Research on a Reductive Deep Chlorine Removal Process for Breaking Through the Solid Film Barrier
Abstract
1. Introduction
2. Materials and Methods
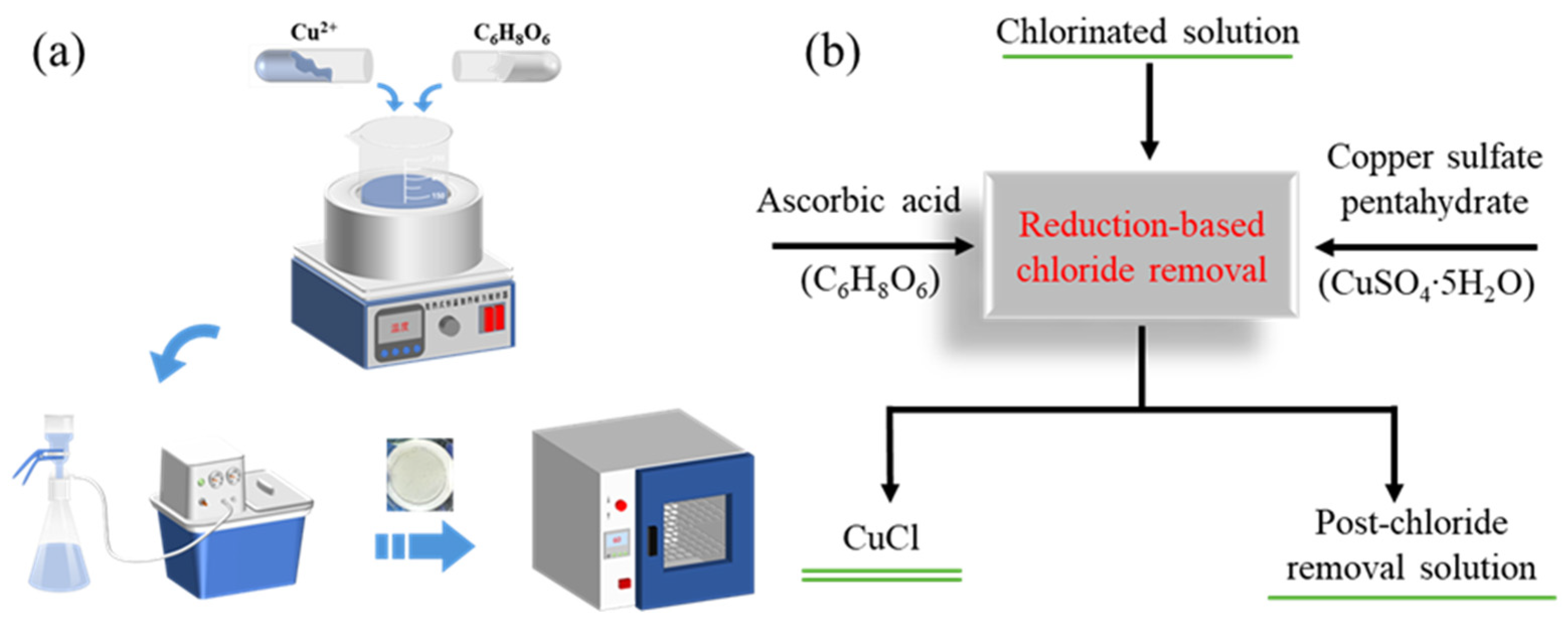
2.1. Experimental Reagents
2.2. Experimental Procedures and Methods
2.3. Analytical Detection Methods
