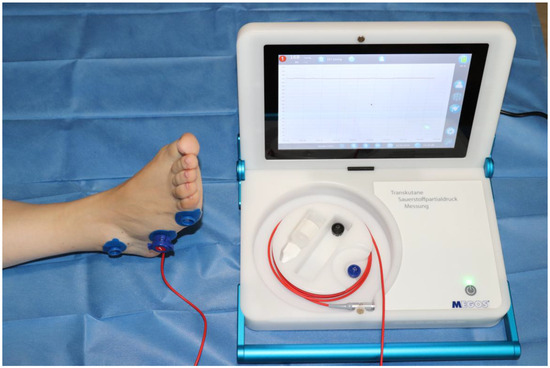
Figure 1.
MediCap© Précise 8001® device.
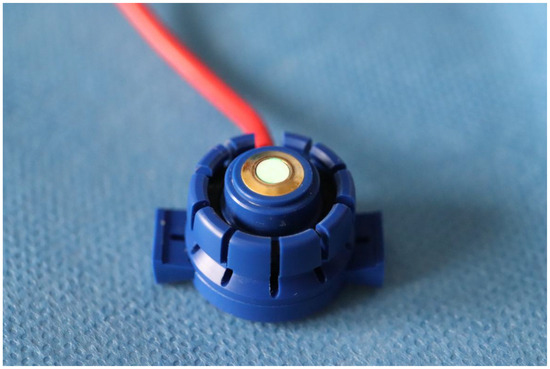
Figure 2.
Sensor detail.
2.4. Procedure
The volunteers underwent a non-invasive local procedure to evaluate TcPO2 at three specific anatomical points: the styloid process, the head of the fifth metatarsal, and the lateral malleolus of both feet. As illustrated in Figure 3, the three anatomical points are marked with sensor application rings.
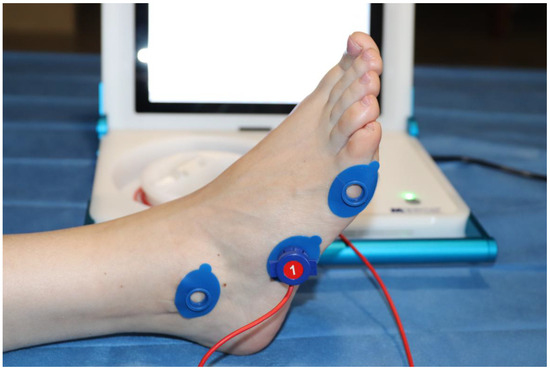
Figure 3.
The three specific anatomical points.
In each session, the subject of study was placed in a supine position on a stretcher in the room where this study was conducted. Prior to measurement, the measurement area was disinfected with alcohol. Then, the probe was firmly attached to the fixing ring, and the contact liquid supplied by the manufacturer was applied. Subjects were advised to abstain from caffeine and tobacco consumption for a period of two hours prior to the measurements [14].
This study’s primary variable was the TcPO2 level in the lateral of the fifth metatarsal head, the styloid process, and the lateral malleolus of both feet. The measurements were obtained in a progressive manner, starting from the distal end and working towards the proximal end. First, we took all measurements of the right foot, then proceeded to measure the left foot. Two sessions were conducted, with an interval of one week between them (Figure 4). For each anatomical point, a measurement was recorded in each session.
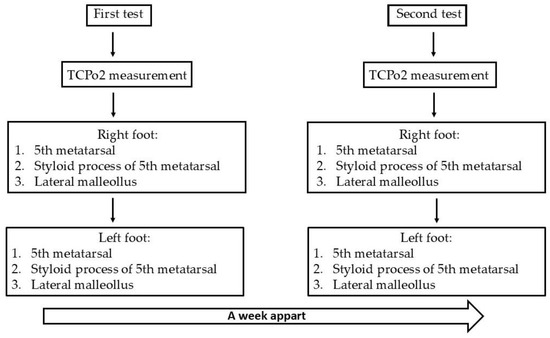
Figure 4.
Diagram of the testing method.
2.5. Statistical Analysis
The statistical analysis was conducted using SPSS® 25.0.2.0 (IBM®, Chicago, IL, USA). The descriptive statistics collected were reported using means and standard deviations (SD). The Shapiro–Wilk statistical model was employed to analyze the difference in paired data, with a p-value greater than 0.05. The Student’s t-test was employed in two key ways: first, to illustrate the presence of statistically significant differences between the results obtained from paired samples, and second, to calculate the effect size (ES) of the standardized mean differences in the repeated samples. The following criterion was used to interpret the ES: the scale ranges from 2.00 (very large), 1.20–2.00 (large), 0.60–1.19 (moderate), 0.2–0.59 (small), and <0.20 (null) [30].
