1. Introduction
Sweet potato cultivation is a widespread practice, with more than 90 million tons grown worldwide, the majority of which is grown in Asian countries [
1]. European sweet potato production is increasing, with Portugal being the leading producer [
2]. The edibility and nutritional value of sweet potatoes extends to their roots, leaves, and stems, making sweet potato cultivation an important part of the food supply in many developing countries [
3,
4,
5].
The root contains considerable amounts of carbohydrates, most of which are starch. The human body can convert this into energy, so the root can be eaten as a staple food. Notably, it exhibits comparatively high fibre [
6,
7] and protein [
8,
9] content among vegetables. In addition, it has a high vitamin content, with some varieties containing high levels of ß -carotene [
10,
11]. Regarding the polyphenolic compounds, it mainly contains flavonoids and phenolic acids [
12,
13], and purple varieties contain anthocyanins [
14,
15,
16]. Notably, sweet potatoes are also rich in minerals, especially magnesium and iron [
17,
18].
The fresh, cleaned, ready-to-use sweet potato, with its minimal processing, is an extremely consumer-friendly, convenient, and easily usable form that represents significant added value. This fresh, nutrient-rich, and easily accessible form of convenience food can be seamlessly integrated into the fast-paced lifestyle of modern living, signifying an expanding market segment [
19].
The quality and safety of freshly marketed, cleaned, and cut products, however, are compromised by two major problems. The first issue is enzymatic browning, which can oxidise biologically valuable phenolic compounds to quinones and polymerize brown pigments, thus degrading the quality of the product [
20]. Microbiological hazards also represent a significant risk. Fresh-cut products are nutrient-rich environments that are favourable for microbial growth and multiplication. As these products typically undergo minimal microbial inactivation during processing, their food safety risk is notably elevated. Visible indications of spoilage may include browning or drying on the cut surfaces, as well as changes in texture such as softening, hardening, or other consistency alterations during storage. Changes in bioactive compounds, antioxidants, and colourants can also contribute to alterations in taste, colour, texture, and nutritional value, ultimately leading to product deterioration. Consequently, these changes can have a negative impact on product quality, shelf life, marketability, and food safety [
21,
22,
23].
Sweet potato tubers, being developed in the soil, are among the most heavily contaminated plant parts, and their microbiota closely resembles that of the soil in which they grow. Processing steps such as washing, peeling, and cutting also influence the microbial susceptibilities and spoilage of freshly cut surfaces. A wide variety of microorganisms can be found on cut fruits and vegetables. These include both Gram-negative and Gram-positive bacteria, as well as yeasts and moulds. Notable among these are certain types of coliform bacteria, such as
Escherichia coli,
Staphylococcus aureus, and
Salmonella spp., which are among the most common foodborne pathogens. Several Gram-positive bacteria, especially lactic acid bacteria, are associated with the spoilage of fresh vegetables. Among the most widespread and significant spoilage-causing microorganisms are fluorescent
Pseudomonas species, such as
P. marginalis [
24,
25].
In order to prevent the enzymatic browning and microbial spoilage of fresh-cut fruits and vegetables, numerous new branches of research have emerged. These research directions focus on natural alternatives to synthetic additives, in line with current consumer trends. These natural components include phenolic acids, flavonoids, sulphur compounds, organic acids, bioactive peptides, and plant extracts [
26,
27,
28].
In response to the growing demands of consumers, a particular focus has been placed on ready-to-eat vegetables. These products require minimal time and energy expenditure during preparation, with examples including pre-processed potatoes and carrots. The products undergo a series of processing steps, including washing, peeling, and cutting. They are then subjected to various treatments and are packaged in vacuum-sealed bags for chilled storage. During the production process, colour retention treatments are also applied to the cleaned raw materials, with the objectives of inhibiting microbial growth and preserving sensory qualities [
29,
30].
Advancements in packaging technology, including (MAP) and vacuum sealing, have emerged as significant methods for safeguarding food items against physical damage, contamination, and environmental factors such as exposure to oxygen. The primary function of these packaging methods is to extend the shelf life while maintaining the quality and safety of the food [
31]. Vacuum packaging is commonly used for the long-term storage of dry foods such as cereals, nuts, sausages, cheese, and smoked fish and for the short-term storage of fresh foods such as vegetables, meat, and liquids. Vacuum packaging of food offers several distinct advantages to the food industry, most notably related to the reduction or even potential elimination of atmospheric oxygen. This leads to a slower rate of oxidative reactions (e.g., lipid and protein oxidation) and restricts the growth of aerobic bacteria and fungi [
32].
The objective of the present study was to determine the effect of vacuum packaging combined with immersion in fruit pomaces and organic acids on the quality of fresh-cut sweet potato (white-fleshed Bonita and orange-fleshed Covington) varieties during cold storage, thus determining their effect on the shelf-life of these products.
2. Materials and Methods
2.1. Investigated Sweet Potato Samples (Preliminary Experiment)
Two different sweet potato varieties (
Figure 1) (10 kg units of each) were selected for our investigations: Bonita (white-fleshed) and Covington (dark-orange-fleshed). They originated from organic farming from Kóka (47°29′11″ N 19°34′44″ E), Hungary. Prior to the examination, the potatoes were washed, peeled, and cut to approximately equal (about 1 × 1 cm) cubes. One batch of the prepared sweet potato cubes was tested on the same day, and two batches were placed in bags, vacuum sealed, and stored in a refrigerator at 5 °C for five days. The main spoilage microorganisms (psychrotrophic microbes and anaerobes at 5 °C,
Enterobacteriaceae, spore-forming bacteria, yeasts/moulds, mesophilic aerobic cells, and facultative anaerobic and anaerobic cells, as well as lactobacilli and lactococci) were then examined in order to identify and select the dominant microorganisms to be tested in subsequent experiments.
2.2. Treatment of the Sweet Potato Varieties with Plant-Based Extracts and Organic Acids
The sweet potato samples (Bonita and Covington) were prepared for the treatments by undergoing a process of washing, peeling, and dicing (about 1 × 1 cm cubes). Two plant-based extracts, apple pomace (AP) and chokeberry pomace (CP), and two organic acids, ascorbic acid (AA) and citric acid (CA), were applied to treat the sweet potato cubes.
