The Effect of Combined Extracts from By-Products, Seaweed, and Pure Phenolics on the Quality of Vacuum-Packed Fish Burgers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Natural Extracts and Pure Compounds
2.2. Preparation of Fish Burgers
2.3. Chemical Analyses
2.4. Microbiological Analyses
2.5. Lipid Extraction and Determination of Fatty Acid Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Results of Chemical Analyses
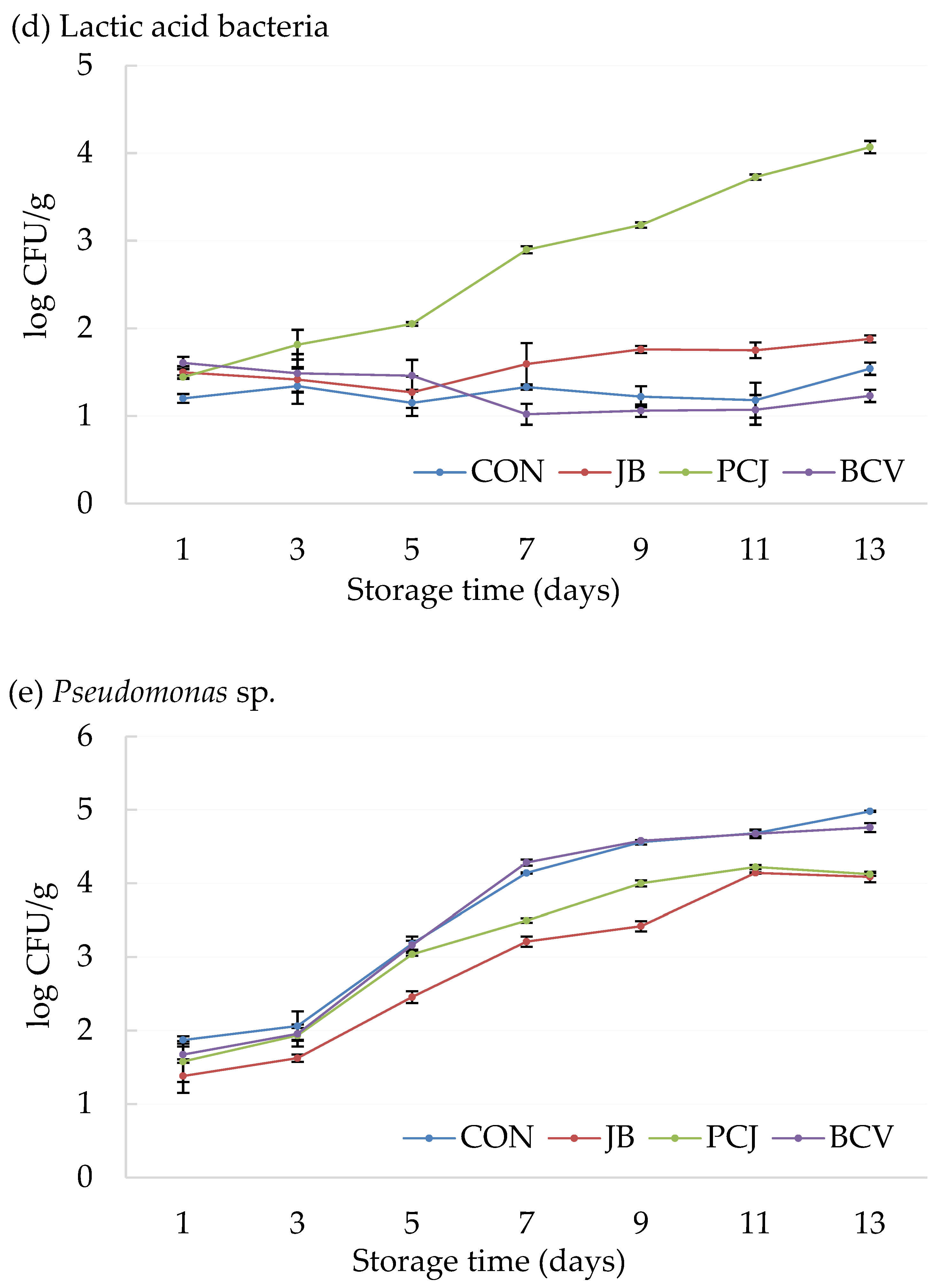
3.2. The Results of Microbiological Analyses
3.3. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Ralston, N.V.C. Seafood and Health: What You Need to Know? In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 275–318. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A Critical Review on the Health Benefits of Fish Consumption and Its Bioactive Constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.O.; Scholderer, J.; Brunsø, K.; Verbeke, W. Exploring the Relationship between Convenience and Fish Consumption: A Cross-Cultural Study. Appetite 2007, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Dalgaard, P. Qualitative and Quantitative Characterization of Spoilage Bacteria from Packed Fish. Int. J. Food Microbiol. 1995, 26, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ucar, Y.; Durmus, M.; Özogul, Y. Natural Preservatives for Fish and Seafood. In Natural Preservatives for Food; Elsevier: Amsterdam, The Netherlands, 2025; pp. 193–220. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, I.C.F.R. (Eds.) Natural Additives in Food; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Šimat, V.; Čagalj, M.; Skroza, D.; Gardini, F.; Tabanelli, G.; Montanari, C.; Hassoun, A.; Özogul, F. Sustainable Sources for Antioxidant and Antimicrobial Compounds Used in Meat and Seafood Products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 55–118. [Google Scholar] [CrossRef]
- Parmar, B.K.; Mohite, A.S.; Pathan, D.I.; Desai, A.S.; Wasave, S.M. The Use of Herbs and Spices in Fish Preservation at Chilled Temperature Storage: Opportunities and Challenges. Int. J. Food Sci. Technol. 2024, 59, 6758–6768. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural Products with Preservative Properties for Enhancing the Microbiological Safety and Extending the Shelf-Life of Seafood: A Review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef]
- Lekshmi, S.; Xavier, K.A.M.; Kumar, S.; Balange, A.K. The Preservative Impact of the Brown Seaweed (Padina tetrastromatica) Extract on the Quality of Tilapia (Oreochromis mossambicus) during Chilled Storage. J. Food Process. Preserv. 2021, 45, e15931. [Google Scholar] [CrossRef]
- Hafez, M.S.M.A.E.; Rashedy, S.H.; Abdelmotilib, N.M.; El-Hassayeb, H.E.A.; Cotas, J.; Pereira, L. Fillet Fish Fortified with Algal Extracts of Codium Tomentosum and Actinotrichia Fragilis, as a Potential Antibacterial and Antioxidant Food Supplement. Mar. Drugs 2022, 20, 785. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.; Miranda, J.M.; Trigo, M.; Rodríguez-Félix, F.; Aubourg, S.P.; Barros-Velázquez, J. Antioxidant and Antimicrobial Effect of Biodegradable Films Containing Pitaya (Stenocereus thurberi) Extracts during the Refrigerated Storage of Fish. Antioxidants 2023, 12, 544. [Google Scholar] [CrossRef]