The standard error of measurement (SEM) was utilized to assess absolute reliability. The interclass correlation coefficient (ICC) was employed to evaluate relative reliability in accordance with the following criteria: CV ≤ 5% indicates high reliability, while CV 5.1 10% indicates acceptable reliability. The coefficient of variation (CV) was also assessed [31]. The relative reliability (ICC) was classified according to the following criteria: values 0.90–1.00 were designated as “excellent reliability”; 0.89–0.75 represent “good”; 0.74–0.5 are “average”; and values ≤ 0.49 are “low” [32]. The presence of systematic bias was quantified using Bland–Altman plots, which established 95% agreement limits between the test and retest results. A coefficient of determination of R2 > 0.1 was defined for the interpretation of the heteroscedasticity of errors in the Bland–Altman plots [33].
Pearson’s correlation coefficient was employed to calculate the correlation of all TcPO2 measurements, and the magnitude of “r” was interpreted according to the following criteria: the scale ranges from 1.00, representing “perfect”, to 0.00, representing “null”. Scores falling between 0.70 and 0.89 are classified as “very large”, those between 0.50 and 0.69 as “large”, those between 0.30 and 0.49 as “moderate”, those between 0.10 and 0.29 as “small”, and those below 0.09 as “null” [34].
The standard error of measurement, coefficient of variation, standard error of bias, and the requisite limits for the Bland–Altman graphs were calculated using a spreadsheet. The remainder of the calculations and graphic representations were conducted utilizing SPSS 25 software.
3. Results
As demonstrated in Table 2, the variables examined during the initial session exhibited higher values in the right foot than in the left. It was noted that the TcPO2 of the right fifth metatarsal was elevated relative to the left (100.62 ± 49.8 mmHg vs. 96.52 ± 51.19 mmHg). The discrepancy between the styloid process and the right and left malleoli was less pronounced (styloid process: 96.24 ± 38.14 mmHg vs. 96.38 ± 41.39 mmHg. lateral malleolus: 82.07 ± 36.43 mmHg vs. 82.69 ± 37.70 mmHg).

Table 2.
The following descriptives of TcPO2 for the various anatomical locations are expressed as both the mean and standard deviation.
The values at the styloid process in the second session exhibited a similar trend to those in the first session, with higher TcPO2 levels recorded in the right foot (right vs. left: 96.93 ± 43.94 mmHg vs. 91.41 ± 37.32 mmHg). In contrast, the remaining anatomical points exhibited divergent behaviors, with elevated values recorded in the left foot (right vs. left of fifth metatarsal head: 93.38 ± 44.23 mmHg vs. 106.14 ± 52.60 mmHg. lateral malleolus: 74.10 ± 32.72 mmHg vs. 81.69 ± 39.59 mmHg).
The data presented in Table 3 indicate the mean duration of the measurements.

Table 3.
The mean and standard deviation (SD) of the measurement time in minutes per anatomical area are presented.
In order to assess the repeatability and reliability of the data obtained with the measuring device, the intraclass correlation coefficients, coefficient of variation (CV), standard error of measurement (SEM), and standard error of the estimate (SE) were calculated, yielding the results detailed below.
No significant differences were identified (p > 0.05), and substantial ES values (ES > 2.00) were observed in nearly all measurements with the exception of the styloid process of the right fifth metatarsal and the left malleolus, where moderate values (0.60–1.19) were recorded for TCPO2 levels in both feet. In accordance with the test–retest reliability measurements undertaken, it was determined that there is an acceptable level of absolute reliability (CV ≤ 10%) and that the results show an ICC ranging from 0.575 to 0.900. As demonstrated in Table 4, the measurements obtained from the right styloid process and the head of the left fifth metatarsal yielded an ICC ranging from 0.575 to 0.624, signifying a moderate degree of reliability. In the other anatomical regions, the ICC values ranged from 0.763 to 0.816, indicating that the degree of reliability was good, with the styloid process of the left foot being the sole point that exhibited an excellent reliability (ICC = 0.900).

Table 4.
Evaluation of the reliability and repeatability of the MediCap Précise 8001® TcPO2 monitor at different anatomical points on the foot.