The apple pomace extract was prepared from the pomace of the Idared variety (AUSTRIA JUICE Hungary Kft., Vásárosnamény, Hungary). After pressing the juice, the fresh pomace was first dried at 60 °C in a conventional atmospheric dryer. Subsequently, 20 g of dried apple pomace was mixed with 60 mL of 80% ethanol and then treated with 20 kHz with Sonorex Super RK 52 ultrasonic bath (Bandelin electronic GmbH, Berlin, Germany) at 25 °C for 30 min [
33,
34].
The chokeberry pomace extract was prepared from the Nero variety (Lajosmizse, 47mace extr N 19 ace e.8′′ E). After pressing the juice, the fresh pomace was lyophilized with a Leybold Heraeus Lyovac CT2 freeze dryer (Labexchanger, Burladingen, Germany). Subsequently, 300 mg of lyophilized pomace and 9 mL of a solvent (50% glycerol + 1% formic acid) were mixed thoroughly and treated at 50 °C for 60 min [
35].
After the treatment times, both extracts were filled into centrifuge tubes and centrifuged for 10 min at 3000 rpm in a Z206A laboratory centrifuge (Hermle Labortechnik, Wehingen, Germany). The solvent from the filtered extracts was evaporated in an oven at 40 °C.
The treatment consisted of soaking the sweet potato cubes in the treatment solutions for 10 min. The concentration of the solutions was 30 g/L for citric acid and ascorbic acid and 10 mg/L for apple and chokeberry pomace extracts. The ratio of sweet potato to treatment solution was 1:5 in each case.
Subsequently, the samples were removed from the treatment solution and allowed to drip onto a filter. At time zero, the samples were subjected to content and microbiological measurements (fresh samples). The remaining samples were placed in vacuum-sealed bags and stored in a refrigerator at 5 °C until the next measurements were taken. Each storage package contained 200 g of sample, and 3 packages were prepared per treatment. Since the Bonita variety proved to be more resistant from a microbiological point of view based on the preliminary experiments (
Section 2.1), this variety was stored for seven days, while the Covington variety was stored for five days (considered as stored samples). Additionally, an untreated control sample was stored and examined as a comparison throughout the experiment.
2.3. Detection of Dominant Microorganisms from Sweet Potato Varieties
Ten grams of washed, peeled, and diced sweet potatoes was weighed into sterile Stomacher bags and diluted tenfold using sterile saline. The samples were then homogenised for 2 min using a BagMixer®, and a decimal dilution series was prepared. In addition, 5 mL of the homogenised samples was heat treated at 80 °C for 15 min for the determination of spore-forming bacteria. The detection of the microbial groups of interest was then performed by pour plating or spread plating of the appropriate dilutions.
For the determination of total aerobic, total anaerobic, and spore-forming bacterial counts, the PGY (0.5% peptone, 0.1% glucose, 0.25% yeast extract, and 1.5% bacteriological agar) medium was used. Covered pour plating with the VRBG medium (BioLab, Budapest, Hungary) was used for the detection of bacteria belonging to the Enterobacteriaceae family. MRS (Biolab, Budapest, Hungary) was used for the detection of the Lactobacillus genus, while the M17 medium (BioLab, Budapest, Hungary) was applied for colony counting of Lactococcus, also with the covered pour plating method. For the detection of yeasts and moulds, the samples were plated onto the PDA medium (Biolab, Budapest, Hungary).
The Petri dishes were incubated at 5 and 30 °C, respectively, after inoculation to determine the psychrotrophic and mesophilic total aerobic and anaerobic microbial counts. Anaerobic tests were performed using anaerobic generator sachets (BD GasPakTM EZ Anaerobic Pouch System with Indicator, BD, Környe, Hungary) to create the appropriate atmosphere. For the culturing of spore-forming bacteria, the Petri dishes were incubated at 30 °C, and plates containing the MRS and M17 media were also incubated at 30 °C. VRBG plates were cultured at 37 °C, while PDA plates were incubated at 25 °C. After the incubation of the cultures for a fixed period of time, colony counting was used for the evaluation.
All microbiological tests were performed in four parallel inoculations.
2.4. Analytical Methods
2.4.1. Determination of Water-Soluble Solid Content
The water-soluble solid content of the ground samples of the treated sweet potatoes and the corresponding control samples was determined using an ATAGO DBX-55 digital refractometer (ATAGO Co., Ltd., Saitama, Japan), expressed in Brix%. Three measurements were made in parallel for each sample.
2.4.2. pH Measurement
The pH of the samples was determined using a TESTO 206 digital pH metre (Testo SE & Co. KGaA, Titisee, Germany). Three measurements were made in parallel for each sample.
2.4.3. Colour Measurement
The colour of the samples was determined using the Konica Minolta CR 400 device (Konica Minolta Business Solutions Ltd., Mississauga, ON, Canada) based on the CIELab system, and the total colour difference (ΔE*) was calculated according to Equation (1).
where L*, a*, and b* are the colour parameters in CIELab.
The colour difference serves to indicate the extent of the difference between two samples. In the case of a difference below 0.5, it is not noticeable. In samples with a difference between 0.5 and 1.5, the distinction is barely noticeable. In cases where the difference is between 1.5 and 3.0, it is noticeable. In samples with a range between 3.0 and 6.0, the difference is clearly visible. In samples with a difference above 6.0, the difference is a large one [
36].
The browning index (BI) can be calculated from the L*, a*, and b* parameters with the following equation: BI = [100(x − 0.31)]/0.172, where x = (a* + 1.75L*)/(5.645L* + a* − 0.3012 b*) [
27].
2.4.4. Determination of Total Polyphenol Content
The total polyphenol content was determined using the Folin–Ciocalteu method, as described by Singleton and Rossi (1965) [
37]. Briefly, 1250 μL of the Folin reagent (diluted to 1:10
v/
v with distilled water) was added to a test tube, followed by 200 μL of methanol (4:1
v/
v methanol/distilled water) and 50 μL of the sample. After one minute, 1000 μL of sodium carbonate was introduced. The mixture was incubated in a water bath at 50 °C for five minutes. Absorbance was then measured at 760 nm using the Hitachi U-2900 spectrophotometer (Hitachi High Technologies Europe GmbH, Krefeld, Germany). The results were expressed as gallic acid equivalents (mg GAE/g of sweet potato). The calibration curve ranged from 0.109 to 0.780, with an R
2 value of 0.873.