- Ozogul, F.; Durmuş, M.; Kosker, A.R.; Özkütük, A.S.; Kuley, E.; Yazgan, H.; Yazgan, R.; Šimat, V.; Ozogul, Y. The Impact of Marine and Terrestrial Based Extracts on the Freshness Quality of Modified Atmosphere Packed Sea Bass Fillets. Food Biosci. 2023, 53, 102545. [Google Scholar] [CrossRef]
- Barros, D.; Nova, P.; Cunha, S.; Monteiro, V.; Fernandes, É.; Pereira-Pinto, R.; Barbosa, C.; Pintado, M.; Gomes, A.; Vaz-Velho, M. Enhancing Storage Stability of Smoke-Flavored Horse Mackerel Filets Using Natural Extracts as Preservatives. Front. Sustain. Food Syst. 2023, 7, 1296265. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Conte, A.; Caroprese, M. Combined Effect of MAP and Active Compounds on Fresh Blue Fish Burger. Int. J. Food Microbiol. 2009, 135, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, L.; Spinelli, S.; Marinelli, V.; Conte, A.; Del Nobile, M.A. Extract from Broccoli By-Products to Extend the Shelf Life of Fish Burgers. J. Food Res. 2019, 8, 56. [Google Scholar] [CrossRef]
- Panza, O.; Conte, A.; Del Nobile, M.A. Zero-Waste Approach Applied to Pomegranates for Prolonging Fish Burger Shelf Life. Foods 2022, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Fuentes, A.; Lerma-García, M.J.; Ayadi, M.A.; Bouaziz, M.; Barat, J.M. Olive Leaf Extracts for Shelf Life Extension of Salmon Burgers. Food Sci. Technol. Int. 2019, 25, 91–100. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Šimat, V.; Skroza, D.; Čagalj, M.; Smole-Možina, S.; Bassi, D.; Gardini, F.; Tabanelli, G. Effects of Rubus fruticosus and Juniperus oxycedrus Derivatives on Culturability and Viability of Listeria monocytogenes. Sci. Rep. 2022, 12, 13158. [Google Scholar] [CrossRef]
- Frleta Matas, R.; Popović, M.; Čagalj, M.; Šimat, V. The Marine Diatom Thalassiosira rotula: Chemical Profile and Antioxidant Activity of Hydroalcoholic Extracts. Front. Mar. Sci. 2023, 10, 1221417. [Google Scholar] [CrossRef]
- Frleta Matas, R.; Radman, S.; Čagalj, M.; Šimat, V. Influence of Nutrient Deprivation on the Antioxidant Capacity and Chemical Profile of Two Diatoms from Genus Chaetoceros. Mar. Drugs 2024, 22, 96. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.d.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Šimat, V.; Čagalj, M.; Generalić Mekinić, I.; Smole Možina, S.; Malin, V.; Tabanelli, G.; Özogul, F.; Skroza, D. Antioxidant and Antimicrobial Activity of Extracts from Selected Mediterranean Agro-Food by-Products, Their Mutual Interaction and Interaction with Phenolic Compounds. Food Biosci. 2024, 61, 104599. [Google Scholar] [CrossRef]