The Bland–Altman graphs reveal a systematic bias low (−9.62; 7.96 mmHg) for the evaluation of TCPO2 in the fifth metatarsal, the styloid process and malleolar values in both the right and left feet, as well as a coefficient of determination of R2 = 0.01; 0.032, as shown in Figure 5.
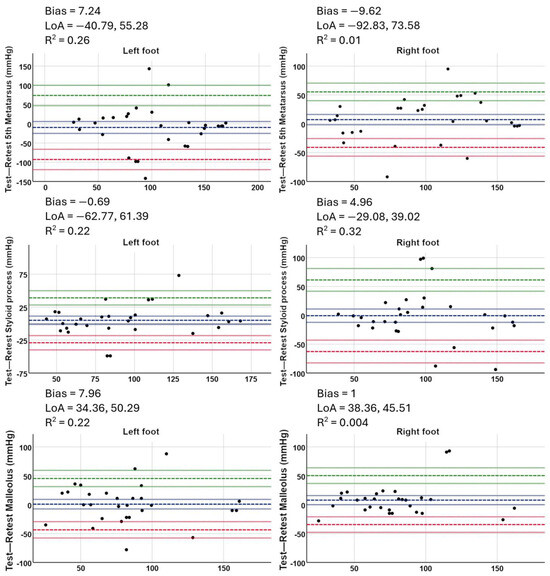
Figure 5.
Bland–Altman test–retest plots for each of the anatomical points (right and left foot) in both test sessions using a fluorescence-sensitive photo-optical sensor.
The magnitude r was very large for the left apophysis, large for the right fifth metatarsal and right and left malleolus, and moderate for the left fifth metatarsal and right apophysis (Figure 6).
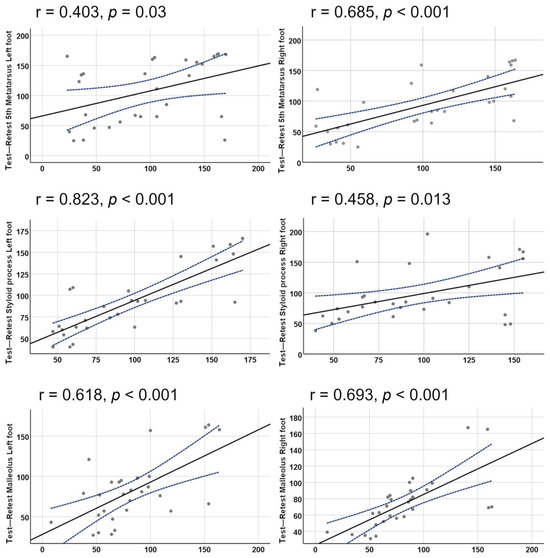
Figure 6.
Relationship between TCPO2 for each anatomical point, with a distinction between right and left legs. The small circles are the TcPO2 (mmHg), the central line is the graph of the Pearson correlation model, and the blue dashed lines are the 95% confidence interval of the model provided by the IBM SPPS© statistical package.
4. Discussion
The primary objective of this study was to assess the reliability and repeatability of a fluorescence-sensitive photo-optical sensor in measuring TcPO2 at sites prone to PIs in the foot. For the right styloid process and the left fifth metatarsal, the ICCs exhibited moderate reliability, with an excellent value for the left styloid process and a satisfactory value for the remaining anatomical regions.
The measurement of PO2 by fluorescence in the field of biology is not a novel concept [35,36]; however, the application of a photo-optical sensor that is sensitive to fluorescence for the measurement of TcPO2 in the clinic is a relatively recent development. The first relevant reference we found is the study carried out by Urban et al. in 2015 [37]. This study examined the concordance between photo-optical and electrochemical TcPO2, at rest and during exercise, in patients with claudication. Subsequently, the research group led by Bernard S. Leenstra published three studies using photo-optical TcPO2 to predict the duration of diabetic foot ulcers [15], in order to study the application in measurements of revascularized patients [38], and for real-time measurements during activity in patients with peripheral arterial disease compared to an electrochemical sensor [16]. All of the articles referenced employed the same photo-optical sensor that has been marketed by MediCap©, which was also utilized in this study. Despite the evident dissimilarity in objectives and rationales underlying the aforementioned studies and the present one, it is noteworthy that two of the studies used a control probe in the subclavicular region when conducting measurements on the lower extremities [16,38]. The feasibility of this endeavor was enabled by the utilization of a MediCap© 8008 multichannel model in both studies. In our specific case, the MediCap© 8001 single-channel model was employed; it lacks the capacity for simultaneous monitoring. Nevertheless, the application of the control probe is constrained to the diagnosis of lower limb arterial disease and remains inadequately substantiated by the extant literature [11].