2.4.5. Determination of Antioxidant Capacity
Antioxidant capacity was assessed using the ferric-reducing ability of plasma (FRAP) method, following the procedure outlined by Benzie and Strain (1996) [
38]. The FRAP reagent was prepared by mixing an acetate buffer (pH 3.6), 2,4,6-tripyridyl-s-triazine (TPTZ), and FeCl
3×6H
2O in appropriate proportions. Following a five-minute incubation period, the absorbance was recorded at 593 nm using the Hitachi U-2900 spectrophotometer (Hitachi High Technologies Europe GmbH, Krefeld, Germany). The results were expressed as ascorbic acid equivalents (mg AAE/g of sweet potato) based on an ascorbic acid standard calibration curve. The calibration curve ranged from 0.327 to 1.578, with an R
2 value of 0.999.
2.5. Statistical Analyses
Data analysis was conducted using IBM SPSS Statistics, version 29.0.1.0 (IBM Corp., New York, NY, USA, 2023). Prior to the analysis of variance (ANOVA), assumptions were verified using the Shapiro–Wilk test for normality and Levene’s test for the homogeneity of variances. Statistical significance was set at p = 0.05.
When ANOVA indicated significant differences, pairwise comparisons were performed. Tukey’s post hoc test was applied when variance homogeneity was confirmed, whereas the Games–Howell post hoc test was used when homogeneity was not met. These approaches ensured robust statistical comparisons and accounted for data variability across factor levels.
3. Results
3.1. Dominant Microorganisms in Sweet Potato Samples and Their Variation with Temperature During Storage
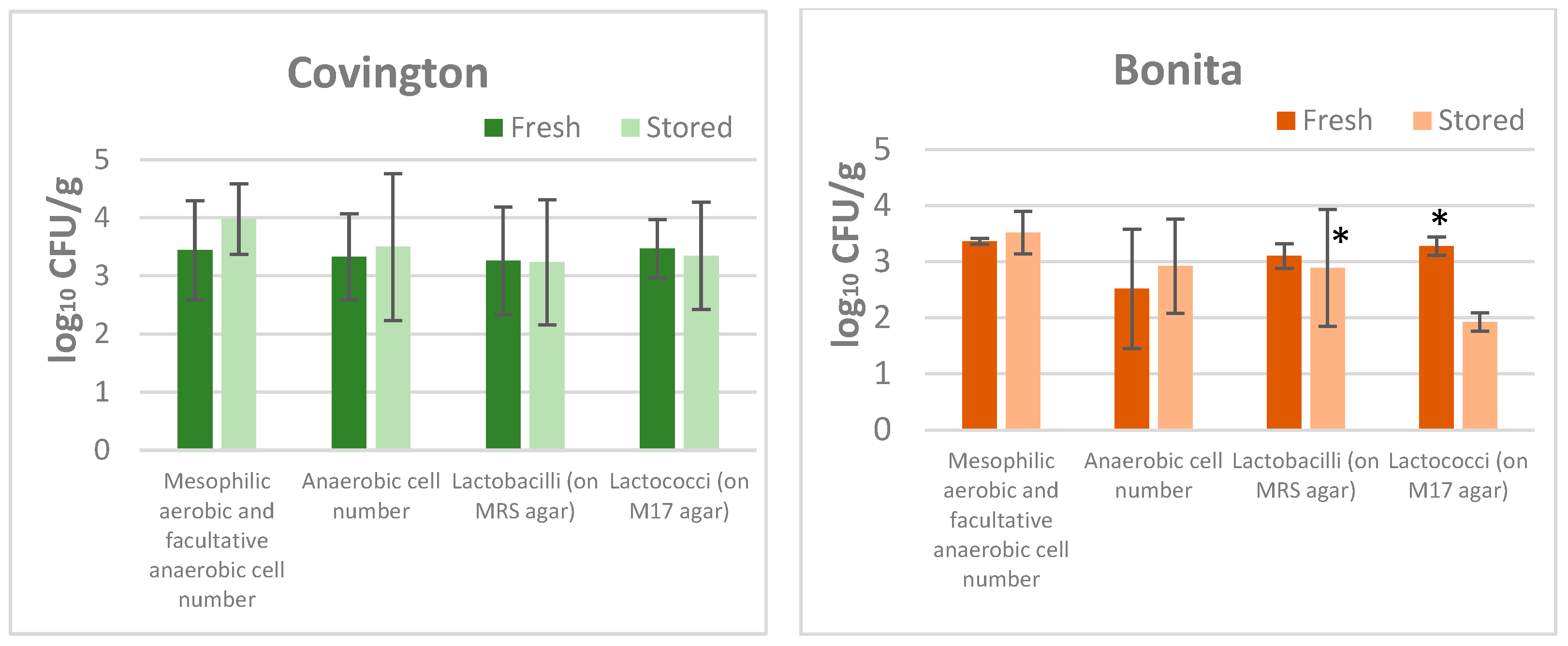
In the case of the sweet potato samples, the presence and concentration of characteristic microbial groups were determined. These microbes are assumed to be present in larger numbers in the products and affect their quality over the shelf life.
The findings yielded from the experiment on sweet potatoes revealed that no proliferation of psychrotrophic and anaerobic microorganisms was observed at a temperature of 5 °C, either on the initial testing day or in the subsequent storage period (
Table 1). Yeasts and moulds were detected in all fresh samples, and their numbers remained relatively constant during storage. Spore-forming bacteria and members of the
Enterobacteriaceae family, if present, were only found in low numbers in the fresh samples. While spore formers tended to stagnate or decrease during storage, members of the
Enterobacteriaceae family increased by 1.5 orders of magnitude. The sweet potato samples were characterised by a considerable number (usually between 2 and 4 orders of magnitude) of the microbial groups, as shown in
Figure 2. It was observed that the population of these microorganisms exhibited an increase during the storage period.