- Čagalj, M.; Fras Zemljič, L.; Kraševac Glaser, T.; Mežnar, E.; Sterniša, M.; Smole Možina, S.; Razola-Díaz, M.d.C.; Šimat, V. Seasonal Changes in Chemical Profile and Antioxidant Activity of Padina Pavonica Extracts and Their Application in the Development of Bioactive Chitosan/PLA Bilayer Film. Foods 2022, 11, 3847. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Šimat, V.; Bogdanović, T.; Krželj, M.; Soldo, A.; Maršić-Lučić, J. Differences in Chemical, Physical and Sensory Properties during Shelf Life Assessment of Wild and Farmed Gilthead Sea Bream (Sparus aurata, L.). J. Appl. Ichthyol. 2012, 28, 95–101. [Google Scholar] [CrossRef]
- Lemon, D.W. An Improved TBA Test for Rancidity; Halifax Laboratory: Halifax, NS, Canada, 1975. [Google Scholar]
- Vyncke, W. Direct Determination of the Thiobarbituric Acid Value in Trichloroacetic Acid Extracts of Fish as a Measure of Oxidative Rancidity. Fette Seifen Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Šimat, V.; Maršić-Lučić, J.; Tudor, M.; Mladineo, I. Long-Term Storage Influence on Volatile Amines (TVB-N and TMA-N) in Sardines and Herring Utilized as Food for Tuna Fattening. J. Appl. Ichthyol. 2009, 25, 766–770. [Google Scholar] [CrossRef]
- Eerola, S.; Hinkkanen, R.; Lindfors, E.; Hirvi, T. Liquid Chromatographic Determination of Biogenic Amines in Dry Sausages. J. AOAC Int. 1993, 76, 575–577. [Google Scholar] [CrossRef]
- Šimat, V.; Dalgaard, P. Use of Small Diameter Column Particles to Enhance HPLC Determination of Histamine and Other Biogenic Amines in Seafood. LWT—Food Sci. Technol. 2011, 44, 399–406. [Google Scholar] [CrossRef]
- Hungerford, J.M. Fish and Other Marine Products. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995; Volume 2, pp. 1–30. [Google Scholar]
- Šimat, V.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Kuley, E.; Kosker, A.R.; Uçar, Y.; Yazgan, H.; Durmuş, M.; Sakarya, Y.; Takadaş, F.; Özkütük, S.T.; Özkütük, A.S.; et al. The Impacts of Biopreservation with Latilactobacillus Sakei Cell-Free Supernatant in Combination with Plant-Based Extracts on the Quality of Modified Atmosphere Packed Sea Bass (Dicentrarchus labrax) Fillets. LWT 2024, 209, 116756. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO: Rome, Italy, 1995. [Google Scholar]
- Pezeshk, S.; Ojagh, S.M.; Alishahi, A. Effect of Plant Antioxidant and Antimicrobial Compounds on the Shelf-Life of Seafood—A Review. Czech J. Food Sci. 2015, 33, 195–203. [Google Scholar] [CrossRef]
- Ahmed, E.-S.S.; Shehata, M.G.; Abd-Rabou, H.S.; El-Menshawy, H. Extend Shelf-Life of Vacuum-Packaged Herring Fish Fillets Using Garlic and Ginger Extracts. J. Pure Appl. Microbiol. 2019, 13, 1571–1581. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Čagalj, M.; Soldo, B.; Generalić Mekinić, I. Effect of Plant Extracts on Quality Characteristics and Shelf-Life of Cold-Marinated Shrimp (Parapenaeus longirostris, Lucas, 1846) under Refrigerated Storage. Food Biosci. 2023, 53, 102673. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Özpolat, E. The Effects of Juniperi Fructus Essential Oil and Vacuum Packing on the Shelf Life of Rainbow Trout Fillets during Storage at 2 ± 1 °C. Cell. Mol. Biol. 2024, 70, 29–34. [Google Scholar] [CrossRef]