A review of the extant literature reveals a paucity of studies measuring TCpO2 in the context of PIs. Most of these studies focus on performing assessments of the sacrum, trochanter, and calcaneus [39,40,41,42,43], but we did not find any reference to the points evaluated in our study. In an effort to locate more comprehensive studies of the foot, a search was conducted within the domain of diabetic foot research, where there is substantial expertise in the assessment of TcPO2 [17,44,45,46,47,48]. Indeed, the measurement of TcP02 was shown to be an effective predictor of the healing of diabetic foot ulcers, with a threshold of 25 mmHg. Higher values were associated with an increased probability of healing, while lower values were linked to a higher probability of major amputation [45]. It is important to reiterate the absence of coincidences in the positioning of the sensors. In diabetic feet, sensors are applied on both the dorsal aspect of the foot, between the first and second metatarsal bones, and on the plantar surface, between the navicular bone and the medial malleolus, with consideration given to the angiosome pattern [17,47].
A preliminary analysis of the study outcomes reveals that the documented values exceed the established benchmarks for healthy skin beneath the knee, which are typically close to 70 mmHg [43,49]. In comparison, the following results were obtained. In the initial session, the mean blood pressure was recorded at 100.62 ± 49.80 mmHg at the right fifth metatarsal and 96.24 ± 38.14 mmHg at the right styloid process. In the subsequent session, the mean blood pressure was recorded at 106.14 ± 52.60 mmHg at the left fifth metatarsal and 96.93 ± 43.94 mmHg at the right styloid process. These values can be explained, first and foremost, in the thickness of the skin at the analyzed points. In their study, Falstie-Jensen et al. measured TcPO2 levels and epidermis thickness in different regions of the body. Their findings demonstrated that, for every micrometer increase in skin thickness, TcPO2 exhibited a decrease of 0.26 mmHg [49]. Given the consideration of the diminished thickness of the skin in the measurement area utilized in this study, the elevated values obtained can be adequately explained. The thickness of the subcutaneous tissue in the malleolus, styloid process, and head of the fifth metatarsal is minimal, and it possesses a thin layer of epidermis. This specific condition would facilitate the diffusion of oxygen through the skin [50]. In the present study, the sensor was positioned directly on the bony prominences. In subsequent research, it would be prudent to affix the sensor around measurement areas to ascertain whether the TcPO2 values diminish and align with the values obtained in other anatomical regions. Second, in the present study, a photo-optical probe was utilized, which, in comparison with electrochemical sensors, has been shown to provide higher TcPO2 values. This is because these photo-optical probes do not consume oxygen during the measurement process [16].
With regard to the CCI, the present study is the first to measure TcPO2 in the styloid process of the fifth metatarsal, the head of the fifth metatarsal, and the lateral malleolus. Consequently, no data exist for comparison. A review of the results of ICC evaluated in other test–retest studies, in which TcPO2 measurements were evaluated with different objectives, reveals that results ranging from moderate to excellent were also obtained [21,22,23,24,26]. Conversely, a recent study sought to ascertain the most efficacious non-invasive measure for diagnosing peripheral arterial disease, with a particular focus on predicting wound healing in revascularized diabetic foot tissues. The investigation revealed that transcutaneous oxygen pressure (TcPO2) and absolute systolic toe pressure emerged as superior indicators of wound healing [44]. These data confirm that measuring TcPO2 is a reliable method for analyzing the skin’s physiological response in various conditions, particularly in the presence of foot wounds.
As shown in Table 2, the time required to obtain the data exhibited variability according to anatomical location. However, the time required to obtain the data was coincident between anatomical locations on both extremities. The duration of the processes required to obtain TcPO2 exhibited a range of variation, with values ranging from 15.16 ± 4.05 min at the right lateral malleolus to 21.40 ± 7.79 min at the right fifth metatarsal head. These values are consistent with the 20 min average reported by studies employing TcPO2 at other anatomical locations [20,21,22,24].