2.4. Calculations Related to Thermodynamic Parameters
2.4.1. Ascorbic Acid Dechlorination
2.4.2. Copper Slag Dechlorination
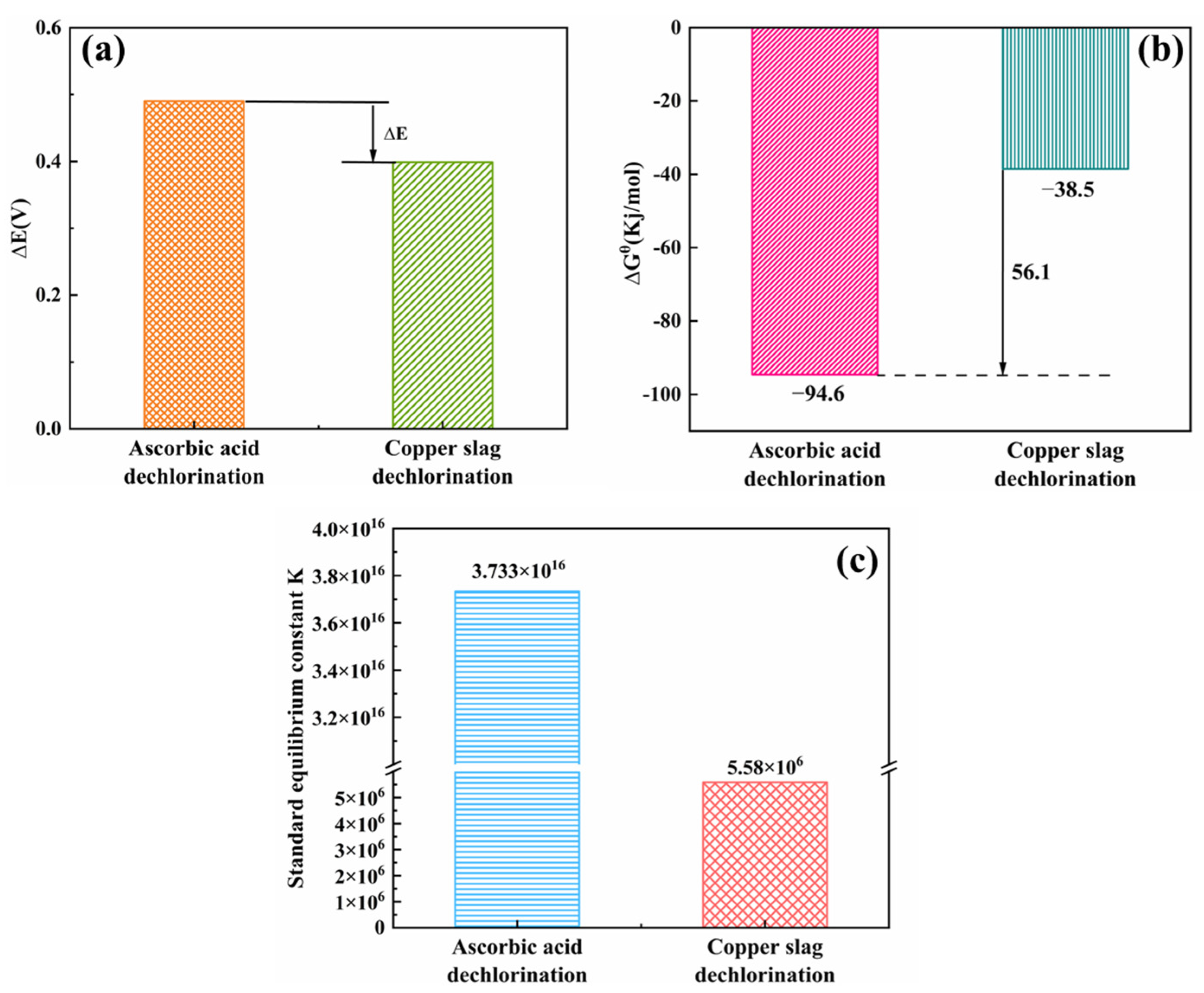
2.4.3. Comparison of Thermodynamic Parameters
3. Results and Discussion
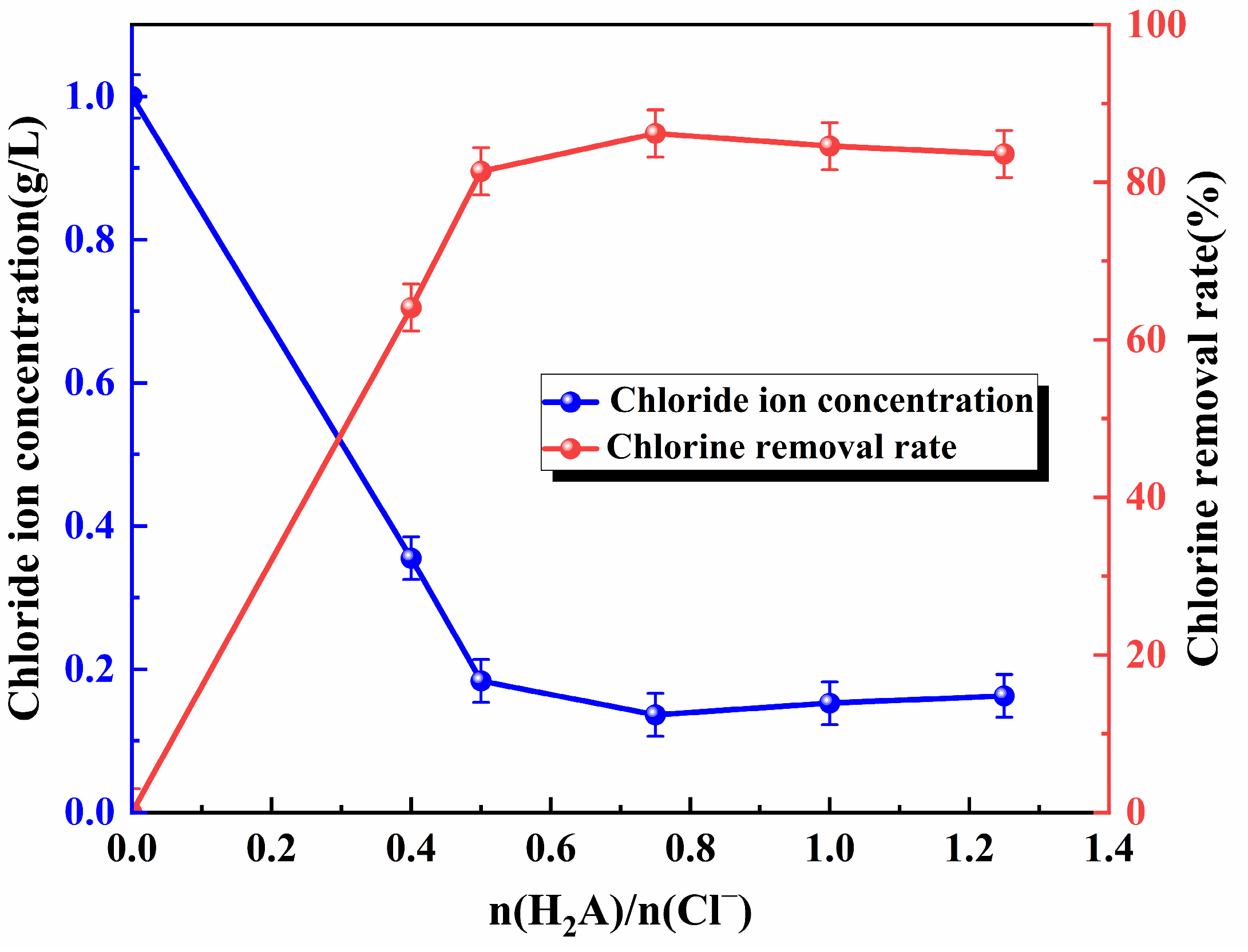