The findings indicate that the predominant culturable microbiota consists of mesophilic lactic acid bacteria, which demonstrated survival and multiplication capabilities in vacuum-packed products. Furthermore, the study suggested that enterobacteria may also play a role in the spoilage process by increasing their cell number by 1.5 orders of magnitude.
The results of the microbiological examination of the tested sweet potato varieties demonstrate that they are characterised by nearly identical microbial contamination after washing, peeling, and slicing, and no significant difference is observed in the initial microbial counts. However, in the case of Bonita (white variety), a significant decrease in lactococci numbers was observed after storage, and this was a variety of sweet potato where initial microbial numbers had not been elevated to a substantial degree.
3.2. Effect of Treatments on the Cultivable Microbiota of Sweet Potato Samples
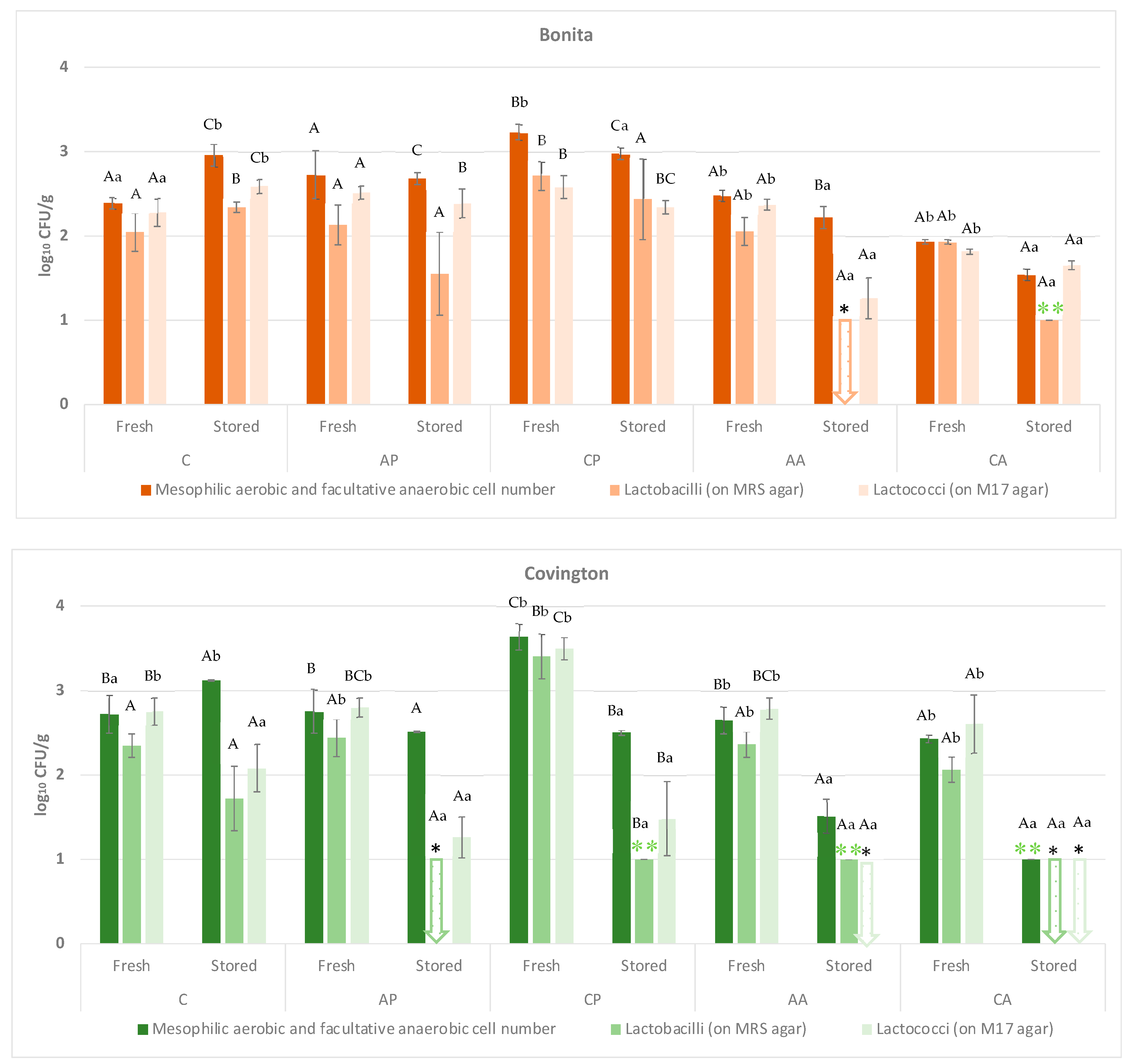
As demonstrated in
Table 2 and
Table 3 and
Figure 3, in the case of the Covington variety, all treatments were effective for the microbial groups that were tested, as the number of cells decreased in almost all cases in comparison to the control sample. Statistical analysis confirmed a significant effect of treatments (F(4;20) = 52.318;
p < 0.001) on total aerobic, total anaerobic, and spore-forming bacterial counts. Storage time also had a significant impact (F(1;20) = 97.943;
p < 0.001), with a notable interaction between treatment and storage duration (F(4;40) = 24.904;
p < 0.001).
In the case of Bonita, the effect of the compounds used for treatment was not as evident, as the treatments did not always result in changes in the microbial groups tested. The statistical data support this, showing a significant treatment effect (F(4;20) = 105.906; p < 0.001), whereas storage time alone had no significant influence (F(1;20) = 2.691; p = 0.117). However, the interaction between treatment and storage time (F(4;40) = 14.834; p < 0.001) regarding the total microbial count was significant. Further analysis revealed significant trends in lactobacilli counts. In Bonita, treatment (F(4;20) = 27.591; p < 0.001), storage time (F(1;20) = 54.456; p < 0.001), and their interaction (F(4;40) = 14.340; p < 0.001) were all significant. Similar patterns were observed in Covington, where treatment (F(4;20) = 11.158; p < 0.001), storage time (F(1;20) = 315.315; p < 0.001), and their interaction (F(4;40) = 24.904; p < 0.001) significantly influenced microbial levels.
