Abstract
Constanta port operations involving the handling of bulk minerals often lead to material losses, resulting in mineral waste, containing a mixture of iron ore, bauxite, and coal, amongst others. In order to recover these minerals, a processing plant was built, which successfully separates most of this waste into its constituents. However, a byproduct obtained from this process is a sludge containing fine particles below 0.5 mm, which are deposited in a reservoir that represents definitive tailings. Since this is a “rich” tailing material, which is difficult to be extracted by using conventional methods due to its small size, the selective flocculation procedure was tested as an alternative method. This paper presents the results obtained for standard methods of selective flocculation tests using polyacrylamide A 100 at a pH value of 10.5. SEM-EDS and XRD analyses were performed, and the chemical composition of the sample components was given. According to preliminary tests, using the selective flocculation procedure, the expected results were obtained in terms of separating the overflow between the content of impurities (with a reduced share of Fe in relation to the input) and sediment with an increased content of Fe (with a reduced share of impurities in relation to the entrance).
1. Introduction
Activities like unloading, depositing, and loading raw materials such as iron ore, coal, and bauxite from vessels, barges, freight trains, or trucks into the harbor area generate losses [1]. The primary causes of these losses are the following:
- Technological (from the usage of long conveyor belts, bunkers, transfer points, etc.);
- Natural (waste originating from dusty materials scattered by strong winds during depositing, waste originating from heavy rains that break some material from piles, etc.);
- Other (accidental discharges, through the occurrence of exploitation incidents, cleaning activities, etc.).
As a result of the manipulation of various raw materials in the technological flow and deposition areas, quantities of different materials accumulated and merged together. This mixture is unusable in its current state, resulting in mineral waste.
The accumulation of this mixture in open-air dumps over a long period of time generates pollution in the Port of Constanta. Once the minerals are merged, they are essentially considered waste. Therefore, effective management of this waste deposit must consider both their recovery and their separation into concentrated materials that can be exploited by various economic entities [1].
To resolve this environmental issue, a mineral processing plant was built that separates this waste into its constituents with very good results [1]. The plant processes the waste through various gravimetric methods, which will not be discussed in this article.
Given the particularities of the waste, the processing plant recovers 95% of the useful minerals contained in it. Considering the environmentally friendly nature of the processing plant, all the process water is recycled through a thickener, from which a sludge of around 5% of all processed waste results. This sludge is currently separated on a spiral concentrator, but this separation is not very effective due to the nature of the sludge, so we had to look further to new separation methods.
In Europe, there are at least two similar facilities for processing waste materials. In Rotterdam, the Netherlands, the bulk port operator deals with waste that is a mixture of coal and iron ore. To recover mineral compounds, they employ a technological process that involves separation in a dense medium, which is magnetite. Flotation is then used to treat the 0–0.5 mm material. This process ensures complete mineral recovery, resulting in iron ore and coal concentrates of high quality, comparable to those of transited bulk goods.
In the port of Brussels, there are large dumps of waste resulting from accidental dumping of stored ores: coal and iron ore. Their recovery aims to recover both iron ore and coal. The iron ore contained in the waste is recovered by magnetic processes, obtaining an iron concentrate with 59.2% Fe. The remaining material undergoes a hydrocyclone operation to separate the coal from the tailings.
For the Constanta plant, the initial testing was performed using a hydrocyclone; however, the results were inconclusive due to the mineral composition of the sludge. Although this method is still being investigated, another alternative method was thought to be suitable that could provide good results—selective flocculation.
Selective flocculation is one of the promising methods for the utilization of fine-grained iron ores, which has been the subject of research for the last few decades in the processing of iron minerals [2,3,4]. The sludge used in this research consists of a mixture of iron ore and other different materials (bauxite, coal, etc.) with particle sizes lower than 0.5 mm. The sludge is not an environmental threat [5], and there is a possibility to produce a material for the industry that could be very useful in the guidelines of a circular economy. The main goal of this research is to examine the possibility of applying selective flocculation to separate iron minerals from other impurities. We wanted to test the performance of this method, and if everything works as expected, we want to implement this method in the process.
2. Materials and Methods
2.1. Materials
These tailings are stored in the Mineral Waste Processing Plant area in the Port of Constanta, Romania. Sampling is performed manually during the forming of the stockpile, with 100 samples taken, consisting of about 100 kg. This makes the sampling representative for the sludge; moreover, the company also performs chemical and physical analyses of the sludge as testing methods for the process’ efficiency, and the results are comparable. After the mass is well mixed and homogenized, a representative sample of the mass of about 5 kg is separated. Representative sample of investigated “harbor waste” is handpicked and is labeled as sample “K” in this article.