- Pan, Z.; Li, L.; Shen, Z.; Chen, Y.; Li, M. Effects of Tea Polyphenol Treatments on the Quality and Microbiota of Crisp Grass Carp Fillets during Storage at 4 °C. Appl. Sci. 2021, 11, 4370. [Google Scholar] [CrossRef]
- Uçak, İ. Investigation of Oxidative, Microbial and Sensory Quality Changes of Fish Burgers Enriched with Pomegranate Seed Extract. Food Health 2020, 6, 238–247. [Google Scholar] [CrossRef]
- Baixas-Nogueras, S.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Suitability of Volatile Amines as Freshness Indexes for Iced Mediterranean Hake. J. Food Sci. 2003, 68, 1607–1610. [Google Scholar] [CrossRef]
- Sánchez-García, F.; Machado, N.D.; Tirado-Fernández, M.; Cejudo-Bastante, C.; Roldán, A.M.; Mantell-Serrano, C.; Casas-Cardoso, L. Hake Fish Preservation Using Plant-Based Impregnated Polylactic Acid Food Films as Active Packaging. Appl. Sci. 2025, 15, 643. [Google Scholar] [CrossRef]
- Kuley, E.; Durmus, M.; Balikci, E.; Ucar, Y.; Regenstein, J.M.; Özoğul, F. Fish Spoilage Bacterial Growth and Their Biogenic Amine Accumulation: Inhibitory Effects of Olive by-Products. Int. J. Food Prop. 2017, 20, 1029–1043. [Google Scholar] [CrossRef]
- Ozogul, Y.; Durmus, M.; Kuley Boga, E.; Uçar, Y.; Ozogul, F. The Function of Emulsions on the Biogenic Amine Formation and Their Indices of Sea Bass Fillets (Dicentrarchus labrax) Stored in Vacuum Packaging. J. Food Sci. 2018, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Tsafack, P.B.; Tsopmo, A. Effects of Bioactive Molecules on the Concentration of Biogenic Amines in Foods and Biological Systems. Heliyon 2022, 8, e10456. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F.; Kuley, E.; Kenar, M. Effects of Rosemary and Sage Tea Extract on Biogenic Amines Formation of Sardine (Sardina pilchardus) Fillets. Int. J. Food Sci. Technol. 2011, 46, 761–766. [Google Scholar] [CrossRef]
- Feng, H.; Liu, J.; Timira, V.; Lin, H.; Wang, H.; Li, Z. Effects of Plant Extracts (Clove, Fennel, Rosemary, Cinnamon, and Tea Polyphenols) on Microbiological, Chemical, and Sensory Quality of Smoke-Flavored Sea Bass during Chilled Storage. ACS Food Sci. Technol. 2022, 2, 1692–1700. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of Vacuum Impregnated Fish Gelatin and Grape Seed Extract on Moisture State, Microbiota Composition, and Quality of Chilled Seabass Fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef]
- Kuley, E.; Yazgan, H.; Özogul, Y.; Ucar, Y.; Durmus, M.; Özyurt, G.; Ayas, D. Effectiveness of Lactobacilli Cell-Free Supernatant and Propolis Extract Microcapsules on Oxidation and Microbiological Growth in Sardine Burger. Food Biosci. 2021, 44, 101417. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food; National Academies Press (US): Washington, DC, USA, 2003. [CrossRef]

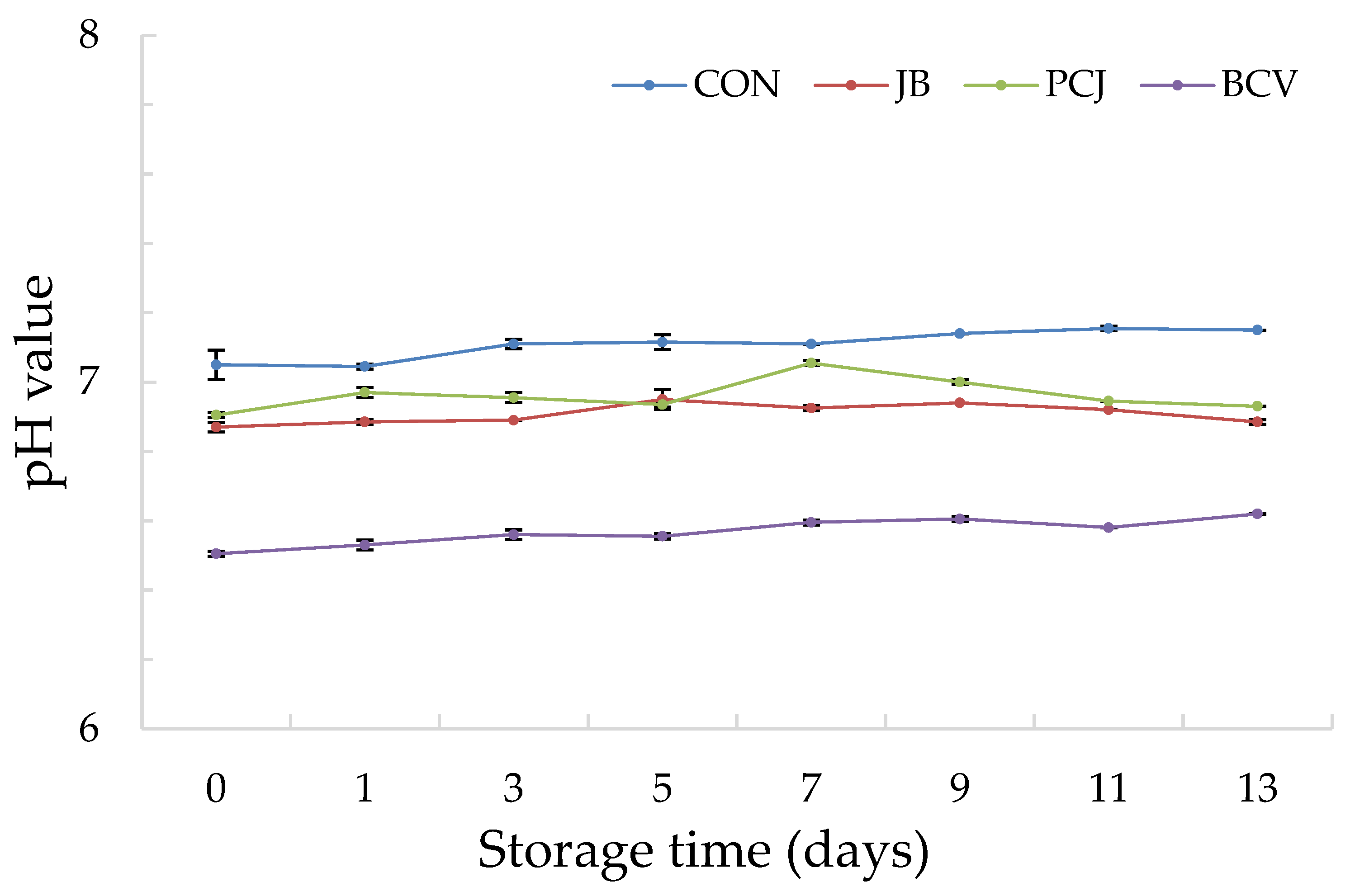
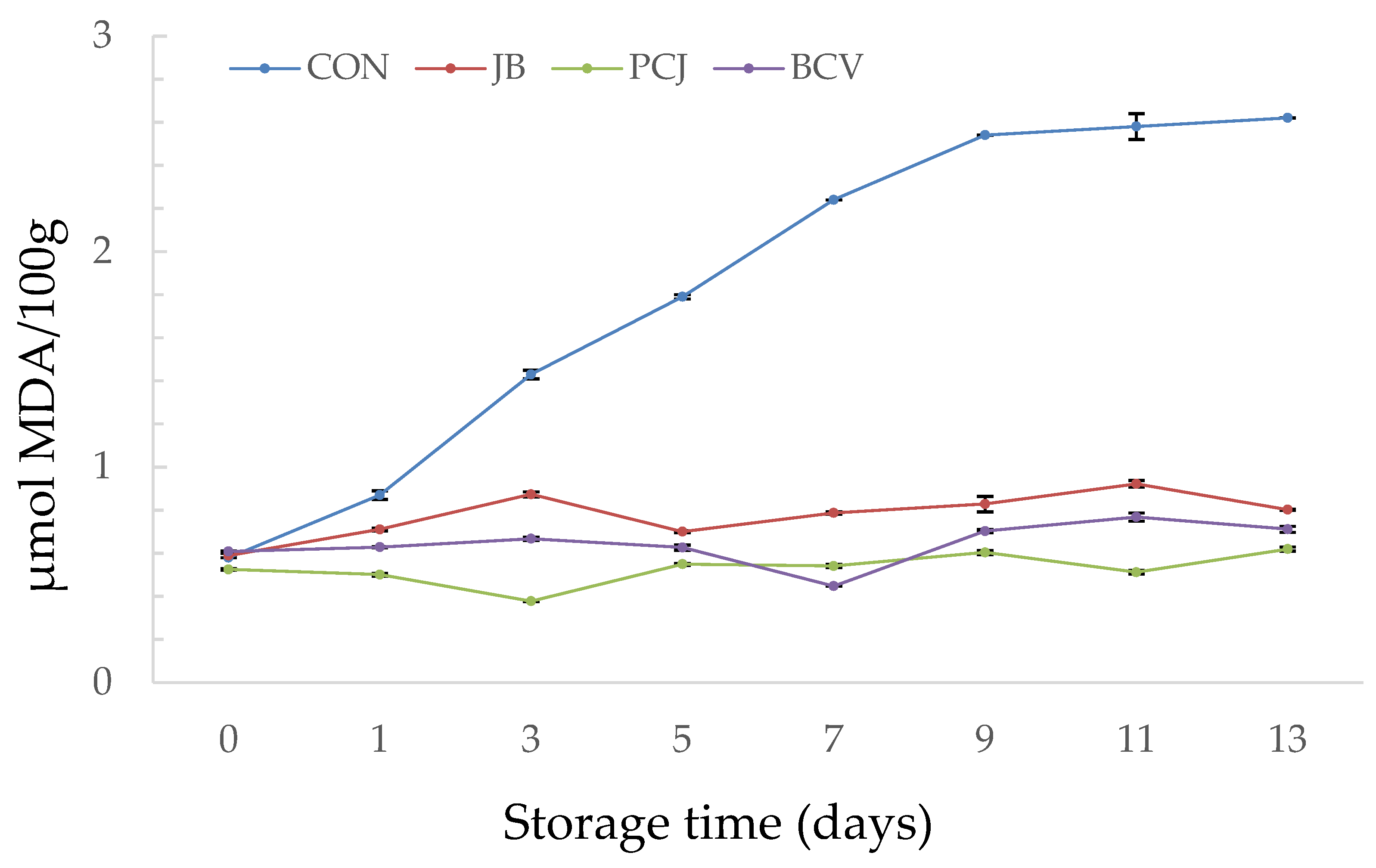