While the findings of this study indicate that the utilized equipment offers reliable measurements for the designated anatomical regions, it is imperative to consider the limitations of this study when interpreting the results. First, this study was conducted on healthy volunteers. This condition was deemed necessary in order to serve as an initial point of reference. However, subsequent investigation is required to ascertain the implications of employing the fluorescence-sensitive photo-optical sensor in clinical practice, with these investigations focusing on a population that exhibits a propensity to develop PIs. Conversely, the CCI exhibited variability between equivalent points in each limb. In the context of this observation, the present study was conducted with a modest sample size, although it remained within the established limits of the sample size calculation. A larger study population could have shown a smaller effect for the limb studied with respect to the analyzed values.
5. Conclusions
The findings of this study demonstrated that the implementation of a fluorescence-sensitive photo-optical sensor for the measurement of TcPO2 in the styloid process of the fifth metatarsal, the head of the fifth metatarsal, and the lateral malleolus yielded reliable and repeatable measurements. It is recommended that the sample size be increased to further refine these results.
Conversely, the results demonstrated elevated TcPO2 levels at the examined anatomical regions in comparison with other areas. It is conceivable that the measurements obtained around the bony prominences could offer more representative values of the oxygen supply in these regions.
In conclusion, the potential implications of this study extend to the domain of clinical practice. This study proposes a novel approach for the utilization of TcPO2 assessment in anatomical regions that exhibit a high risk of developing PIs and have yet to be thoroughly investigated. The findings of this study demonstrate the viability of performing TcPO2 measurements in the designated anatomical regions to facilitate the early detection of ischemia. This advancement is pivotal in the prevention of PIs in patients who present with restricted mobility and vascular diseases. Conversely, it must be noted that the photo-optical TcPO2 is a mobile device that has the advantage of being easily transported. Furthermore, it is less expensive than electrochemical TcPO2. The duration of the measurements ranged from 15 to 20 min. In the case of bedridden patients, the measurement points on the foot are usually accessible without modifying the subject’s posture; therefore, this time does not represent a limitation for TcPO2 measurements. As such, the fluorescence-sensitive photo-optical sensor could offer significant benefits for bedridden patients who are at risk of developing PIs.
The investigation of TCPO2 measurements in susceptible PI areas in the feet is necessary to determine the prognostic capacity of this measurement. This investigation follows previous studies of TCPO2 measurements in diabetic foot ulcers.
Author Contributions
Conceptualization, M.M.-J., M.I.-R. and I.J.-R.; methodology, M.M.-J., M.I.-R. and I.J.-R.; software, M.M.-J. and M.I.-R.; validation, M.M.-J. and M.I.-R.; formal analysis, M.M.-J. and M.I.-R.; investigation, M.M.-J., M.I.-R. and I.J.-R.; resources, M.M.-J. and M.I.-R.; data curation, M.M.-J. and M.I.-R.; writing—original draft preparation, M.M.-J. and M.I.-R.; writing—review and editing, M.M, M.I.-R. and I.J.-R.; visualization, M.M, M.I.-R. and I.J.-R.; supervision, M.I.-R. and I.J.-R.; project administration, M.I.-R. and I.J.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the University of Valencia (2024-ENFPOD-3198972, approval date 12 March 2024).
Informed Consent Statement