Since the starting raw material (“harbor waste”—sample K) is a mixture of different phases with a wide range of particle sizes, the sample is subjected to the sieving method to obtain three fractions of different particle sizes (K1, K2, and K3) and thus facilitates the analyzing process. Three classes are selected to determine the mass fraction of the largest (K1), medium-sized (K2), and smallest (K3) size classes. At the same time, the chemical composition of each class is determined individually. The fraction K1 consists of particles with a size of 450–250 µm, the particles in fraction K2 are of 250–25 µm in size, and particles in K3 are smaller than 25 µm.
After sieving sample K, which was previously dried in atmospheric conditions, the material of different fractions is treated with a hand-magnet. The use of a hand-permanent magnet on loose material is a simple standard manual procedure that can be used to separate ferromagnetic minerals.
A magnet protected by cellophane is passed over the very surface of the material, which is scattered on paper, repeating the process until the ferromagnetic component is separated from the material.
The magnetic fraction (marked with the suffix M) is roughly separated from the non-magnetic fraction at K1 and K2, while the smallest fraction is almost completely separated with a magnet. A total of 6 samples are obtained for SEM-EDS analysis, (K1, K1M, K2, K2M, K3, and K3M), of which 5 samples (K1, K1M, K2, K2M, and K3M) are analyzed using the X-ray powder diffraction method.
All reagents used are of analytical grade, and they are prepared as solutions in distilled water.
The 0.1% PAM A100 (anionic polyacrylamide—PAM-type SUPERFLOC A100, manufactured by Kemira, Helsinki, Finland) is used as flocculant. Preparation of flocculant for all experiments is carried out in the same way according to the instructions provided by the manufacturer of reagents [6]. As pH modifier, 0.1 M NaOH is used. Flocculant consumption is 250 g/t based on previous research [7].
The 0.02 M SHMP (sodium hexametaphosphate, Na6P6O18, manufactured by Lach-Ner, s.r.o. (Neratovice, Czech Republic)) is used as dispersant. The effect of dispersant on the stabilization of system is studied by conducting dispersion tests in 500 mL graduated glass cylinder.
Dispersant consumption is 80 g/t, which, according to earlier research, is considered optimal [7].
2.2. Methods
2.2.1. Theoretical Principles of Selective Flocculation
Selective flocculation is a cornerstone in the field of wastewater treatment, focusing primarily on the aggregation of fine particles into sedimentable masses. This process is particularly important when it comes to separating specific particles from complex suspensions, leveraging the intrinsic properties of the particles and the strategic use of coagulation additives [7,8].
Flocculation involves the addition of chemical agents, such as coagulants and flocculants, which neutralize the charges on the surfaces of the colloidal particles, facilitating their aggregation into flocs. Coagulants, usually metal salts such as aluminum sulfate and ferric chloride, reduce the electrostatic repulsion between particles. Flocculants, often organic polymers, enhance the formation of bonds between particles, thereby increasing the size and weight of flocs to accelerate sedimentation [9,10].
Selective flocculation is a chemical process used to separate and remove specific particles from a colloidal suspension, such as minerals or impurities, without affecting other particles present. This process is based on the use of chemical additives (called flocculants or coagulants) that selectively interact with certain particles, causing them to agglomerate (form flocs) and settle, thus allowing them to separate from the rest of the suspension.
Selective flocculation uses specific additives designed to interact exclusively with targeted particle types, allowing for their efficient separation from mixed suspensions. The selection of suitable coagulants and flocculants depends on the nature of the particles, the slurry concentration, and the operating conditions such as pH and temperature. The pH adjustments and the use of complexing agents can further optimize the efficiency of selective flocculation [11,12].
In the field of industrial and municipal water treatment, selective flocculation is vital for efficient separation of specific particles, improvement of treated water quality, and reduction in operational costs. Control over process parameters, including mixing speed and reaction time, is crucial to achieving efficient and selective flocculation results [13,14,15].
2.2.2. Characterization Methods
Basic chemical analysis was performed using Brucker XRF CTX 800 (Brucker Nano Analytics, Berlin, Germany) device on a −0.5 mm representative sample “K” before sieving as well as on three obtained fractions of different granulations after sieving.