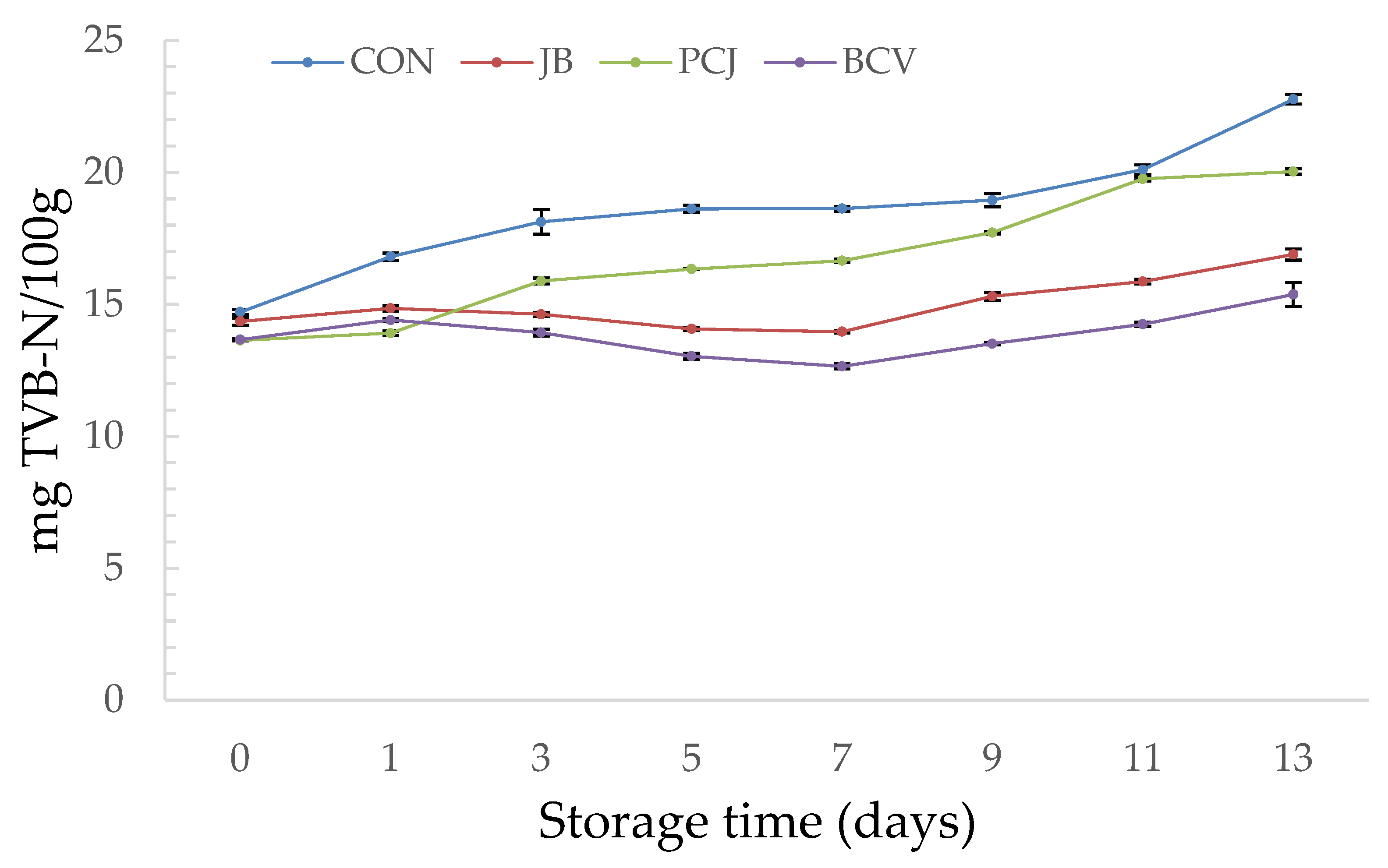
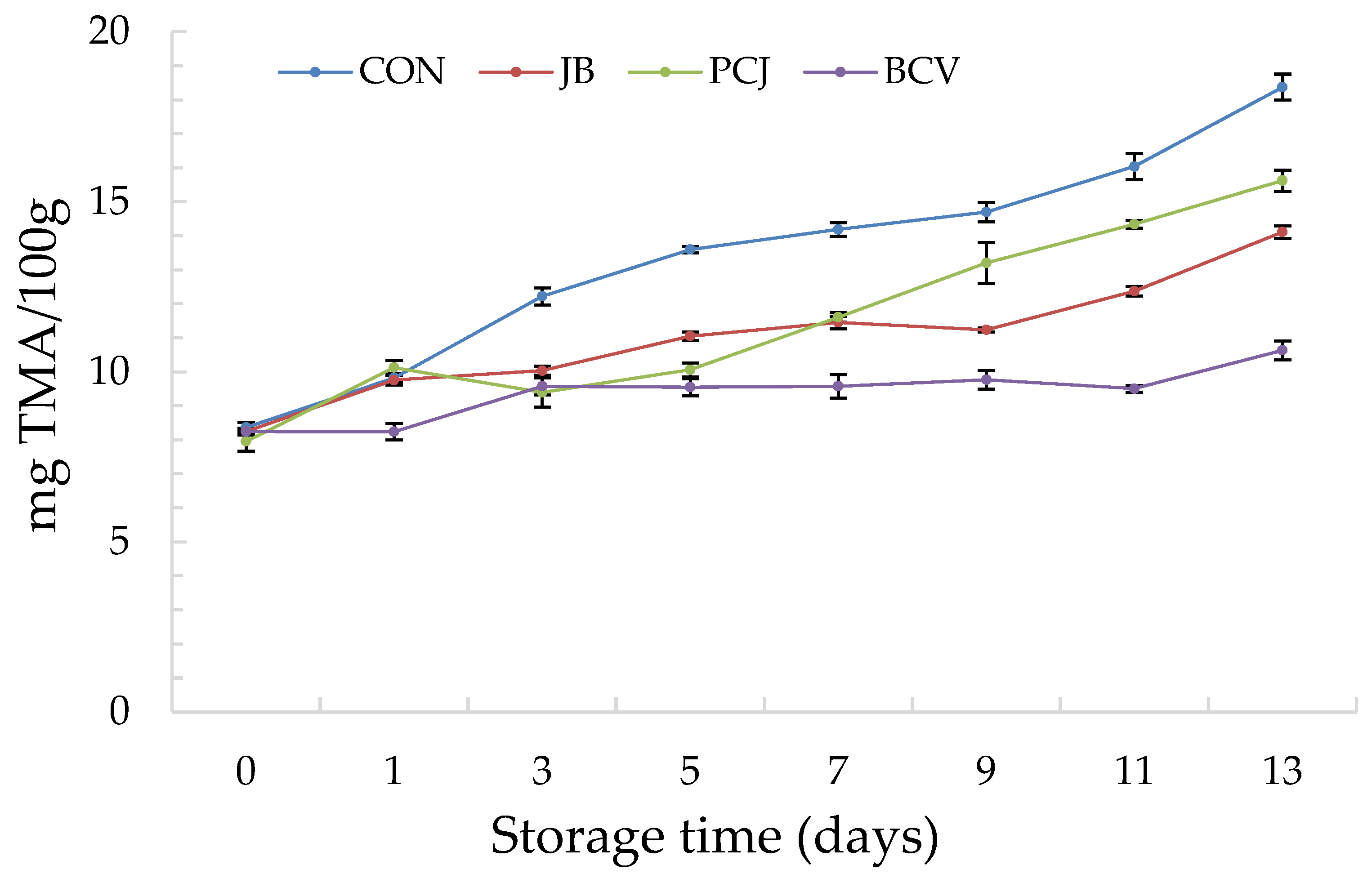

| Fish Burger Sample | Abbreviation |
|---|---|
| Burger without natural extracts (control) | CON |
| Burger with 2% (w/w) common juniper by-product and blackberry leaf extract (1:2 ratio) | JB |
| Burger with 2% (w/w) Padina pavonica and prickly juniper needle extract (1:1 ratio) | PCJ |
| Burger with 2% (w/w) blackberry leaf extract, catechin, and vanillic acid (2:1:1 ratio) | BCV |
| Treatment Group | Storage Time | Biogenic Amine (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β-Phenethylamine | Tryptamine | Putrescine | Cadaverine | Histamine | Tyramine | Spermidine | Spermine | ||
| CON | 0 | 6.44 ± 0.19 a | 0.24 ± 0.05 a | 0.52 ± 0.10 a | 0.78 ± 0.07 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.84 ± 0.02 a | 2.12 ± 0.08 a |
| 1 | 4.58 ± 0.13 b | 0.18 ± 0.02 a | 0.46 ± 0.08 a | 0.89 ± 0.09 a | 0.00 ± 0.00 | 0.62 ± 0.10 a | 0.82 ± 0.03 a | 1.48 ± 0.11 b | |
| 3 | 4.82 ± 0.11 b | 1.22 ± 0.11 b | 0.48 ± 0.05 a | 1.22 ± 0.12 b | 0.00 ± 0.00 | 1.80 ± 0.13 b | 0.64 ± 0.02 a | 2.12 ± 0.10 a | |
| 5 | 5.24 ± 0.22 a | 3.54 ± 0.09 c | 0.61 ± 0.07 a | 2.49 ± 0.13 c | 0.00 ± 0.00 | 0.90 ± 0.10 a | 0.87 ± 0.02 a | 2.08 ± 0.13 a | |
| 7 | 1.10 ± 0.27 c | 3.71 ± 0.54 c | 0.92 ± 0.04 b | 2.52 ± 0.08 c | 0.00 ± 0.00 | 0.78 ± 0.11 a | 0.01 ± 0.01 b | 1.19 ± 0.06 b | |
| 9 | 1.38 ± 0.32 c | 3.14 ± 0.48 c | 0.98 ± 0.15 b | 2.50 ± 0.19 c | 0.00 ± 0.00 | 0.58 ± 0,09 a | 0.00 ± 0.00 | 1.39 ± 0.05 a | |
| 11 | 0.29 ± 0.06 d | 3.27 ± 0.38 c | 1.44 ± 0.11 c | 3.66 ± 0.03 d | 0.00 ± 0.00 | 0,67 ± 0.08 a | 0.00 ± 0.00 | 1.28 ± 0.03 a | |
| 13 | 0.81 ± 0.18 d | 6.34 ± 0.41 d | 3.05 ± 0.06 d | 3.61 ± 0.00 d | 0.00 ± 0.00 | 0.73 ± 0.07 a | 0.00 ± 0.00 | 0.97 ± 0.01 c | |
| JB | 0 | 8.44 ± 0.36 a | 0.32 ± 0.09 a | 0.25 ± 0.07 a | 0.59 ± 0.05 a | 0.00 ± 0.00 | 1.12 ± 0.34 a | 0.98 ± 0.21 a | 2.17 ± 0.59 a |
| 1 | 4.33 ± 0.21 b | 0.18 ± 0.04 a | 0.26 ± 0.05 a | 1.39 ± 0.11 b | 0.00 ± 0.00 | 1.47 ± 0.48 a | 1.98 ± 0.26 b | 4.78 ± 0.22 b | |
| 3 | 6.62 ± 0.17 c | 1.72 ± 0.11 b | 0.14 ± 0.02 a | 1.54 ± 0.12 b | 0.00 ± 0.00 | 2.98 ± 0.29 b | 1.20 ± 0.18 b | 3.02 ± 0.17 c | |
| 5 | 8.37 ± 0.33 a | 1.75 ± 0.12 b | 0.54 ± 0.09 a | 1.21 ± 0.01 b | 0.00 ± 0.00 | 5.48 ± 0.46 c | 1.37 ± 0.31 b | 3.10 ± 0.04 c | |
| 7 | 4.13 ± 1.16 b | 2.37 ± 0.16 c | 0.51 ± 0.26 a | 1.24 ± 0.14 b | 0.00 ± 0.00 | 5.79 ± 1.29 c | 0.31 ± 0.11 c | 2.27 ± 0.18 a | |
| 9 | 3.70 ± 0.23 b | 3.62 ± 0.99 d | 0.81 ± 0.13 b | 1.48 ± 0.18 b | 0.00 ± 0.00 | 5.90 ± 1.21 c | 0.27 ± 0.19 c | 2.16 ± 0.01 a | |
| 11 | 2.97 ± 0.03 d | 3.51 ± 0.28 d | 1.38 ± 0.06 c | 2.76 ± 0.29 c | 0.00 ± 0.00 | 6.16 ± 0.59 c | 0.33 ± 0.11 c | 2.71 ± 0.10 a | |
| 13 | 2.66 ± 0.03 d | 6.38 ± 0.56 e | 3.49 ± 0.09 d | 2.68 ± 0.16 c | 0.00 ± 0.00 | 5.67 ± 0.04 c | 0.18 ± 0.03 c | 1.86 ± 0.01 a | |
| PCJ | 0 | 3.10 ± 0.09 a | 0.74 ± 0.12 a | 0.00 ± 0.00 | 0.42 ± 0.04 a | 0.00 ± 0.00 | 1.34 ± 0.04 a | 0.00 ± 0.00 | 1.05 ± 0.04 a |