Written informed consent was obtained from the patient(s) to publish this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Edsberg, L.E.; Black, J.M.; Goldberg, M.; McNichol, L.; Moore, L.; Sieggreen, M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J. Wound Ostomy Cont. Nurs. 2016, 43, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Mervis, J.S.; Phillips, T.J. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J. Am. Acad. Dermatol. 2019, 81, 881–890. [Google Scholar] [CrossRef]
- Khor, H.M.; Tan, J.; Saedon, N.I.; Kamaruzzaman, S.B.; Chin, A.V.; Poi, P.J.; Tan, M.P. Determinants of mortality among older adults with pressure ulcers. Arch. Gerontol. Geriatr. 2014, 59, 536–541. [Google Scholar] [CrossRef]
- Noie, A.; Jackson, A.C.; Taheri, M.; Sayadi, L.; Bahramnezhad, F. Determining the frequency of pressure ulcers incidence and associated risk factors in critical care patients: A 3-year retrospective study. Int. Wound J. 2024, 21, e70120. [Google Scholar] [CrossRef]
- Xiao, F.; Peng, H.; Li, Y. The preventive effect of seamless nursing care on pressure ulcer and related complications in elderly inpatients. Am. J. Transl. Res. 2021, 13, 3515–3521. [Google Scholar]
- Moore, Z.; Avsar, P.; Conaty, L.; Moore, D.H.; Patton, D.; O’Connor, T. The prevalence of pressure ulcers in Europe, what does the European data tell us: A systematic review. J. Wound Care 2019, 28, 710–719. [Google Scholar] [CrossRef]
- García-Fernández, F.P.; Soldevilla-Agreda, J.J.; Pancorbo-Hidalgo, P.L.; Torra-Bou, J.E.; López-Franco, M.D. Prevalencia de las lesiones cutáneas relacionadas con la dependencia en adultos hospitalizados en España: Resultados del 6.o Estudio Nacional del GNEAUPP 2022. Gerokomos 2023, 34, 250–259. [Google Scholar]
- Torra-Bou, J.E.; Soldevilla-Agreda, J.J.; Pancorbo-Hidalgo, P.L.; López-Franco, M.D.; García-Fernández, F.P. Prevalencia de las lesiones cutáneas relacionadas con la dependencia en residencias de mayores y centros sociosanitarios de España: Resultados del 6.o Estudio Nacional del GNEAUPP 2022. Gerokomos 2023, 34, 269–276. [Google Scholar]
- Dehghani, M.; Pourmontaseri, H. Aetiology, risk factors and treatment of typical and atypical pressure ulcers in patients with traumatic brain injury: A narrative review. Int. Wound J. 2024, 21, e14788. [Google Scholar] [CrossRef]
- Hoogendoorn, I.; Reenalda, J.; Koopman, B.F.J.M.; Rietman, J.S. The effect of pressure and shear on tissue viability of human skin in relation to the development of pressure ulcers: A systematic review. J. Tissue Viability 2017, 26, 157–171. [Google Scholar] [CrossRef]
- Catella, J.; Mahé, G.; Leftheriotis, G.; Long, A. Reference Probe for TcpO2 at Rest: A Systematic Review. Diagnostics 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.A.; Nieuwstraten, J.A.; Schepers, A.; van Schaik, J.; van den Hoven, P.; van der Vorst, J.R.; Hamming, J.F.; Brouwers, J.J.W. Prognostic performance of bedside tests for predicting ulcer healing and wound healing after minor amputation in patients prone to medial arterial calcification: A systematic review. Vasc. Med. 2025, 30, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.L. Evaluation of the clinimetrics of transcutaneous oxygen measurement and its application in wound care. Int. Wound J. 2015, 12, 625–629. [Google Scholar] [CrossRef]
- Catella, J.; Long, A.; Mazzolai, L. What Is Currently the Role of TcPO2 in the Choice of the Amputation Level of Lower Limbs? A Comprehensive Review. J. Clin. Med. 2021, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, B.; de Kleijn, R.; Kuppens, G.; Verhoeven, B.A.N.; Hinnen, J.W.; de Borst, G.J. Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study. J. Clin. Med. 2020, 9, 3291. [Google Scholar] [CrossRef]
- Leenstra, B.S.; Kuppens, G.Z.L.; van Bergen, A.; de Kleijn, R.; de Borst, G.J.; Verhoeven, B. Comparison of Photo-optical Transcutaneous Oxygen Tension Measurement with Electro-Chemical Transcutaneous Oxygen Tension Measurement in Patients with Arterial Claudication. Ann. Vasc. Surg. 2021, 77, 274–279. [Google Scholar] [CrossRef]