Morphological and compositional features of unpolished polyphase samples were analyzed by scanning electron microscopy with a JEOL JSM6610LV SEM microscope (JEOL Ltd., Tokyo, Japan) combined with energy-dispersive X-ray spectrometry (EDS)—X-max (Oxford Instruments plc, Oxford, UK) at the University of Belgrade—Faculty of Mining and Geology. The samples were coated in gold using coating device LEICA EM SCD005. The results were collected under high-vacuum conditions with acceleration voltage of 20 kV. Internal standards were applied for semiquantitative analyses. The detection limit was 0.1% by weight. Using this method, it was not possible to determine the content of the first five elements of the periodic system of chemical elements. The morphology and chemical composition were analyzed in points and fields on the grains as well as the concentrations of individual elements in the form of maps.
In order to obtain accurate data on the mineral composition, the samples were tested on a PHILIPS PW 1710 powder X-ray diffractometer (Philips Analytical, Eindhoven, The Netherlands) at room temperature using Bragg–Brentano geometry and copper anode—CuKα radiation (154,178 Å). The diffractometer was operated at 40 kV and 30 mA, while the scan range was from 3 to 75° 2θ with a step size of 0.02° 2θ. The obtained data on the position of the diffraction peaks 2θ (°) with corresponding relative intensities I/Imax and lattice spacings dhkl (Å) are given graphically. The present crystalline phases were identified by comparing the values of relative intensity I/Imax and d-values with the literature data and ICDD (International Center for Diffraction Data) standards (PDF # card number).
2.2.3. Settling and Flocculation Experiments
The natural settling and settling with the addition of polyacrylamide as flocculant were determined. The flocculation experiments were performed at a pulp density with 8% of solid, which is adequate to the simulation of natural industrial conditions. To examine the effect of flocculant dosage, (i) a beaker of 500 mL volume, (ii) 40 g of dry sample in 500 mL of distilled water for the experiment, and (iii) 250 g/t of PAM and 80 g/t of dispersant SHMP were used. All the tests were performed at pH = 4, pH = 6, and pH = 10.5. The first tests were performed without the presence of reagents at three different values in order to determine the influence of reagents on settling. After that, all dispersion and flocculation tests were carried out at a pH value of 10.5 because it was estimated by visual observation that the dispersion exists only at that pH value. The 5% NaOH was used to adjust the pH value. All tests were carried out on two parallel samples, and the average value was taken into consideration.
The test procedure was as follows:
- Mixing time without dispersant 2 min;
- Mixing time with dispersant 5 min;
- Mixing time with flocculants 5 min;
- Settlement time 1 min;
- Separation of the float from the settled;
- Drying samples (sediment) at 105 °C;
- Weighed the sediment material;
- Chemical analysis.
3. Results
3.1. Chemical Analysis
Results of the basic chemical analysis of −0,5 mm sample K using Brucker XRF CTX 800 device are given in Table 1.

Table 1.
Basic chemical composition of −0,5 mm sample K.
The density of the raw sample was also determined: 2.279 g/cm3.
Results of the basic chemical analysis of the samples with three different size classes, i.e., −450 + 250 µm, −250 + 25 µm, and −25 + 0 µm, are given in Table 2.

Table 2.
Basic chemical composition of sample K by size class (K1, K2, and K3).
Table 2 shows that the Fe content in all three classes is higher than in the initial sample. The reason for this could be that the grinding achieves a higher release or that the three selected classes are below 450 µm. It should be noted that the initial sample size class was −500 + 0 µm. This means that the largest fractions (−500 + 450 µm) do not have a significant Fe content.
3.2. Particle Size Distribution
The starting raw material is a heterogenous mixture with a wide range of particle sizes.
Results of the particle size analyses by size class (given in Table 3 and Figure 1) show that the highest mass percentage (70.15%) belongs to the medium-sized fraction (−250 + 25 µm).

Table 3.
Particle size distribution of sample K.
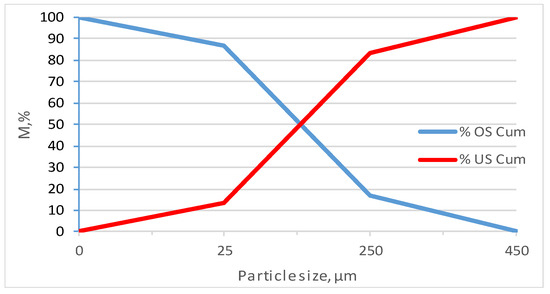
Figure 1.