| 1 | 3.19 ± 0.04 a | 0.82 ± 0.16 a | 0.00 ± 0.00 | 0.10 ± 0.06 a | 0.00 ± 0.00 | 1.69 ± 0.10 a | 0.00 ± 0.00 | 0.50 ± 0.05 b | |
| 3 | 1.47 ± 0.06 b | 2.48 ± 0.21 b | 0.00 ± 0.00 | 0.63 ± 0.05 a | 0.00 ± 0.00 | 3.20 ± 0.19 b | 0.00 ± 0.00 | 0.63 ± 0.07 b | |
| 5 | 4.22 ± 0.11 c | 4.01 ± 0.43 c | 0.00 ± 0.00 | 2.99 ± 0.07 b | 0.00 ± 0.00 | 5.70 ± 0.14 c | 0.00 ± 0.00 | 1.41 ± 0.11 a | |
| 7 | 2.29 ± 0.01 b | 5.74 ± 0.60 d | 2.78 ± 0.10 a | 2.95 ± 0.11 b | 0.00 ± 0.00 | 6.01 ± 0.05 c | 0.00 ± 0.00 | 0.66 ± 0.02 b | |
| 9 | 1.13 ± 0.14 b | 7.26 ± 1.04 e | 17.66 ± 0.19 b | 3.06 ± 0.22 b | 0.00 ± 0.00 | 9.12 ± 0.20 d | 0.00 ± 0.00 | 0.86 ± 0.07 b | |
| 11 | 2.12 ± 0.10 b | 5.15 ± 1.09 d | 25.15 ± 0.73 c | 4.74 ± 0.19 c | 0.00 ± 0.00 | 12.21 ± 0.06 e | 0.00 ± 0.00 | 0.56 ± 0.12 b | |
| 13 | 0.98 ± 0.00 d | 6.28 ± 1.02 e | 28.02 ± 0.16 c | 8.69 ± 0.29 d | 0.00 ± 0.00 | 15.89 ± 0.31 f | 0.00 ± 0.00 | 0.50 ± 0.01 b | |
| BCV | 0 | 5.87 ± 0.18 a | 0.36 ± 0.06 a | 0.23 ± 0.04 a | 0.41 ± 0.03 a | 0.00 ± 0.00 | 1.96 ± 0.22 a | 0.69 ± 0.07 a | 2.20 ± 0.16 a |
| 1 | 5.91 ± 0.14 a | 0.64 ± 0.05 a | 0.24 ± 0.07 a | 0.18 ± 0.01 a | 0.00 ± 0.00 | 1.92 ± 0.28 a | 1.26 ± 0.12 b | 2.36 ± 0.15 a | |
| 3 | 7.07 ± 0.52 b | 3.11 ± 0.23 b | 0.32 ± 0.03 a | 0.26 ± 0.02 a | 0.00 ± 0.00 | 2.24 ± 0.18 a | 1.05 ± 0.13 b | 2.30 ± 0.21 a | |
| 5 | 8.36 ± 0.24 c | 3.88 ± 0.28 b | 0.00 ± 0.00 | 1.32 ± 0.03 b | 0.00 ± 0.00 | 6.38 ± 0.24 b | 0.76 ± 0.05 a | 1.44 ± 0.09 b | |
| 7 | 9.56 ± 0.76 c | 6.03 ± 1.03 c | 0.00 ± 0.00 | 0.45 ± 0.01 a | 0.00 ± 0.00 | 4.63 ± 0.11 c | 0.45 ± 0.17 a | 3.73 ± 0.92 c | |
| 9 | 7.11 ± 0.68 b | 6.88 ± 1.11 c | 0.00 ± 0.00 | 0.10 ± 0.00 c | 0.00 ± 0.00 | 4.17 ± 0.04 c | 1.74 ± 0.41 c | 2.93 ± 0.30 a | |
| 11 | 7.94 ± 0.04 b | 6.77 ± 1.68 c | 0.00 ± 0.00 | 0.09 ± 0.01 c | 0.00 ± 0.00 | 4.83 ± 0.13 c | 1.00 ± 0.03 b | 3.64 ± 0.21 c | |
| 13 | 3.15 ± 0.16 a | 9.88 ± 0.36 d | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.52 ± 0.05 c | 0.89 ± 0.33 b | 2.42 ± 0.16 a | |
| Treatment Group | CON | JB | PCJ | BCV | ||||
|---|---|---|---|---|---|---|---|---|
| Storage Time (Days) | 1 | 11 | 1 | 11 | 1 | 11 | 1 | 11 |
| Fatty Acids (%) | ||||||||
| C6:0 | 0.20 ± 0.05 a | 0.02 ± 0.02 b | 0.20 ± 0.04 a | 0.03 ± 0.03 | 0.15 ± 0.15 c | 0.15 ± 0.10 c | 0.13 ± 0.06 c | 0.08 ± 0.08 b |
| C8:0 | 0.01 ± 0.01 a | n.d. | 0.04 ± 0.01 a | 0.02 ± 0.02 a | 0.01 ± 0.01 a | 0.08 ± 0.06 a | 0.01 ± 0.01 a | n.d. |
| C10:0 | n.d. | n.d. | 0.01 ± 0.00 a | n.d. | n.d. | 0.02 ± 0.01 a | n.d. | n.d. |
| C11:0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.01 ± 0.01 | n.d. | n.d. |
| C12:0 | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a |
| C13:0 | 0.01 ± 0.00 a | n.d. | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | n.d. | 0.01 ± 0.00 a |
| C14:0 | 1.60 ± 0.19 a | 1.64 ± 0.09 a | 1.55 ± 0.06 a | 1.66 ± 0.21 a | 1.66 ± 0.13 a | 1.77 ± 0.17 b | 1.55 ± 0.03 a | 1.70 ± 0.12 b |