3.1. Ascorbic Acid Addition
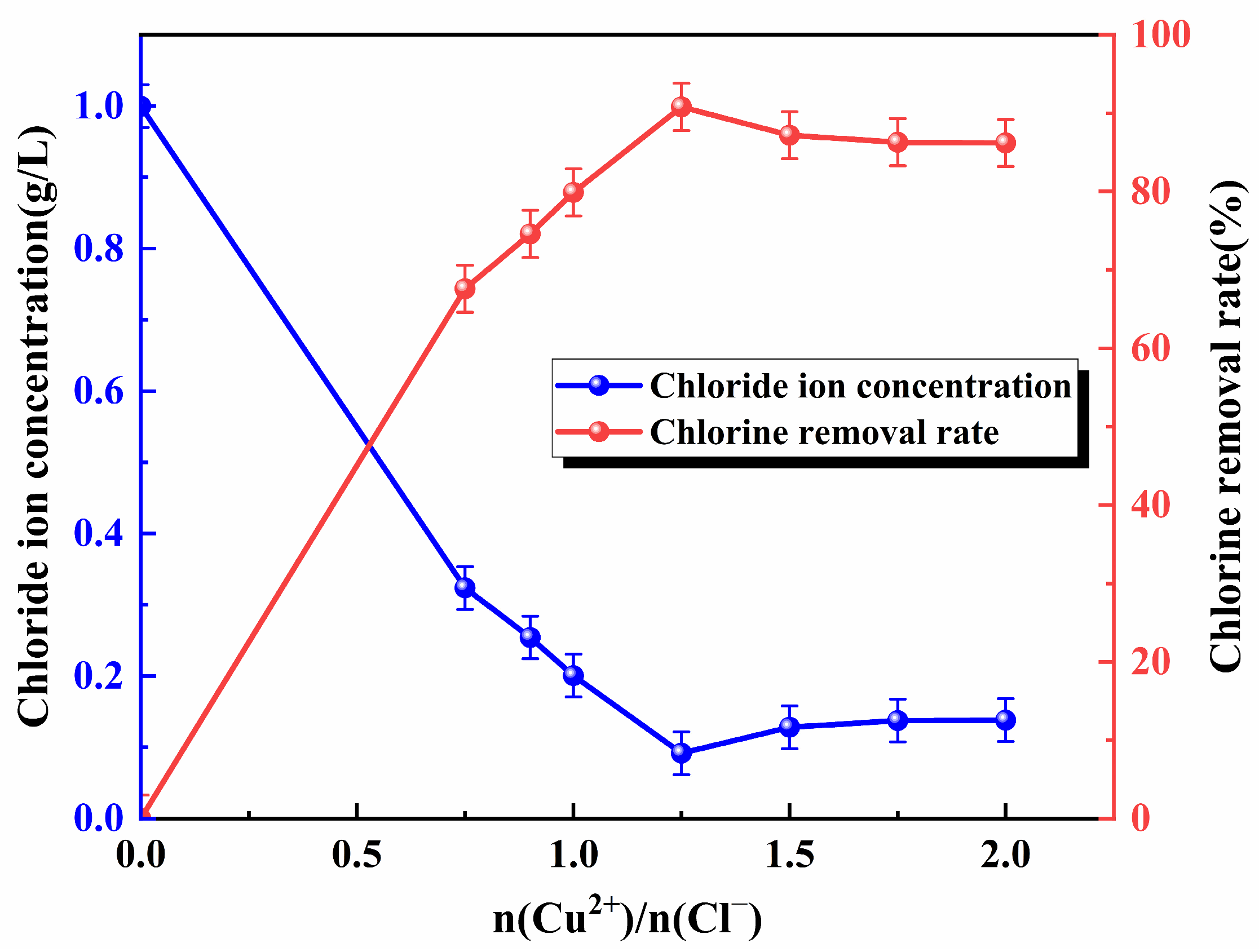
3.2. Copper Ion Addition
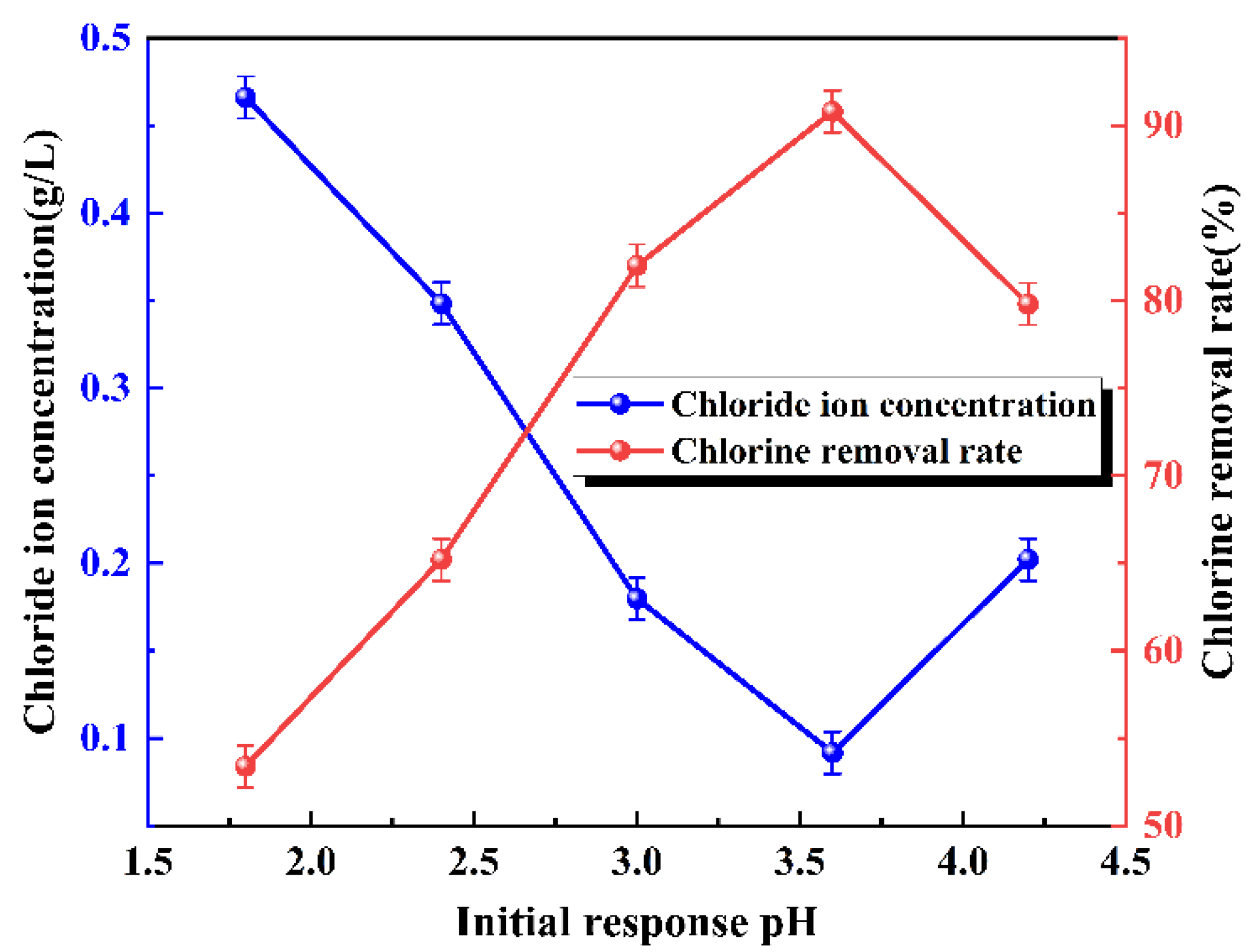
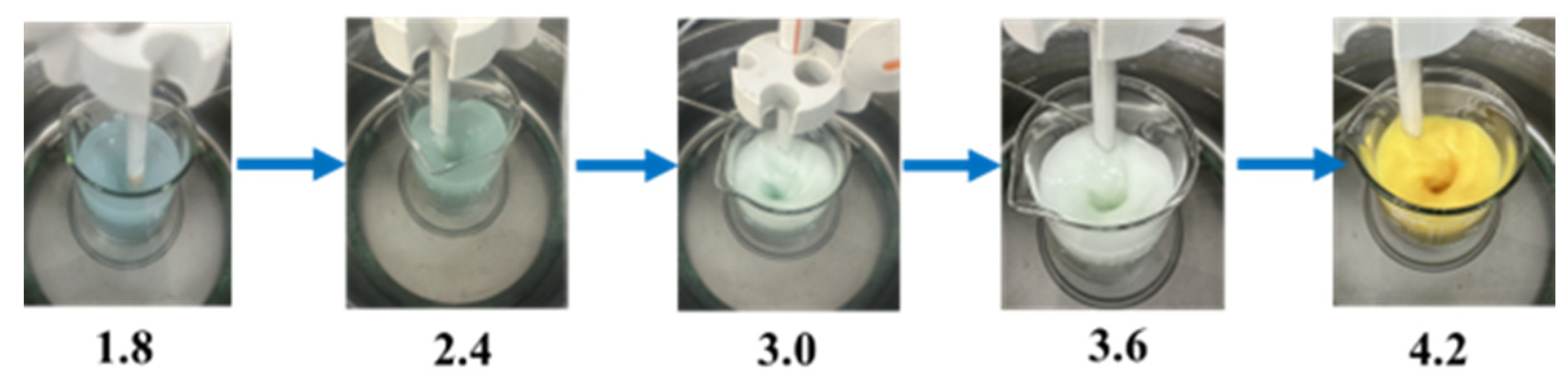
3.3. Initial Reaction pH
3.4. Reaction Temperature
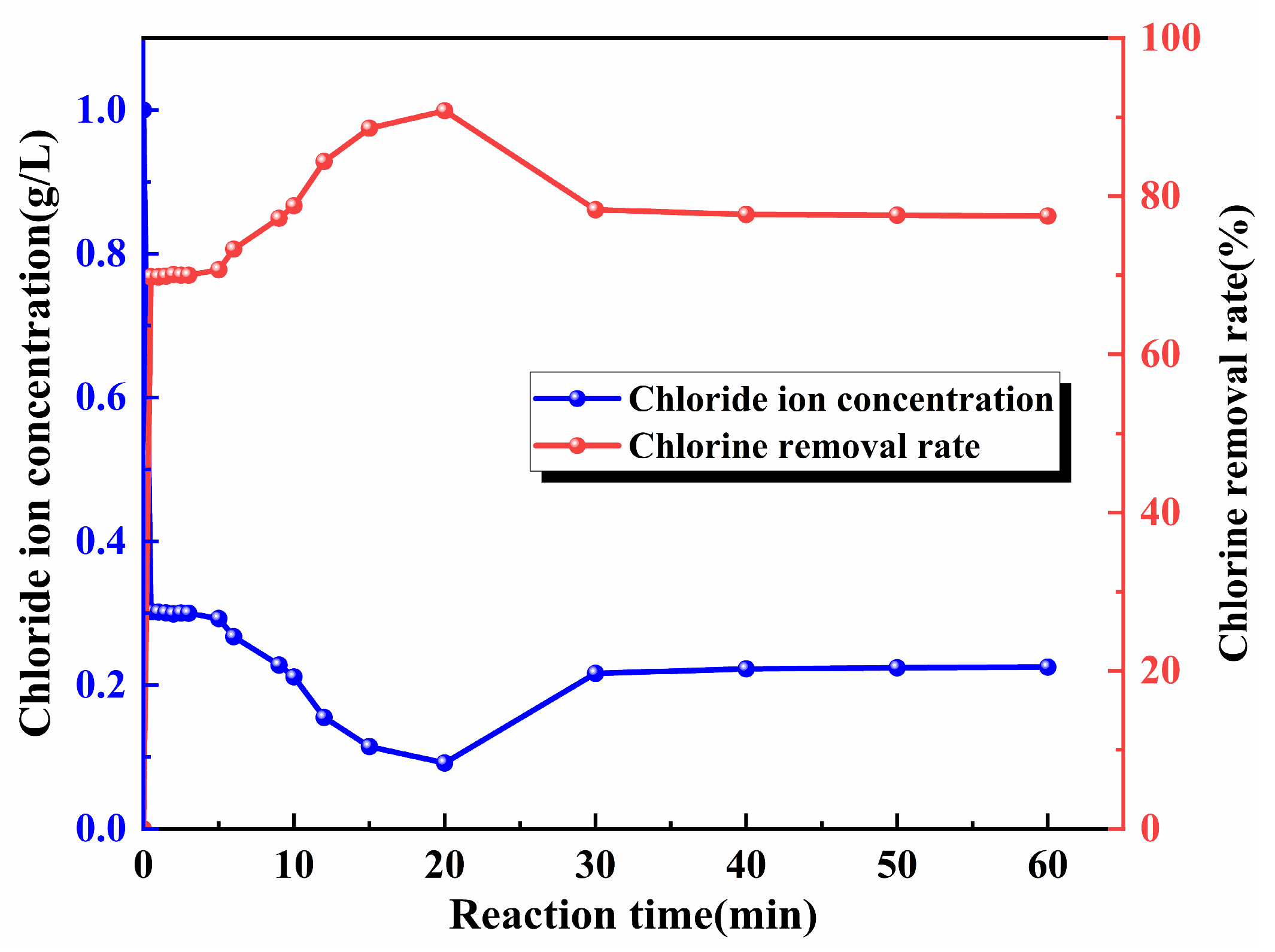