Particle size distribution of sample K.
3.3. SEM-EDS and XRD Analyses
Scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS) and X-ray diffraction (XRD) are complementary techniques that have been extensively employed in previous studies for the detailed characterization of mineralogical samples. Their combined use has significantly advanced the understanding of mineral compositions and microstructures, particularly in studies involving mineral separation and waste characterization [16].
For mineral processing and waste management, knowing the exact composition and structure of the waste is crucial. Previous studies have used the insights gained from both techniques to optimize separation methods—such as adjusting the selective flocculation parameters—to target specific mineral phases more effectively [17].
SEM-EDS analysis of the unpolished polymineral aggregate—sample K—revealed the presence of grains with a maximum size of up to about 400 μm. On the surface of the most grains, agglomeration of powders of small particle sizes, different in chemical compositions, can be observed. Grains with clearly visible heterogeneity were analyzed in the form of points, as shown in Figure 2, while the results of semiquantitative EDS chemical analysis normalized to 100% are given in Table 4.

Figure 2.
SEM micrograph (BSE mode) of unpolished grains (sample K) with marked analyzed points: (a) spot chemical analyzes on one grains marked with 1, 2 and 3 and (b) point chemical analyzes on two grains marked with 4, 5 and 6.

Table 4.
Semiquantitative chemical composition of sample K, obtained by EDS analysis.
EDS analysis determined that the grains representing the dominant phase of sample K mostly composed of carbon (up to 80% by weight) and oxygen, while smaller individual grains were composed of iron and oxygen. A low content of Al and Si was observed in both phases. Their presence may be the result of fine-grained clay aggregates or mica minerals (with lower potassium and magnesium content) on the surface. Titanium is sporadically found in concentrations lower than 0.5% and cannot be associated with any analyzed phase. The morphology of the grains that contain carbon and oxygen is most similar to coal—anthracite grains. They are fragments irregular and polyhedral in shape with sharp corners and rough surfaces as well as flat surfaces.
Using the SEM-EDS method on unpolished separated fractions (K1, K1M, K2, K2M, K3, and K3M), the morphology and chemical composition were analyzed in points and fields on the present grains as well as the concentrations of individual elements in the form of maps. Given that the examined samples were unpolished, the mineralogical identification of the phases with this method was further complicated because even after sieving and separation of fractions by size and separation with a magnet, completely homogeneous grains could rarely be observed. There are mostly grains that represent a kind of composite composed of at least two phases as well as grains that contain fine-grained particles of other phases on their surface. This is present in all investigated fractions, especially in K1 (Figure 3). Such examples are significantly less represented in the smallest fraction, and the grains are more homogeneous, which facilitated the identification of mineral phases.
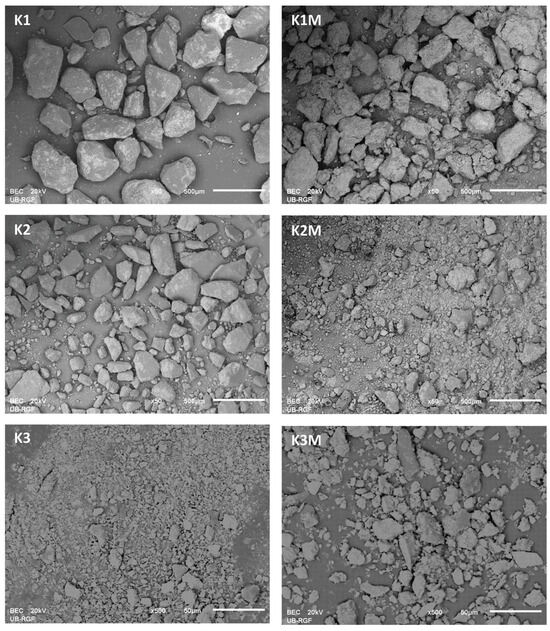
Figure 3.
SEM-BSE images of the selected samples (K1, K1M, K2, and K2M with magnification of 50× and scale bar of 500 μm, and K3 and K3M with magnification of 500× and scale bar of 50 μm).
Considering the previously stated observations, it is clear that the results of mineralogical determination obtained by X-ray powder diffraction analysis of the questioned material differ in relation to the phases that could be determined by EDS analysis performed in the form of point analysis.
Figure 4 shows sample K1 with analyzed points and corresponding EDS spectra. It can be seen that the gray-colored grains are built only of carbon and oxygen, while the lighter phases present on the described grains are aluminosilicate with iron (spectrum 2) and iron oxide (spectrum 3).