Lactococci counts followed a comparable trend, with Bonita exhibiting significant effects due to the treatment (F(4;20) = 50.903; p < 0.001), storage time (F(1;20) = 32.906; p < 0.001), and interaction (F(4;40) = 24.839; p < 0.001). Covington also demonstrated statistical significance in treatment (F(4;20) = 36.229; p < 0.001), storage time (F(1;20) = 540.340; p < 0.001), and interaction (F(4;40) = 21.389; p < 0.001).
Enterobacteriaceae counts provided additional insights. In Bonita, the treatment (F(4;20) = 5.657; p < 0.01), storage time (F(1;20) = 8.221; p < 0.05), and interaction (F(4;40) = 12.253; p < 0.05) were significant. However, for Covington, while the treatment (F(4;20) = 3.171; p < 0.05) and interaction (F(4;40) = 8.905; p < 0.001) were significant, the storage time did not exert a notable influence (F(1;20) = 0.670; p = 0.423).
Short-term storage effects varied depending on the treatment applied. In Bonita, the ascorbic acid treatment resulted in no significant changes in Enterobacteriaceae (p = 0.116) or yeast and mould counts (p = 0.519). The apple pomace treatment also had no impact on the total aerobic, total anaerobic, and spore-forming bacterial count (p = 0.811); the lactococci count (p = 0.300); the lactobacilli count (p = 0.139); the Enterobacteriaceae count (p = 0.379), or the yeast and mould count (p = 0.118). Similarly, the chokeberry pomace treatment did not alter the lactococci (p = 0.400), lactobacilli (p = 0.059), or yeast and mould counts (p = 0.374). When no treatment was applied, short-term storage had no effect on the lactococci (p = 0.093) or yeast and mould counts (p = 0.924).
For Covington, the ascorbic acid treatment had no effect on the Enterobacteriaceae (p = 0.123) or yeast and mould counts (p = 0.374). The apple pomace treatment left the total aerobic, total anaerobic, and spore-forming bacterial count (p = 0.174); the Enterobacteriaceae count (p = 0.374), and the yeast and mould counts (p = 0.116) unchanged. The citric acid treatment did not impact Enterobacteriaceae counts (p = 0.121), while the chokeberry pomace treatment had no effect on yeast and mould counts (p = 0.374). When no treatment was applied, short-term storage did not affect lactobacilli counts (p = 0.054).
Regarding yeast and mould counts, Bonita showed no significant effect due to the treatment (F(4;20) = 1.846; p = 0.160), storage time (F(1;20) = 0.001; p = 0.982), or their interaction (F(4;40) = 1.304; p = 0.302). In contrast, Covington exhibited a significant treatment effect (F(4;20) = 5.548; p < 0.01), with storage time (F(1;20) = 5.780; p < 0.05) and the interaction of factors (F(4;40) = 2.895; p < 0.05) playing a role.
In the majority of cases where microbial counts exhibited alterations during the storage period, an increase was detected. Exceptions to this were observed in Enterobacteriaceae in Bonita with the citric acid treatment; yeast and mould counts in Bonita with chokeberry pomace; total aerobic, total anaerobic, and spore-forming bacterial counts in untreated Bonita; and lactococci and lactobacilli counts in Bonita treated with apple pomace. Furthermore, a decline in total aerobic, total anaerobic, and spore-forming bacterial counts, along with lactococci and lactobacilli, was observed in Bonita following treatments with chokeberry pomace, ascorbic acid, or citric acid. In Covington, total aerobic, total anaerobic, and spore-forming bacterial counts declined with the chokeberry pomace, ascorbic acid, or citric acid treatments. Similarly, lactococci and lactobacilli exhibited a decline in response to ascorbic acid and citric acid treatments.
In the course of this study, it was demonstrated that the number of lactic acid bacteria and enterobacteria, which likely function as the predominant agents in spoilage processes, can be decreased concomitantly with yeast as a consequence of the applied treatments. With regard to the compounds employed in the treatments, it was found that organic acids exhibited greater efficacy in comparison to plant extracts, while in the context of microbes, it was observed that bacteria demonstrated a higher degree of sensitivity to the treatments when compared to fungi.
In conclusion, our findings demonstrate that plant extracts exhibited a diminished antimicrobial effect on the examined samples. Nonetheless, apple pomace extract emerged as slightly more effective against microbes than the chokeberry extract. The findings unequivocally corroborate the efficacy of organic acids, underscoring the pronounced antimicrobial potency of citric acid.