3.5. Reaction Time
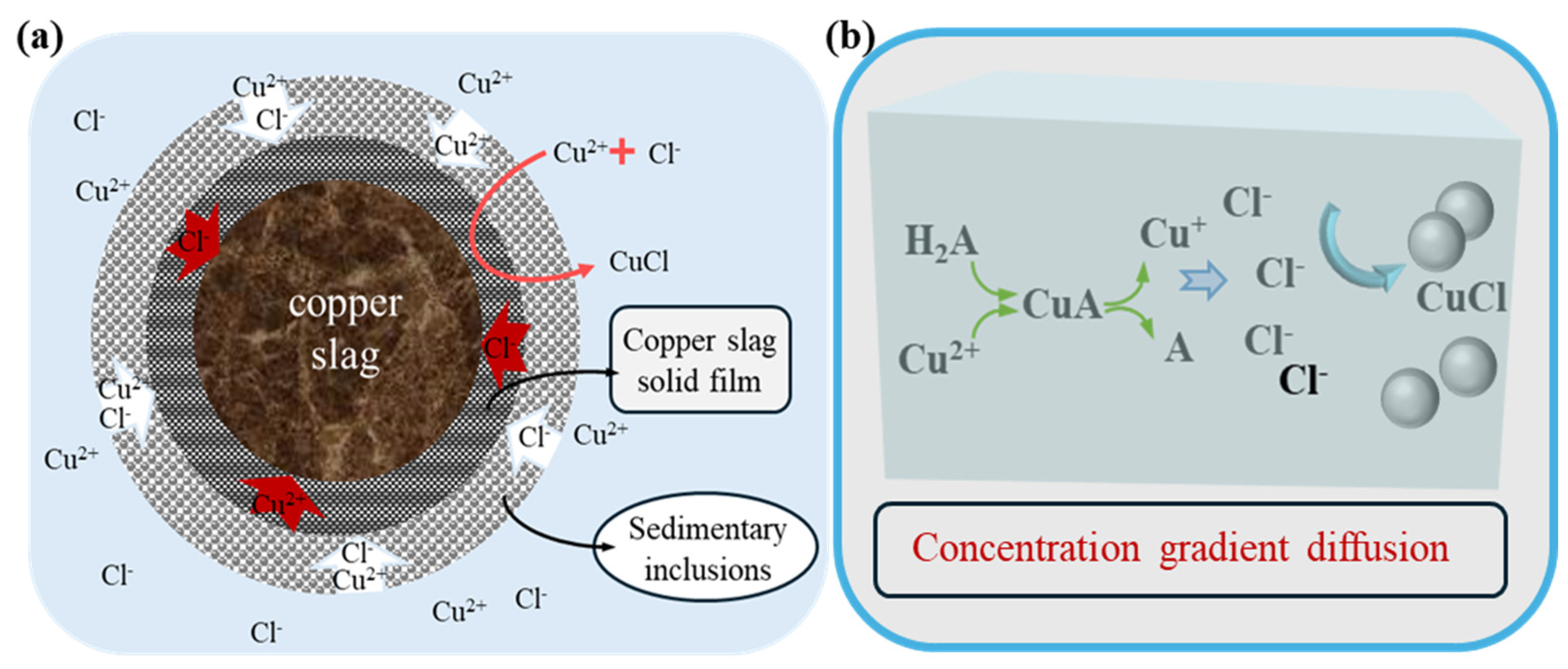
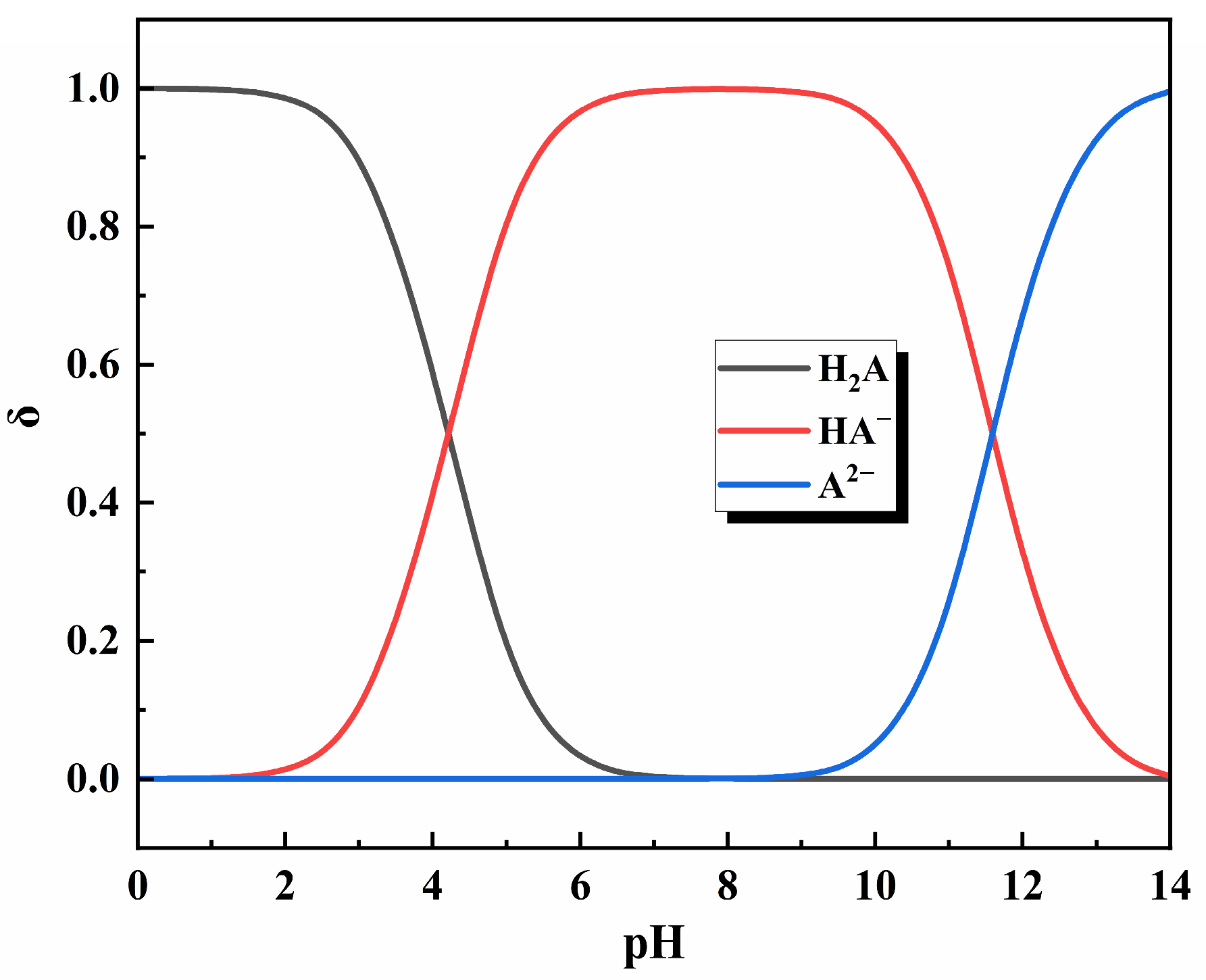
3.6. Ascorbic Acid Dechlorination Reaction Mechanism
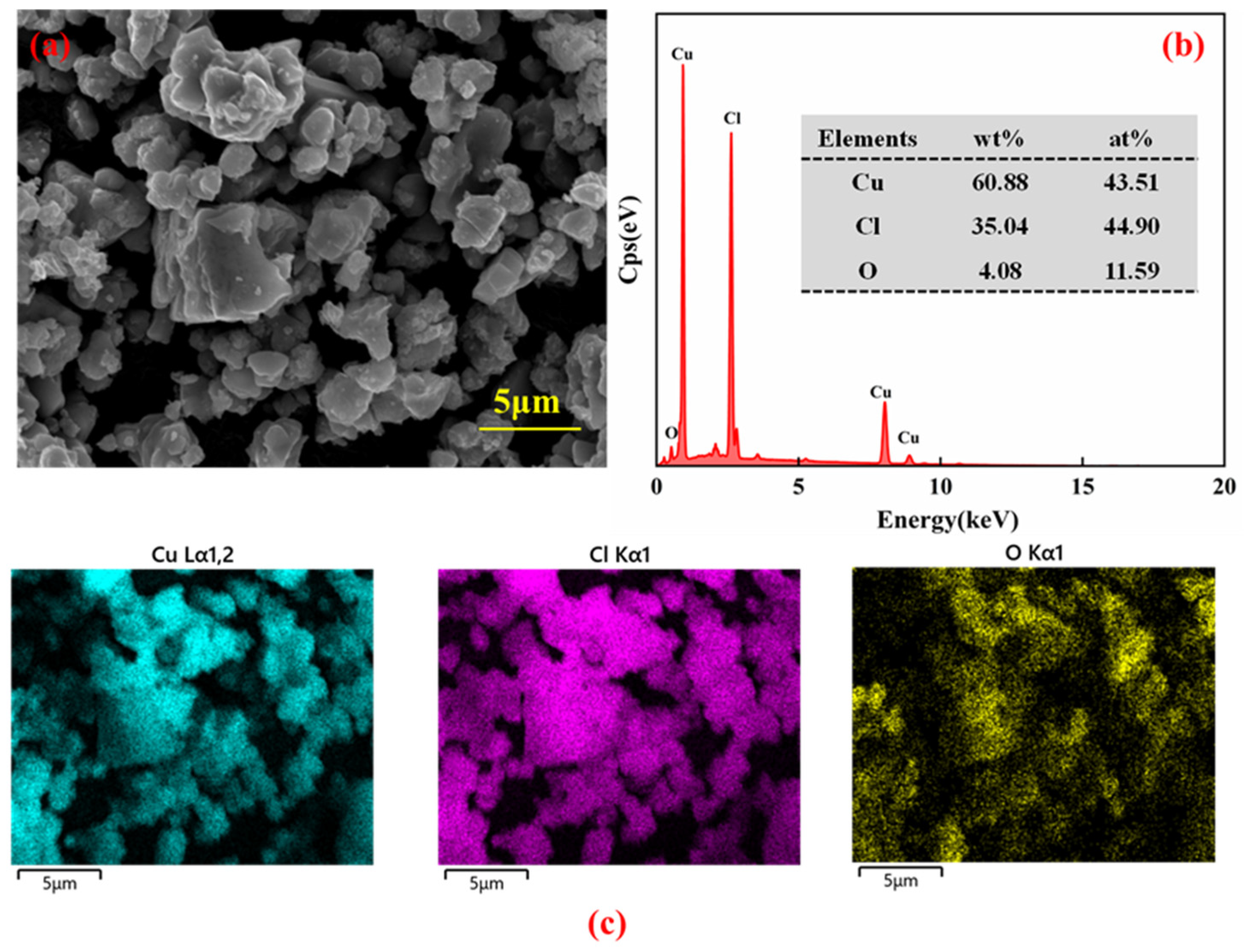
3.6.1. Analysis of Products of Dechlorination
3.6.2. Dechlorination Process Through Reduction of Cu2+ with Ascorbic Acid
4. Conclusions
- (1)
- The ascorbic acid dechlorination process showed significant advantages over copper slag dechlorination. Its potential difference ΔE was 0.091 V higher, the reaction-driving force was enhanced by 18.6%, the standard Gibbs free energy ΔGθ was 59.3% lower, and the equilibrium constant K was 6.7 × 109 times higher. This indicates that the dechlorination of ascorbic acid has stronger spontaneity and a more complete reaction, and a higher purification depth can be achieved.