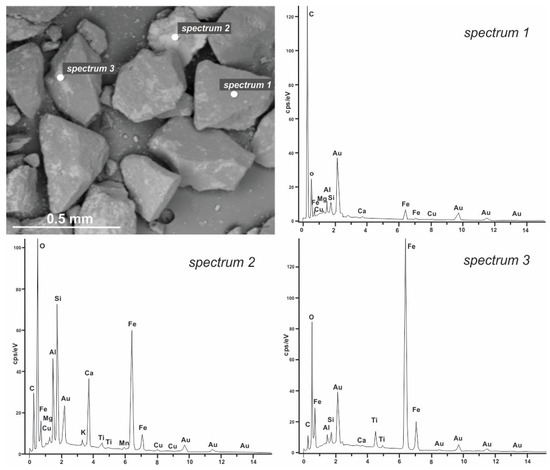
Figure 4.
BSE image of sample K1 with the marked analyzed points and obtained EDS spectra.
The mineral phases identified in the investigated samples by XRD and SEM-EDS analyses are shown in Table 4. X-ray diffraction analysis revealed the presence of magnetite in all magnetic fractions, while this phase was absent in non-magnetic fractions, which was expected. Hematite, gibbsite, and quartz are present in both fractions (Figure 5).
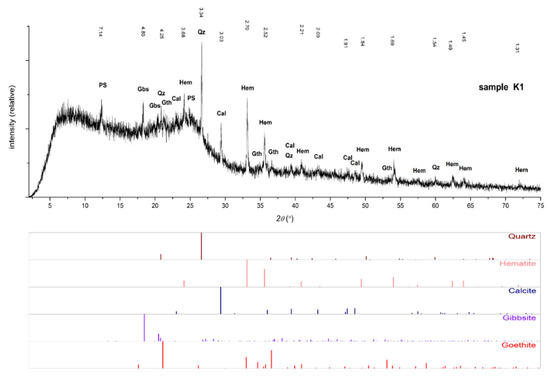
Figure 5.
Diffractogram of sample K1. Legend: Qz—quartz, Hem—hematite, Cal—calcite, Gabs—gibbsite, Gth—goethite, and PS—phyllosilicate.
Results of SEM-EDS analysis in Table 5 are given based on individual points analysis on the chosen grains that showed homogeneity in composition.

Table 5.
Mineral phases identified in investigated samples by XRD and SEM-EDS analyses.
EDS analysis showed high and elevated concentrations of carbon in all analyzed samples. Individual grains consisting mainly of carbon and oxygen were also observed. X-ray analysis determined that the phase that most corresponds to amorphous matter is present only in sample K1. Its presence is clearly visible as a raised base line on the diffractogram itself (Figure 5).
In general, the mineralogical composition of coal mainly includes phases such as quartz, muscovite, kaolinite, carbonates (calcite, dolomite, and siderite), oxides (magnetite and hematite), and pyrite, unless combustion has been carried out [18,19,20,21,22]. The presence of quartz, hematite, magnetite, calcite, dolomite, goethite, and layered silicates as the main phases, which may correspond to the composition of coal, is confirmed by X-ray analysis, with the fact that phases containing sulfur (e.g., sulfides) are missing in our samples. Gibbsite was also identified by this analysis, but its presence is unrelated to the previously described coal composition.
The heterogeneous nature of the material is more noticeable after elemental EDS mapping. The results of the representation of dominant elements in the samples are shown in Figure 6, Figure 7 and Figure 8.
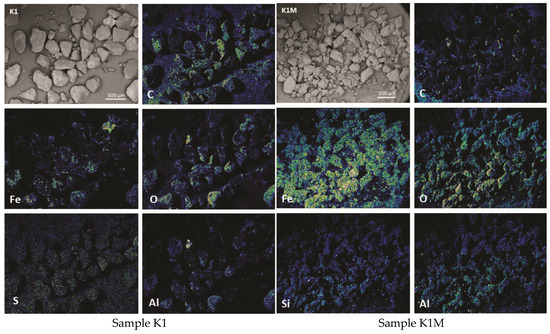
Figure 6.
Elemental maps showing major elements and BSE images of samples K1 and K1M.