| C14:1 | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.06 ± 0.03 a | 0.04 ± 0.00 a | 0.03 ± 0.01 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a |
| C15:0 | 0.19 ± 0.00 a | 0.18 ± 0.02 a | 0.19 ± 0.01 a | 0.20 ± 0.04 a | 0.20 ± 0.01 a | 0.24 ± 0.04 b | 0.18 ± 0.02 a | 0.19 ± 0.01 a |
| C15:1 | n.d. | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a | n.d. | 0.01 ± 0.01 a | n.d. | 0.01 ± 0.01 a |
| C16:0 | 15.42 ± 0.65 a | 15.63 ± 0.31 a | 15.07 ± 0.14 a | 15.46 ± 0.57 a | 15.59 ± 0.37 a | 15.61 ± 0.46 a | 15.38 ± 0.14 a | 15.45 ± 0.41 a |
| C16:1 | 2.88 ± 0.09 a | 2.84 ± 0.08 a | 2.75 ± 0.03 a | 2.83 ± 0.21 a | 2.86 ± 0.05 a | 2.92 ± 0.23 b | 2.76 ± 0.04 a | 3.10 ± 0.11 b |
| C17:0 | 0.17 ± 0.01 a | 0.18 ± 0.00 a | 0.16 ± 0.00 a | 0.21 ± 0.05 b | 0.18 ± 0.01 a | 0.17 ± 0.00 a | 0.18 ± 0.00 a | 0.17 ± 0.00 a |
| C17:1 | 0.20 ± 0.02 a | 0.22 ± 0.00 a | 0.07 ± 0.12 b | 0.21 ± 0.01 a | 0.19 ± 0.00 a | 0.13 ± 0.11 c | 0.21 ± 0.01 a | 0.15 ± 0.06 c |
| C18:0 | 4.50 ± 0.07 a | 4.52 ± 0.14 a | 4.30 ± 0.08 a | 4.69 ± 0.23 b | 4.47 ± 0.13 a | 4.86 ± 0.04 b | 4.32 ± 0.12 a | 4.42 ± 0.03 a |
| C18:1 n-9t | 0.07 ± 0.02 a | 0.11 ± 0.06 b | 0.12 ± 0.08 b | 0.10 ± 0.01 a | 0.11 ± 0.08 a | 0.07 ± 0.01 a | 0.14 ± 0.08 b | 0.06 ± 0.03 a |
| C18:1 n-9c | 36.80 ± 0.25 a | 36.88 ± 0.37 | 36.75 ± 0.13 | 36.67 ± 0.61 | 36.91 ± 0.29 | 36.51 ± 0.38 | 36.97 ± 0.20 | 36.41 ± 0.27 |
| C18:2 n-6t | 0.03 ± 0.02 a | 0.03 ± 0.01 a | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | n.d. | n.d. |
| C18:2 n-6t | 25.80 ± 0.26 a | 25.50 ± 0.16 a | 25.65 ± 0.23 a | 25.32 ± 0.26 a | 25.62 ± 0.25 a | 25.53 ± 0.29 a | 25.56 ± 0.23 a | 25.75 ± 0.13 a |
| C18:3 n-6 | 0.36 ± 0.01 a | 0.38 ± 0.01 a | 0.38 ± 0.01 a | 0.40 ± 0.03 a | 0.36 ± 0.01 a | 0.37 ± 0.01 a | 0.38 ± 0.01 a | 0.36 ± 0.01 a |
| C18:3 n-3 | 3.90 ± 0.07 a | 3.72 ± 0.17 a | 3.89 ± 0.10 a | 3.85 ± 0.05 a | 3.86 ± 0.08 a | 3.90 ± 0.12 a | 3.84 ± 0.04 a | 4.01 ± 0.08 b |
| C20:0 | n.d. | 0.03 ± 0.03 a | n.d. | 0.07 ± 0.03 a | 0.04 ± 0.04 a | n.d. | 0.02 ± 0.02 a | 0.03 ± 0.03 a |
| C20:1 | 2.00 ± 0.13 a | 2.05 ± 0.05 a | 2.11 ± 0.15 a | 2.01 ± 0.18 a | 1.98 ± 0.08 a | 2.01 ± 0.09 a | 2.15 ± 0.01 a | 1.87 ± 0.08 b |
| C20:2 | 1.15 ± 0.05 a | 1.20 ± 0.06 a | 1.22 ± 0.04 a | 1.14 ± 0.08 a | 1.19 ± 0.07 a | 1.14 ± 0.04 a | 1.15 ± 0.03 a | 1.20 ± 0.02 a |
| C20:3 | 0.56 ± 0.03 a | 0.54 ± 0.02 a | 0.64 ± 0.05 b | 0.53 ± 0.01 a | 0.56 ± 0.03 a | 0.51 ± 0.04 a | 0.58 ± 0.04 a | 0.55 ± 0.03 a |
| C20:3 n-6 | 0.15 ± 0.00 a | 0.16 ± 0.05 a | 0.21 ± 0.06 b | 0.19 ± 0.10 b | 0.14 ± 0.00 a | 0.14 ± 0.01 a | 0.15 ± 0.03 a | 0.16 ± 0.02 a |
| C20:4 n-6 | 0.30 ± 0.01 a | 0.33 ± 0.11 a | 0.37 ± 0.06 b | 0.33 ± 0.08 a | 0.29 ± 0.01 a | 0.28 ± 0.01 a | 0.32 ± 0.01 a | 0.32 ± 0.04 a |
| C20:5n-3 (EPA) | 1.27 ± 0.03 a | 1.04 ± 0.03 b | 1.37 ± 0.13 c | 1.34 ± 0.03 c | 1.22 ± 0.03 d | 1.21 ± 0.04 d | 1.32 ± 0.02 a | 1.28 ± 0.08 a |
| C22:0 | 0.21 ± 0.03 a | 0.18 ± 0.04 a | 0.23 ± 0.04 a | 0.14 ± 0.04 b | 0.26 ± 0.13 c | 0.21 ± 0.01 a | 0.18 ± 0.05 a | 0.21 ± 0.02 a |