- López-Moral, M.; García-Madrid, M.; Molines-Barroso, R.J.; García-Álvarez, Y.; Tardáguila-García, A.; Lázaro-Martínez, J.L. Analyses of transcutaneous oxygen pressure values stratified for foot angiosomes to predict diabetic foot ulcer healing. J. Tissue Viability 2023, 32, 480–486. [Google Scholar] [CrossRef]
- Bucki, M.; Luboz, V.; Perrier, A.; Champion, E.; Diot, B.; Vuillerme, N.; Payan, Y. Clinical workflow for personalized foot pressure ulcer prevention. Med. Eng. Phys. 2016, 38, 845–853. [Google Scholar] [CrossRef]
- Sugathapala, R.D.U.P.; Latimer, S.; Balasuriya, A.; Chaboyer, W.; Thalib, L.; Gillespie, B.M. Prevalence and incidence of pressure injuries among older people living in nursing homes: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2023, 148, 104605. [Google Scholar] [CrossRef]
- Daviet, J.-C.; Dudognon, P.; Preux, P.-M.; Rebeyrotte, I.; Lacroix, P.; Munoz, M.; Salle, J.-Y. Reliability of transcutaneous oxygen tension measurement on the back of the hand and complex regional pain syndrome after stroke1. Arch. Phys. Med. Rehabil. 2004, 85, 1102–1105. [Google Scholar] [CrossRef]
- Gélis, A.; Fattal, C.; Dupeyron, A.; Pérez-Martin, A.; Colin, D.; Pelissier, J. Reproducibility of Transcutaneous Oxygen Pressure Measurements in Persons with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2009, 90, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Henni, S.; Semporé, Y.W.; Le Meliner, T.; Ouedraogo, N.; Hamel, J.F.; Abraham, P. Intra-test and test-retest reliability of exercise oximetry in arterial claudication. Microvasc. Res. 2018, 117, 44–49. [Google Scholar] [CrossRef]
- Henni, S.; Hersant, J.; Ammi, M.; Mortaki, F.-E.; Picquet, J.; Feuilloy, M.; Abraham, P. Microvascular Response to the Roos Test Has Excellent Feasibility and Good Reliability in Patients with Suspected Thoracic Outlet Syndrome. Front. Physiol. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Laroche, D.; Barnay, J.L.; Tourlonias, B.; Orta, C.; Obert, C.; Casillas, J.M. Microcirculatory Assessment of Arterial Below-Knee Stumps: Near-Infrared Spectroscopy Versus Transcutaneous Oxygen Tension-A Preliminary Study in Prosthesis Users. Arch. Phys. Med. Rehabil. 2017, 98, 1187–1194. [Google Scholar] [CrossRef]
- Bouyé, P.; Picquet, J.; Jaquinandi, V.; Enon, B.; Leftheriotis, G.; Saumet, J.-L.; Abraham, P. Reproducibility of proximal and distal transcutaneous oxygen pressure measurements during exercise in stage 2 arterial claudication. Int. Angiol. 2004, 23, 114–121. [Google Scholar]
- Tueguem Moyo, T.; Jéhannin, P.; Le Pabic, E.; Le Faucheur, A.; Omarjee, L.; Mahe, G. Test-retest Reliability and Minimal Detectable Change in Exercise Oximetry in Claudicants. Ann. Vasc. Surg. 2024, 99, 19–25. [Google Scholar] [CrossRef]
- Munro, B.H. Statistical Methods for Health Care Research; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 518p. [Google Scholar]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Pavlů, D.; Pánek, D.; Kuncová, E.; Thung, J.S. Effect of Blood Circulation in the Upper Limb after Flossing Strategy. Appl. Sci. 2021, 11, 1634. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Medica 2021, 31, 010502. [Google Scholar] [CrossRef]
- Atkinson, G.; Nevill, A.M. Statistical Methods for Assessing Measurement Error (Reliability) in Variables Relevant to Sports Medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gerke, O. Reporting Standards for a Bland-Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef]
- Andrades-Ramírez, O.; Ulloa-Díaz, D.; Rodríguez-Perea, A.; Araya-Sierralta, S.; Guede-Rojas, F.; Muñoz-Bustos, G.; CHIROSA RÍOS, L.J. Test–Retest Reliability of Concentric and Eccentric Muscle Strength in Knee Flexion–Extension Controlled by Functional Electromechanical Dynamometry in Female Soccer. Appl. Sci. 2024, 14, 8744. [Google Scholar] [CrossRef]
- Shonat, R.D.; Johnson, P.C. Oxygen tension gradients and heterogeneity in venous microcirculation: A phosphorescence quenching study. Am. J. Physiol.-Heart Circ. Physiol. 1997, 272, H2233–H2240. [Google Scholar] [CrossRef]
- Torres Filho, I.P.; Intaglietta, M. Microvessel PO2 measurements by phosphorescence decay method. Am. J. Physiol-Heart Circ. Physiol. 1993, 265, H1434–H1438. [Google Scholar] [CrossRef]