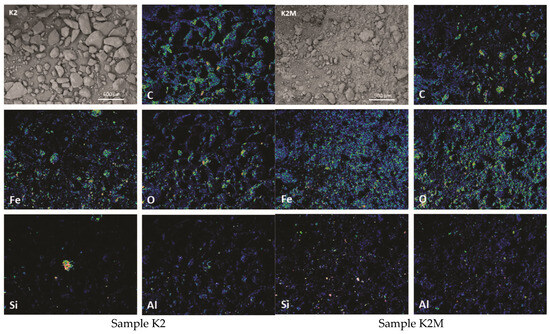
Figure 7.
Elemental maps showing major elements and BSE images of samples K2 and K2M.
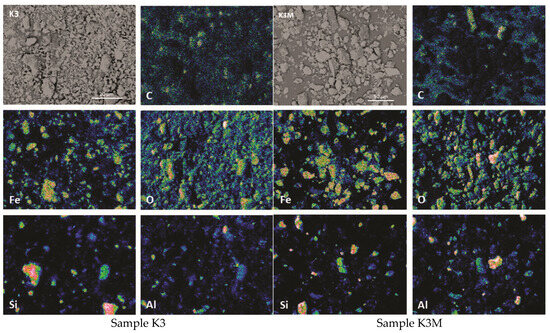
Figure 8.
Elemental maps showing major elements and BSE images of samples K3 and K3M.
In conclusion, SEM-EDS analysis of the samples revealed significant heterogeneity with dominant carbon and oxygen phases along with iron-rich grains, indicating a complex composition suitable for selective flocculation to recover valuable fine particles. The initial tests consisted of flotation and hydrocycloning separation trials, but the results were irrelevant, mainly regarding the separation of iron minerals from aluminum minerals. For this reason, selective flocculation was chosen as the separation method.
3.4. Selective Flocculation Tests
In solid–liquid systems, the rate of deposition of solids depends on the size and shape of grains, fluid and grain density, charge density, etc. Decreasing the particle size decreases its settling velocity. For this reason, a faster settling of fine particles using the flocculation technique is necessary. The main goal of this research was to use the selective flocculation procedure to obtain the highest possible percentage of iron oxide in the precipitated part and main impurities in the overflow. For selective flocculation, reagents (dispersant and flocculant) were chosen, which in earlier research showed good results when it came to iron oxides [23].
3.4.1. Initial Selective Flocculation Tests Under Different Conditions
In this chapter, the influences of pH, presence/absence of flocculant (PAM), and presence/absence of flocculant (PAM) and dispersant (SHMP) on the selectivity were tested. All flocculation tests were performed on starting raw material—sample K. Flocculation behavior was tested by monitoring the weight distribution and the content grade of basic chemical composition between the settling and floating parts. Each test was repeated five times in similar conditions, and the average was calculated. The results are presented in Table 6 and Figure 9.

Table 6.
Weight distribution and the content grade of basic chemical composition between the settling and floating parts of sample “K” (−0.5 mm).

Figure 9.
Selective flocculation at different pH (4, 6 and 10.5, from left to right).
Initial tests have shown that deposition at different pH values without the presence of reagents takes place slowly. At pH values of four and six, a clear zone above the precipitated sample is visible, while at pH 10.5 values, turbidity and poor visibility of the separation zone of the overflow and sediment occur.
With the addition of flocculant, A 100, at pH 10.5, a significant difference in mass distribution between settling and floating parts is visible. However, there is no difference in the content of the basic components. This indicates that the flocculant acts but non-selectively.
The test results with dispersants and flocculants showed that precipitation occurs at pH values of four and six and that there is no selectivity. Therefore, further tests were focused on pH 10.5, taking into account the mass distribution and the content of all tested components in the settling and floating parts. Due to the poor visibility of the separation zone between the overflow and sediment, the deposition time was extended to 5 min.
The best selectivity was achieved under these conditions, considering that the aim of this research was to increase the content of iron and reduce the content of impurities in the settling part and to reduce the content of iron and increase the content of other components in the floating part.
3.4.2. Selective Flocculation Tests Depending on Particle Size Classes
Further research went in the direction of performing a selective flocculation test on three different particle size classes (−450 + 250 µm, −250 + 25 µm, and −25 + 0 µm), samples labeled K1, K2, and K3. The goal was to determine content of the basic components depending on the size classes. The results are presented in Table 7.

Table 7.
Weight distribution and content grade of basic chemical composition between the settling and floating parts of K1, K2, and K3 samples depending on particle size classes.