- (2)
- Under optimized conditions (initial pH 3.6, n(Cu2+)/ n(Cl−)/n(H2A) = 1.25:1:0.75, 20 °C, 20 min), the chloride concentration was reduced from 1 g/L to 0.0917 g/L with 90.8% removal. The ascorbic acid dechlorination process achieved rapid dechlorination through its unique enol structure, and the reaction process was a homogeneous aqueous phase reaction, with simple and easy-to-control diffusion steps, which avoided the limitations posed by the solid film barrier and possessed higher flexibility and reaction efficiency than copper slag dechlorination.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoeber, L.; Steinlechner, S. A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Clean. Eng. Technol. 2021, 4, 100214. [Google Scholar] [CrossRef]
- Zeng, W.; Hu, X.; Yan, Y.; Chen, B.; Chen, Y.; Tang, C.; Yang, J. Study on the Cavitation and Dissociation of Sulfur from Zinc Leaching Residue. JOM 2024, 76, 1394–1407. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y.; Zhang, W. A Review on the Removal of Magnesium and Fluoride in Zinc Hydrometallurgy. J. Sustain. Metall. 2022, 8, 25–36. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, R.; Liu, Z.; Li, C. Removal of chloride from simulated zinc sulfate electrolyte by ozone oxidation. Hydrometallurgy 2016, 160, 147–151. [Google Scholar] [CrossRef]
- Turan, M.D.; Altundoğan, H.S.; Tümen, F. Recovery of zinc and lead from zinc plant residue. Hydrometallurgy 2004, 75, 169–176. [Google Scholar] [CrossRef]
- Duan, L.; Yun, Q.; Jiang, G.; Teng, D.; Zhou, G.; Cao, Y. A review of chloride ions removal from high chloride industrial wastewater: Sources, hazards, and mechanisms. J. Environ. Manag. 2024, 353, 120184. [Google Scholar] [CrossRef]
- Cheng, H.; Xiao, H.-F.; Chen, Q.; Li, X.-M.; Qin, W.-M.; Chen, B.-S.; Xiao, D.; Zhang, W.-M. Significantly enhanced dehalogenation selectivity in near-neutral zinc sulfate electrolytes by diffusion dialysis. J. Membr. Sci. 2018, 563, 142–148. [Google Scholar] [CrossRef]
- Kong, X.-P. Discussion on the harm and removal method of impurity chlorine in wet zinc smelting. World Nonferrous Metals 2018, 2, 18–19. [Google Scholar]
- Liu, W.; Lü, L.; Lu, Y.; Hu, X.; Liang, B. Removal of chloride from simulated acidic wastewater in the zinc production. Chin. J. Chem. Eng. 2019, 27, 1037–1043. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.-Y.; Chai, L.-Y.; Xiao, H.-J.; Pei, F.; Shu, Y.-D. Effect of impurities in recycling water on Pb-Ag anode passivation in zinc electrowinning process. Trans. Nonferrous Met. Soc. China 2011, 21, 1665–1672. [Google Scholar] [CrossRef]
- Farahbod, F. Experimental study of order and constant rate of chlorine removal reaction using ion exchange resin. Appl. Water Sci. 2023, 13, 167. [Google Scholar] [CrossRef]
- Kameda, T.; Yoshioka, T.; Mitsuhashi, T.; Uchida, M.; Okuwaki, A. The simultaneous removal of calcium and chloride ions from calcium chloride solution using magnesium–aluminum oxide. Water Res. 2003, 37, 4045–4050. [Google Scholar] [CrossRef]
- Rahmati, N.O.; Pourafshari Chenar, M.; Azizi Namaghi, H. Removal of free active chlorine from synthetic wastewater by MEUF process using polyethersulfone/titania nanocomposite membrane. Sep. Purif. Technol. 2017, 181, 213–222. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Liu, X. Chloride ion removal from Zinc sulfate aqueous solution by electrochemical method. Hydrometallurgy 2013, 134–135, 62–65. [Google Scholar] [CrossRef]
- Ahmadi, R.; Khoshkhati, F.; Kia, M.R. Dechlorination from sulphate aqueous solutions of zinc electrolysis by electrochemical method. Asian J. Water Environ. Pollut. 2018, 15, 23–28. [Google Scholar] [CrossRef]
- Rudnik, E.; Bayaraa, E. Electrochemical dissolution of smelted low-grade electronic scraps in acid sulfate-chloride solutions. Hydrometallurgy 2016, 159, 110–119. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Hong, R.; Qiu, W.; Deng, C.; Yu, C. Electrochemical chlorine evolution reaction to improve the desalination of sea sand. Sci. Total Environ. 2024, 945, 174063. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Jassby, D.; An, A.K. Conductive reverse osmosis membrane for electrochemical chlorine reduction and sustainable brackish water treatment. Chem. Eng. J. 2022, 435, 134858. [Google Scholar] [CrossRef]