| C22:1 n-9 | 0.34 ± 0.02 a | 0.33 ± 0.04 a | 0.42 ± 0.06 b | 0.36 ± 0.08 a | 0.33 ± 0.03 a | 0.32 ± 0.02 a | n.d. | 0.34 ± 0.04 a |
| C22:2 | n.d. | 0.31 ± 0.03 a | 0.30 ± 0.05 a | 0.34 ± 0.11 a | 0.10 ± 0.14 b | 0.20 ± 0.16 c | 0.29 ± 0.02 a | 0.30 ± 0.03 a |
| C22:6n-3 (DHA) | 1.56 ± 0.11 a | 1.23 ± 0.01 b | 1.61 ± 0.02 a | 1.58 ± 0.09 a | 1.44 ± 0.03 c | 1.36 ± 0.02 c | 1.57 ± 0.02 a | 1.52 ± 0.02 a |
| C24:1 | 0.23 ± 0.03 a | 0.20 ± 0.02 a | 0.26 ± 0.02 a | 0.19 ± 0.02 b | 0.21 ± 0.02 a | 0.20 ± 0.03 b | 0.23 ± 0.02 a | 0.26 ± 0.06 a |
| ∑ SFA | 22.34 ± 1.01 a | 22.43 ± 0.67 a | 21.79 ± 0.37 a | 22.50 ± 1.22 b | 22.58 ± 0.99 b | 23.16 ± 0.91 b | 21.99 ± 0.46 a | 22.30 ± 0.72 b |
| ∑ MUFA | 42.56 ± 0.57 a | 42.69 ± 0.65 a | 42.53 ± 0.61 a | 42.46 ± 1.16 a | 42.64 ± 0.56 a | 42.19 ± 0.87 a | 42.50 ± 0.35 a | 42.26 ± 0.66 a |
| ∑ PUFA | 35.09 ± 0.59 a | 34.46 ± 0.72 a | 35.64 ± 0.76 a | 35.04 ± 0.86 a | 34.78 ± 0.65 a | 34.66 ± 0.83 a | 35.16 ± 0.45 a | 35.42 ± 0.57 a |
| EPA | 1.27 ± 0.03 a | 1.04 ± 0.03 b | 1.37 ± 0.13 c | 1.34 ± 0.03 c | 1.22 ± 0.03 d | 1.21 ± 0.04 d | 1.32 ± 0.02 c | 1.28 ± 0.08 c |
| DHA | 1.56 ± 0.11 a | 1.23 ± 0.06 b | 1.61 ± 0.02 c | 1.58 ± 0.09 ac | 1.44 ± 0.03 d | 1.36 ± 0.12 e | 1.57 ± 0.02 a | 1.52 ± 0.12 a |
| EPA+DHA | 2.83 ± 0.15 a | 2.72 ± 0.09 b | 2.98 ± 0.16 c | 2.92 ± 0.12 c | 2.66 ± 0.06 d | 2.58 ± 0.16 d | 2.89 ± 0.05 a | 2.80 ± 0.20 ab |
| n-3 | 7.39 ± 0.23 a | 6.70 ± 0.38 | 7.62 ± 0.32 | 7.50 ± 0.28 | 7.17 ± 0.16 | 7.12 ± 0.30 | 7.42 ± 0.10 | 7.48 ± 0.33 |
| n-6 | 32.25 ± 0.45 a | 32.19 ± 0.63 | 32.66 ± 0.60 | 32.12 ± 0.73 | 32.12 ± 0.59 | 32.08 ± 0.67 | 32.27 ± 0.41 | 32.65 ± 0.37 |
| n-3/n-6 * | 0.23 | 0.21 | 0.23 | 0.23 | 0.22 | 0.22 | 0.23 | 0.23 |
| PUFA/SFA * | 1.57 | 1.53 | 1.64 | 1.56 | 1.54 | 1.50 | 1.6 | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimat, V.; Skroza, D.; Frleta Matas, R.; Radelić, D.; Bogdanović, T.; Čagalj, M. The Effect of Combined Extracts from By-Products, Seaweed, and Pure Phenolics on the Quality of Vacuum-Packed Fish Burgers. Appl. Sci. 2025, 15, 5508. https://doi.org/10.3390/app15105508
Šimat V, Skroza D, Frleta Matas R, Radelić D, Bogdanović T, Čagalj M. The Effect of Combined Extracts from By-Products, Seaweed, and Pure Phenolics on the Quality of Vacuum-Packed Fish Burgers. Applied Sciences. 2025; 15(10):5508. https://doi.org/10.3390/app15105508
Chicago/Turabian StyleŠimat, Vida, Danijela Skroza, Roberta Frleta Matas, Dilajla Radelić, Tanja Bogdanović, and Martina Čagalj. 2025. "The Effect of Combined Extracts from By-Products, Seaweed, and Pure Phenolics on the Quality of Vacuum-Packed Fish Burgers" Applied Sciences 15, no. 10: 5508. https://doi.org/10.3390/app15105508
APA StyleŠimat, V., Skroza, D., Frleta Matas, R., Radelić, D., Bogdanović, T., & Čagalj, M. (2025). The Effect of Combined Extracts from By-Products, Seaweed, and Pure Phenolics on the Quality of Vacuum-Packed Fish Burgers. Applied Sciences, 15(10), 5508. https://doi.org/10.3390/app15105508