3.3. Results of Analytical Methods
3.3.1. Results of Measurement of Total Polyphenol Content
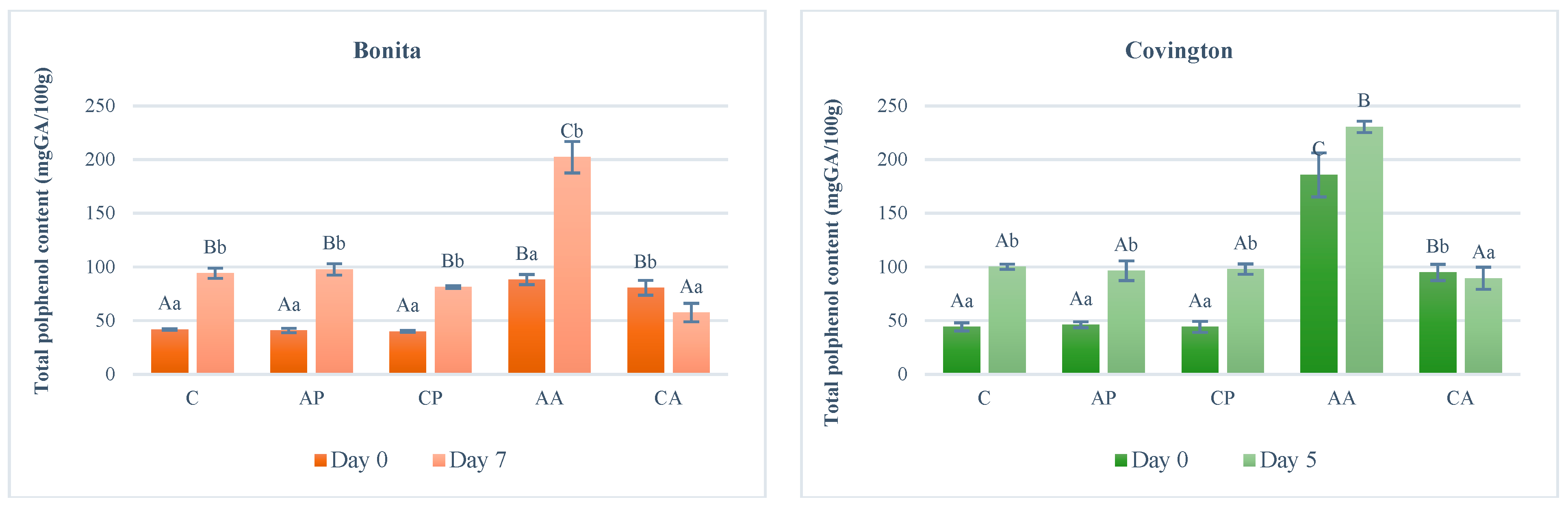
The total polyphenol content of the Bonita (white flesh) cultivar was found to be similar (40.01–41.87 mg GAE/100 g) for samples prepared without treatment (C) and treated with apple pomace (AP) or chokeberry pomace (CP) extracts, as shown in
Figure 4. In comparison, samples treated with citric acid (CA) or ascorbic acid (AA) solutions showed double values (80.69 mgGAE/100 g and 88.32 mgGAE/100 g). The treatment had a significant effect (F(4;10) = 202.909;
p < 0.001). Following a week of storage, the polyphenol content of the untreated (C) and plant extract-treated (AP and CP) samples exhibited an approximate twofold increase, while the ascorbic acid (AA) treatment resulted in more than double the amount of polyphenolic compounds. The ascorbic acid treatment yielded the highest value (202.25 mgGAE/100 g). In contrast, the polyphenol content of the sample treated with the citric acid solution (CA) decreased (by 28.5%) and was only 57.65 mg GAE/100 g at the end of storage. The storage time had a significant effect (F(1;10) = 475.856;
p < 0.001). The increase in polyphenol content after storage was highest for the sample treated with the apple pomace extract (AP) (139%), followed by the sample treated with the ascorbic acid solution (AA) (129%), the untreated (C) sample (124%), and the sample treated with the chokeberry pomace extract (CP) (103%). The interaction of treatment and storage time had a significant effect (F(4;20) = 97.192;
p < 0.001).
The Covington (orange flesh) variety exhibited a comparable trend to that of the Bonita variety. At the time of sample preparation, the polyphenol content of the untreated (C), apple pomace (AP), and chokeberry pomace (CP) extract-treated samples was nearly the same (44.31–46.27 mg GAE/100 g). These values were more than doubled for the sample soaked in the citric acid solution (CA) (94.94 mgGAE/100 g) and more than quadrupled for the sample soaked in the ascorbic acid solution (AA) (185.81 mgGAE/100 g). The treatment had a significant effect (F(4;10) = 283.850; p < 0.001)
The results of the five-day storage experiment demonstrated a similar trend to that observed for the Bonita variety. The untreated (C) and plant extract-treated (AP, CP) samples exhibited an increase in polyphenol content of approximately two times (96.57–100.26 mg GAE/100 g), while the ascorbic acid (AA) treatment caused a smaller increase (230.55 mg GAE/100 g). The storage time had a significant effect (F(1;10) = 159.528; p < 0.001)
The citric acid solution (CA) treatment exhibited a slight decrease (5.7%), while the remaining samples demonstrated a more prominent increase. This increase was most significant in the untreated (C) sample and the samples soaked with chokeberry extract (CP) (126% and 121%), followed by the sample treated with malic extract (AP) (109%) and the sample treated with the ascorbic acid solution (AA) (24%). The interaction of treatment and storage time had a significant effect (F(4;20) = 13.225; p < 0.001).
The ascorbic acid (AA) treatment resulted in the highest total polyphenol content in both varieties, primarily due to the protective effect of ascorbic acid in the treatment solution on polyphenolic compounds. A comparison of the two cultivars revealed comparable behaviours in the immediate aftermath of treatment and after storage, with similar changes in total polyphenol content. After examining 14 sweet potato varieties, it was found that the phenolic content of the yellow- and orange-fleshed varieties was lower (4.8 to 90 mgGE/100 g), while that of the purple-fleshed varieties was between 68.1 and 780.6 mgGE/100 g [
39]. In the case of the white- and orange-fleshed varieties, the phenol content values are 20–40 mgGE/100 g [
40], 20–50 mgGE/100 g [
41], and 15–20 mgGE/100 g [
42].