- Dou, W.; Song, Y.; Zhang, Y.; Liu, T.; Kong, L.; Hu, X.; Li, D. Removal of fluoride and chloride from waste acid by Bi2O3: Comparison between the synchronous and two-step methods. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zhang, L. Removal of chlorine from zinc sulfate solution: A review. Environ. Sci. Pollut. Res. 2022, 29, 62839–62850. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-J.; Kong, J.-H.; Zhang, X.-Y. Discussion on the Method of Removing Fluorine and Chlorine from Zinc Sulfate Solution in Zinc Refinery. Sustain. Min. Metall. 2018, 34, 30–33. [Google Scholar]
- Liu, Z.-L. Application practice of copper slag chlorine removal in electric zinc process. Min. Metall. 2020, 29, 84–87. [Google Scholar]
- Ke, X.; Xie, B.; Zhang, J.; Wang, J.; Li, W.; Ban, L.; Hu, Q.; He, H.; Wang, L.; Wang, Z. Study on the preparation of ascorbic acid reduced ultrafine copper powders in the presence of different protectants and the properties of copper powders based on methionine protection. Nanoscale Adv. 2024, 6, 1135–1144. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Fitzner, K. Gold Nanoparticles Formation via Au(III) Complex Ions Reduction with l-Ascorbic Acid. Int. J. Chem. Kinet. 2017, 49, 789–797. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Zhang, P.; Hou, L.; Sun, Y.; Lu, Z.; Tang, Y.; Tian, H.; Shi, T. Antioxidative Reactivity of L-Ascorbic Acid and D-Isoascorbic Acid Species towards Reduction of Hexachloroiridate (IV). J. Chem. 2021, 2021, 5505741. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W.; Van Der Westhuizen, W.A. Recovery of manganese from ferruginous manganese ore using ascorbic acid as reducing agent. Miner. Eng. 2020, 154, 106406. [Google Scholar] [CrossRef]
- Song, K.; Su, H.; Liu, M.; Wang, R.; Hu, L.; Liu, W.; Lv, X.; Xin, Y. A novel ammonium-free vanadium precipitation process for the integrated actions of ascorbic acid reduction and enhanced hydrolysis under hydrothermal influence. J. Environ. Chem. Eng. 2024, 12, 111842. [Google Scholar] [CrossRef]
- Karković, A.; Brala, C.J.; Pilepić, V.; Uršić, S. Solvent-induced hydrogen tunnelling in ascorbate proton-coupled electron transfers. Tetrahedron Lett. 2011, 52, 1757–1761. [Google Scholar] [CrossRef]
- Go, S.-H.; Kim, H.; Yu, J.; You, N.-H.; Ku, B.-C.; Kim, Y.-K. Synergistic effect of UV and l-ascorbic acid on the reduction of graphene oxide: Reduction kinetics and quantum chemical simulations. Solid State Sci. 2018, 84, 120–125. [Google Scholar] [CrossRef]
- Yokoyama, S.; Takahashi, H.; Itoh, T.; Motomiya, K.; Tohji, K. Synthesis of metallic Cu nanoparticles by controlling Cu complexes in aqueous solution. Adv. Powder Technol. 2014, 25, 999–1006. [Google Scholar] [CrossRef]
- Zaib, Q.; Park, H.S.; Kyung, D. Experimental modeling and optimization for the reduction of hexavalent chromium in aqueous solutions using ascorbic acid. Sci. Rep. 2021, 11, 13146. [Google Scholar] [CrossRef] [PubMed]






| Redox Electric Pair | Reduction Half-Reaction Equation | Eθ (V) |
|---|---|---|
| Cu2+/CuCl | Cu2+ + e− + Cl−→CuCl | 0.57 |
| C6H8O6/C6H6O6 | C6H8O6 − 2e−→C6H6O6 + 2H+ | 0.08 |
| Redox Electric Pair | Reduction Half-Reaction Equation | Eθ (V) |
|---|---|---|
| Cu2+/CuCl | Cu2+ + e− + Cl−→CuCl | 0.57 |
| CuCl/Cu | Cu0 − e− + Cl−→CuCl | 0.171 |
| Dechlorination Process | Thermodynamic Parameter | ||
|---|---|---|---|
| Reaction Potential Difference (ΔE) | Standard Gibbs Free Energy (ΔGθm) | Standard Equilibrium Constant (K) | |
| Ascorbic acid dechlorination | 0.49 V | −94.6 kJ/mol | 3.733 × 1016 |
| Copper slag dechlorination | 0.399 V | −38.5 kJ/mol | 5.58 × 106 |
| Process comparison | ΔE1 > ΔE2 | K1 K2 | |
| Element | Cu | Cl | O |
|---|---|---|---|
| Concentration (%) | 67.82 | 32.09 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wen, A.; Li, J. Research on a Reductive Deep Chlorine Removal Process for Breaking Through the Solid Film Barrier. Appl. Sci. 2025, 15, 5673. https://doi.org/10.3390/app15105673
Li R, Wen A, Li J. Research on a Reductive Deep Chlorine Removal Process for Breaking Through the Solid Film Barrier. Applied Sciences. 2025; 15(10):5673. https://doi.org/10.3390/app15105673
Chicago/Turabian StyleLi, Rui, Ailin Wen, and Jing Li. 2025. "Research on a Reductive Deep Chlorine Removal Process for Breaking Through the Solid Film Barrier" Applied Sciences 15, no. 10: 5673. https://doi.org/10.3390/app15105673
APA StyleLi, R., Wen, A., & Li, J. (2025). Research on a Reductive Deep Chlorine Removal Process for Breaking Through the Solid Film Barrier. Applied Sciences, 15(10), 5673. https://doi.org/10.3390/app15105673