As can be seen from Table 7, in the finest size class (−25 + 0 µm), there is the highest content of Fe in the input but also by products compared to the other size classes in the settled part. Also, in the finest class, there is a higher content of other tested components.
We observed that there are some differences between the input and output compositions. These discrepancies can arise due to various factors, and we are still investigating what the cause was. However, from a qualitative perspective, the results can be confirmed.
According to the obtained results, different particle sizes do not play a significant role in the distribution of masses between the settling and floating parts, especially in the case of the finer class. An increase in iron ore content after flocculation by comparison to the initial sample can be seen in the settling part. In the floating part, the Fe content decreases compared to the initial sample. Considering the mass distribution, the iron concentration in the settling part is quite satisfactory compared to the Fe content in the initial sample (34.18%) for all three size classes and especially in the finest class (38.05%). The best Fe recovery was achieved in the medium-sized class, but it was also satisfactory in other classes.
At the same time, there is an increase in the content of other elements in the floating part (and thus a decrease in the settling part), which was one of the goals of this research and indicates the possibility of selective flocculation of iron ore from other impurities in such complex systems. Theoretically, selective flocculation depends on many parameters, such as surface chemistry of particles, particle size distribution, degree of dispersion of particles, functional groups of the polymer, etc. According to the obtained results, we can consider this method as suitable.
4. Conclusions
The mineral phases in the sludge generated from processing “harbor waste” (sample K from the Constanta tailings plant) were examined. Chemical analyses, along with SEM-EDS and XRD, indicate that the material is mainly a mixture of iron ore, coal, and bauxite (gibbsite). The EDS analysis revealed that the dominant grains are primarily composed of carbon (up to 80% by weight) and oxygen, whereas the smaller grains mainly consist of iron and oxygen.
XRD confirmed the presence of quartz, hematite, magnetite, calcite, dolomite, goethite, and layered silicates—phases typically associated with coal—while sulphur-containing phases (e.g., sulfides) were not detected.
Additionally, studies were carried out to assess the feasibility of using selective flocculation to separate iron minerals from other impurities, employing a commercial flocculant based on anionic polyacrylamide. The findings indicate that improved selectivity of the settled material is achievable at pH 10.5 and when the deposition time is extended from one to five minutes.
An increase in iron ore content after flocculation by comparison to the initial sample (34.18%) can be seen, especially in the case of the −25 + 0 µm class (41.38%), depending on the particle size class, as presented in Table 7.
Considering the results obtained and the numerous factors influencing the selective flocculation process, further investigation is essential to optimize outcomes in such complex systems. Future research will explore extending the settling time, testing alternative flocculants and dispersants, and varying their concentrations and dosages.
These preliminary tests have undoubtedly marked the beginning of a new phase in the study of selective flocculation for “harbor waste” (sample K). This advancement is expected to enhance the recovery of iron ore and consequently reduce waste sludge deposition, thereby contributing to the protection of both the living and working environment.
Author Contributions
Conceptualization, A.-F.M. and L.T.; methodology, L.T., A.-F.M. and M.L.; validation, A.-F.M., L.T. and S.S.; formal analysis, A.Z.; resources, C.T. and E.T.; data curation A.-F.M. and L.T.; writing—original draft preparation, L.T.; writing—review and editing, A.-F.M. and L.T.; supervision, A.-F.M., L.T., M.L., A.Z. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mihai, A.; Lazar, M.; Badulescu, C.; Traista, C. The Origin of Mineral Waste from Constanta Port and the Necessity to Introduce it into the Circular Economy. Min. Rev. 2024, 29, 87–95. [Google Scholar] [CrossRef]
- Tammishetti, V.; Kumar, D.; Rai, B.; Pradip Shukla, V.; Patra, A.S.; Chakraborty, D.P.; Kumar, A. Selective Flocculation of Iron Ore Slimes: Results of Successful Pilot Plant Trials at Tata Steel, Noamundi. Trans. Indian Inst. Met. 2017, 70, 411–419. [Google Scholar] [CrossRef]
- Sarma, J.; Rajkhowa, S.; Mahiuddin, S. Upgradation of Iron Ore Fines and Slime by Selective Flocculation Using Surface-Active Agents, Settling Study, and Characterization of the Beneficiation Waste for Value Addition. J. Chem. 2022, 2022, 6451187. [Google Scholar] [CrossRef]
- Alvim, E.S.; Fernandes Lima, R.M. Selective Flocculation/Magnetic Separation of Ultrafine Iron Ore Particles with Corn Starch and Polyacrylamides: A Comparative Study. Miner. Process. Extr. Metall. Rev. 2023, 45, 606–615. [Google Scholar] [CrossRef]
- Mihai, A.; Mărin, N.; Tankosic, L. Agrochemical Effects of Tailings from Mineral Waste Processing Plant on Wheat Crop. In Proceedings of the XIX Balkan Mineral Processing Congress (XIX BMPC), Mitrovica, Kosovo, 28–31 May 2023; pp. 215–220. [Google Scholar]
- Cytec Industries Inc. Cytec Mining Chemicals Handbook; Cytec Industries Inc.: Woodland Park, NJ, USA, 2010; pp. 264–273. Available online: https://pdfcoffee.com/mining-chemicals-handbook-revised-edition-pdf-free.html, (accessed on 13 May 2025).