- Urban, M.; Fouasson-Chailloux, A.; Signolet, I.; Colas Ribas, C.; Feuilloy, M.; Abraham, P. Comparison of two devices for measuring exercise transcutaneous oxygen pressures in patients with claudication. VASA 2015, 44, 355–362. [Google Scholar] [CrossRef]
- Leenstra, B.S.; Meerkerk, C.; Conte, M.; Hinnen, J.W.; de Borst, G.J.; Verhoeven, B. Feasibility of Photo-Optical Transcutaneous Oxygen Tension Measurement During Revascularization of the Lower Extremity. Ann. Vasc. Surg. 2021, 77, 127–131. [Google Scholar] [CrossRef]
- Chai, C.Y.; Sadou, O.; Worsley, P.R.; Bader, D.L. Pressure signatures can influence tissue response for individuals supported on an alternating pressure mattress. J. Tissue Viability 2017, 26, 180–188. [Google Scholar] [CrossRef]
- Gómez-González, A.J.; Morilla-Herrera, J.C.; Lupiáñez-Pérez, I.; Morales-Asencio, J.M.; García-Mayor, S.; León-Campos, Á.; Marfil-Gómez, R.; Aranda-Gallardo, M.; Moya-Suárez, A.B.; Kaknani-Uttumchandani, S. Perfusion, tissue oxygenation and peripheral temperature in the skin of heels of healthy participants exposed to pressure: A quasi-experimental study. J. Adv. Nurs. 2020, 76, 654–663. [Google Scholar] [CrossRef]
- Knight, S.L.; Taylor, R.P.; Polliack, A.A.; Bader, D.L. Establishing predictive indicators for the status of loaded soft tissues. J. Appl. Physiol. 2001, 90, 2231–2237. [Google Scholar] [CrossRef]
- Lupiáñez-Pérez, I.; Gómez-González, A.J.; Marfil-Gómez, R.M.; Morales-Asencio, J.M.; García-Mayor, S.; León-Campos, Á.; Kaknani-Uttumchandani, S.; Moya-Suárez, A.B.; Aranda-Gallardo, M.; Morilla-Herrera, J.C. Tissue temperature, flux and oxygen of sacral and trochanteric area under pressure of healthy subjects: A quasi-experimental study. J. Tissue Viability 2021, 30, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V. The influence of local heating on skin microcirculation in pressure ulcers, monitored by a combined laser Doppler and transcutaneous oxygen tension probe. Clin. Physiol. 2000, 20, 413–421. [Google Scholar] [CrossRef]
- Alvaro-Afonso, F.J.; Garcia-Alvarez, Y.; Garcia-Morales, E.A.; Flores-Escobar, S.; De Benito-Fernandez, L.; Alfayate-Garcia, J.; Sánchez-Ríos, J.P.; Puras-Mallagray, E.; Malo-Benages, E.J.; Ramírez-Ortega, M.; et al. Determining the Best Noninvasive Test for Peripheral Arterial Disease Diagnosis to Predict Diabetic Foot Ulcer Healing in Patients Following Endovascular Revascularization. Healthcare 2024, 12, 1664. [Google Scholar] [CrossRef]
- Chen, P.; Vilorio, N.C.; Dhatariya, K.; Jeffcoate, W.; Lobmann, R.; McIntosh, C.; Piaggesi, A.; Steinberg, J.; Vas, P.; Viswanathan, V.; et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3644. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Bostan, L.E.; Worsley, P.R.; Bader, D.L. Evaluating the effects of sedentary behaviour on plantar skin health in people with diabetes. J. Tissue Viability 2020, 29, 277–283. [Google Scholar] [CrossRef]
- Nam, H.J.; Wee, S.Y.; Kim, S.Y.; Jeong, H.G.; Lee, D.W.; Byeon, J.; Park, S.; Choi, H.J. The correlation between transcutaneous oxygen pressure (TcPO2) and forward-looking infrared (FLIR) thermography in the evaluation of lower extremity perfusion according to angiosome. Int. Wound J. 2023, 21, e14431. [Google Scholar] [CrossRef]
- Yang, C.; Weng, H.; Chen, L.; Yang, H.; Luo, G.; Mai, L.; Jin, G.; Yan, L. Transcutaneous Oxygen Pressure Measurement in Diabetic Foot Ulcers: Mean Values and Cut-Point for Wound Healing. J. Wound Ostomy Cont. Nurs. 2013, 40, 585–589. [Google Scholar] [CrossRef]
- Falstie-Jensen, N.; Brøchner-Mortensen, J.; Spaun, E.; Falstie-jensen, S. The influence of epidermal thickness on transcutaneous oxygen pressure measurements in normal persons. Scand. J. Clin. Lab. Investig. 1988, 48, 519–523. [Google Scholar] [CrossRef]
- Lübbers, D.W. Theoretical basis of the transcutaneous blood gas measurements. Crit. Care Med. 1981, 9, 721–733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).