3.3.2. Results of Measurement of Antioxidant Capacity
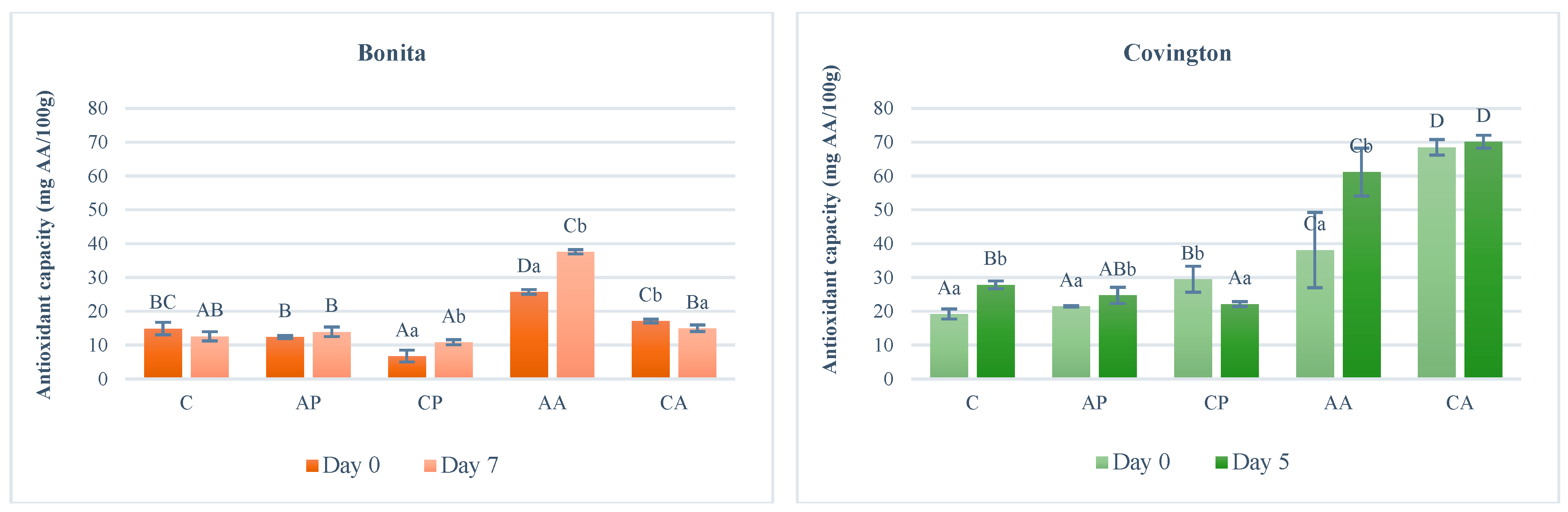
For the Bonita (white flesh) variety, the antioxidant capacity at the time of sample preparation was nearly equivalent for untreated samples (C), samples treated with apple pomace extract (AP), and samples treated with citric acid (CA) (12.40–17.14 mgAA/100 g) (
Figure 5). In comparison, the value of the sample soaked in the extract of chokeberry (CP) was approximately half (6.78 mgAA/100 g), while that of the sample soaked in the ascorbic acid solution (AA) was twice as high (25.75 mgAA/100 g). The treatment had a significant effect (F(4;10) = 384.173;
p < 0.001). A study of 11 varieties found that the FRAP value ranged from 2.5 to 7.5 mgAA/100 g [
43].
After storage, samples treated with no treatment (C), the citric acid solution (CA), and plant extracts (AP and CP) had similar values (10.89–15.02 mgAA/100 g). The highest value was obtained for the sample treated with the ascorbic acid solution (AA) (37.64 mgAA/100 g). The storage time had a significant effect (F(1;10) = 43.005; p < 0.001).
In terms of storage trends, the control (C) and citric acid solution-treated (CA) samples showed a slight decrease (15.3% and 12.4%, respectively). The sample soaked in apple pomace extract (AP) exhibited a slight increase (12.5%), while the ascorbic acid solution-treated sample demonstrated a more significant increase (46%). The most pronounced increase was observed in the sample treated with chokeberry pomace extract, reaching 61%. The interaction of treatment and storage time had a significant effect (F(4;20) = 42.344; p < 0.001).
For the Covington variety (orange-fleshed), the antioxidant capacity at the time of sample preparation was nearly similar to the untreated (C) and plant extract-treated (AP, CP) samples (19.22–29.50 mgAA/100 g). In comparison, the sample soaked in the ascorbic acid solution (AA) exhibited nearly double the value (38.15 mgAA/100 g), while the sample treated with the citric acid solution (CA) demonstrated approximately three times the value (68.51 mgAA/100 g). The treatment had a significant effect (F(4;10) = 798.087; p < 0.001).
After a duration of five days, the antioxidant capacity exhibited similar values in the control (C) and plant extract-treated (AP, CP) samples (22.18–27.86 mgAA/100 g), whereas in the samples treated with ascorbic acid (AA) and citric acid (CA), the values were about twice as high (61.19–70.19 mgAA/100 g). The storage time had a significant effect (F(1;10) = 83.571; p < 0.001).
With regard to storage trends, the sample treated with chokeberry pomace extract (CP) showed a decrease of 25%. In all other cases, an increase was observed, with the smallest change observed in the citric acid solution-treated sample (2.4%), followed by the apple pomace extract-treated sample with a 15% increase. The antioxidant capacity of the control sample exhibited a 45% increase, while the ascorbic acid-treated sample demonstrated a 60% increase. The interaction of treatment and storage time had a significant effect (F(4;20) = 55.940; p < 0.001).
3.3.3. Results of Measurement of Water-Soluble Solid Content, pH Value, and Colour
For the Bonita (white-fleshed) sweet potato samples, the water-soluble dry matter content of the untreated sample measured in its fresh state (10.97 °Brix) was higher than that of the treated samples (9.60–10.07 °Brix) (
Table 4). During the storage period, a decline in the water-soluble dry matter content was observed (9.40, 9.30, 9.67, and 9.33 °Brix), except for the control sample, where an increase was detected (11.37 °Brix). Notably, no statistically significant differences were found in the dry matter contents measured among the treated samples. The treatment had a significant effect (F(4;
,10) = 138.084;
p < 0.001), and the storage time had a significant effect (F(1;10) = 7.412;
p < 0.05), and their interaction was also significant (F(4;20) = 10.857;
p < 0.001). For the Covington (dark, orange-fleshed) sweet potato samples, the water-soluble dry matter content of the untreated sample in its fresh state (9.70 °Brix) was higher than that of the treated samples (8.40–8.53 °Brix).
During the storage period, no statistically significant differences were detected in the measured dry matter content values. The treatment had a significant effect (F(4;10) = 100.462; p < 0.001), but storage time had no significant effect (F(1;10) = 0.010; p = 0.429), while the interaction of treatment and storage time was significant (F(4;20) = 2.882; p < 0.05)
In summary, the findings indicate that, in the fresh state, the treatments led to a modest reduction in water-soluble dry matter content for both sweet potato varieties. During the storage period, the Covington samples were not affected regarding their water-soluble dry matter content. Higher total soluble solids were detected in orange cultivars (8.52–9.72 °BRIX) than in white (5.57 °BRIX) sweet potato tubers [
44].