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; Volume 15, ISBN 9781780407500. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Lawler, D.F. Water Quality Engineering: Physical/Chemical Treatment Processes; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-16965-0. [Google Scholar]
- Zhu, G.; Zheng, H.; Zhang, Z.; Tshukudu, T.; Zhang, P.; Xiang, X. Characterization and coagulation–flocculation behavior of polymeric aluminum ferric sulfate (PAFS). Chem. Eng. J. 2011, 178, 50–59. [Google Scholar] [CrossRef]
- Lal, K.; Garg, A. Effectiveness of synthesized aluminum and iron based inorganic polymer coagulants for pulping wastewater treatment. J. Environ. Chem. Eng. 2019, 7, 103204. [Google Scholar] [CrossRef]
- Mathur, S.; Singh, P.; Moudgil, B.M. Advances in selective flocculation technology for solid-solid separations. Int. J. Miner. Process. 2000, 58, 201–222. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.; Hadoudi, N.; Charki, A.; Bensitel, N.; Ouarghi, H.E.; Amhamdi, H.; Ahari, M. Advancements in the chemical treatment of potable water and industrial wastewater using the coagulation–flocculation process. Sep. Sci. Technol. 2023, 58, 2619–2630. [Google Scholar] [CrossRef]
- Kruyt, H.R.; Overbee, J.T.G. Introductory Paper: Theoretical Treatment of the Double Layer. 1940, pp. 110–116. Available online: https://overbeek.sites.uu.nl/wp-content/uploads/sites/863/2022/08/012.pdf (accessed on 13 May 2025).
- Dukhin, A.S.; Dukhin, S.S.; Goetz, P.J. Gravity as a factor of aggregative stability and coagulation. Adv. Colloid Interface Sci. 2007, 134–135, 35–71. [Google Scholar] [CrossRef] [PubMed]
- Carmignano, O.R.; Vieira, S.S.; Teixeira, A.P.C.; Lameiras, F.S.; Brandão, P.R.G.; Lago, R.M. Iron Ore Tailings: Characterization and Applications. J. Braz. Chem. Soc. 2021, 32, 1895–1911. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Černík, M.; Kromková, Z.; Vranec, P.; Mašlejová, A. X-Ray Diffraction Analysis of Coal Materials. Structure 2015, 22, 172–174. [Google Scholar]
- Ritz, M.; Klika, Z. Determination of minerals in coal by methods based on the recalculation of the bulk chemical analysis. Acta Geodyn. Geomater. 2010, 7, 453–460. [Google Scholar]
- Siddigui, I.; Shah, M.T.; Ahmed, I. X-Ray Diffraction Analyses of Thar, Sonda and Meting—Jhimpir Coalfields, Sindh. Sindh Univ. Res. J. 2009, 41, 67–74. [Google Scholar]
- Silva, L.F.O.; Sampaio, C.H.; Guedes, A.; de Vallejuelo, S.F.-O.; Madariaga, J.M. Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel 2012, 94, 52–63. [Google Scholar] [CrossRef]
- Guiling, X.; Ping, L.; Cai, L.; Pan, X.; Liu, S.; Daoyin, L.; Xiaoping, C. Effect of powder properties on discharge characteristics of cohesive carbonaceous fuel powders from a top discharge blow tank at high pressure. Chem. Eng. Commun. 2018, 205, 1604–1621. [Google Scholar] [CrossRef]
- Tankosić, L.; Tančić, P.; Sredić, S.; Nedić, Z. Comparative Study of the Mineral Composition and Its Connection with Some Properties Important for the Sludge Flocculation Process-Examples from Omarska Mine. Minerals 2018, 8, 119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).