For the Bonita (white-fleshed) variety, the untreated (C), apple pomace (AP), and chokeberry pomace (CP) samples had almost identical pH values (5.81–5.83), while the ascorbic acid (AA) and citric acid (CA) samples had lower pH values (5.36 and 4.45, respectively) (
Table 4). During the storage period, the control and plant extract-treated samples exhibited a slight increase in pH (5.92, 5.85, and 5.90). Conversely, the pH values of the ascorbic acid (5.36) and citric acid (4.45) samples exhibited a decrease during storage, reaching 5.16 and 4.25, respectively. The treatment had a significant effect (F(4;10) = 34,105.970;
p < 0.001); the storage time had a significant effect (F(1;10) = 161.783;
p < 0.001), and their interaction was also significant (F(4;20) = 444.391;
p < 0.001).
A similar trend was observed for the Covington variety. Conversely, the untreated sample exhibited an increase during storage, reaching 5.96, while the apple pomace (5.31) and chokeberry pomace (5.69) samples showed an increase (5.96, 5.92, and 5.72, respectively). Conversely, the ascorbic acid (4.85) and citric acid (4.42) samples exhibited lower initial pH values, which underwent a decline during storage (to 4.65 and 4.11, respectively). The treatment had a significant effect (F(4;10) = 76,643.667; p < 0.001), while the storage time had a significant effect (F(1,10) = 998.756; p < 0.001), and their interaction was also significant (F(4;20) = 5351.756; p < 0.001).
In summary, for both varieties, the pH values of the untreated (C), apple pomace (AP), and chokeberry pomace (CP) samples were higher than those of the ascorbic acid (AA)- and citric acid (CA)-treated samples. This difference can be attributed to the acidic treatments. A comparison of the various treatments in the Bonita (white-fleshed) samples revealed that the differences were less pronounced, and the changes during storage were less significant than in the Covington (dark-orange-fleshed) variety.
The colour change was characterised using the ΔE* values (
Table 5). A comparison of the colour values on day 7 with their respective values and on day 0 for the Bonita (white-fleshed) variety was made. The difference was found to be noticeable for the untreated (C) and chokeberry pomace-treated (CP) samples, while it was very noticeable for the apple pomace (AP)-, ascorbic acid (AA)-, and citric acid (CA)-treated samples. On day 7, the difference was noticeable for the apple pomace (AP) and ascorbic acid (AA) samples, while it was very noticeable for the chokeberry pomace (CP) and citric acid (CA) samples compared to the untreated (C) sample. In light of these observations, it can be concluded that colour changes occurred during storage for all samples, both untreated and treated. On day 7, a comparison of the treated samples to the control revealed no significant differences among the treatments. However, a notable distinction emerges when day 7 values are compared with day 0 values for the treatment with chokeberry pomace extract (CP). This observation is further supported by the low browning index (BI) values of the samples treated with chokeberry pomace extract (CP).
For the Covington (dark-orange-fleshed) variety, the ΔE* values indicate that a noticeable colour difference was observed between day 0 and day 5 samples for both the untreated and treated samples. On day 5, a very noticeable colour difference was observed for the apple pomace (AP) sample compared to the untreated sample, while it was only noticeable for the chokeberry pomace (CP), ascorbic acid (AA), and citric acid (CA) samples. These findings suggest that all samples underwent colour changes during storage, yet no notable differences in browning index (BI) values were observed between treatments, in contrast to the Bonita variety.
In summary, the colour-stabilising effect of the extracts utilised in the treatments was more pronounced in the Bonita (white-fleshed) variety.
Rocculi et al. [
45] investigated the alterations in the colour coordinates of freshly cut potato slices when applying anti-browning agents. Their findings indicated that increasing the concentration of ascorbic acid and citric acid resulted in higher L* and a* values, and very high concentrations of these acids led to the whitening of the samples. In the present study, a similar effect was observed for the Covington (dark-orange-fleshed) variety, where ascorbic acid and citric acid increased the colour coordinate values, particularly the a* values. In contrast, for the Bonita variety, which is characterised by white flesh, a modest rise in L* values was recorded, accompanied by a pronounced increase in a* values. This was reflected in the changes observed in the ΔE* values and the browning index (BI).
By inhibiting the enzymatic browning reaction on the skin, the reaction on the peripheral and central parts of sweet potato slices was decreased by acidic electrolyzed water. The browning index (BI) of fresh-cut sweet potato slices ranged from 1.95 to 2.81, showing similar results with the effects of apple pomace extract (AP) and ascorbic acid (AA) used in the present study [
40]. A study investigated the effect of quercetin treatment on the colour quality and phenolic content of fresh-cut potatoes. The results showed that quercetin played an important role in preventing enzymatic browning, extending shelf life, and maintaining the quality of fresh-cut potatoes, but ascorbic acid (AA) was more effective based on the browning index (BI) values [
46]. Four browning-susceptible cultivars and three browning-resistant cultivars were investigated to understand the browning process of potato tubers. The browning index (BI) values ranged from −2.5 to 1.8 in the case of the browning-resistant varieties, while the values were higher (2–12) in the case of the browning-susceptible potatoes. The total polyphenol content was lower (76–90 mgGE/100 g) for the browning-resistant varieties, while it was higher (68–121 mgGE/100 g) for the susceptible varieties [
47].
4. Discussion
In the course of the measurements, an initial series of experiments was conducted to examine the dominant and spoilage-causing microbes of the two tested sweet potato varieties. Subsequently, an investigation was conducted to ascertain the growth and survival of these microbes during storage and as a consequence of the various treatments with the extracts.
As demonstrated in the scientific literature, the initial microbiota of fresh vegetables consists of
Aeromonas,
Bacillus,
Clostridium, coryneforms,
Enterobacter,
Erwinia,
Flavobacterium,
Pantoea,
Pseudomonas, lactic acid bacteria, and
Xanthomonas. Studies have observed that predominant spoilage microorganisms of refrigerated minimally processed vegetables stored under VP and modified atmosphere packaging (MAP) conditions are lactic acid bacteria, with
Leuconostoc mesenteroides being a notable representative [
48].
It has been established that a wide variety of Gram-positive bacteria, most notably LAB, is associated with the spoilage of fresh-cut fruits and vegetables that are packaged under a modified atmosphere and stored at a lower temperature. The process of lactic acid bacterial fermentation results in a decrease in pH, a consequence of the production of lactic acid. In addition, the fermentation process gives rise to the production of acetyl methylcarbinol and diacetyl, which are responsible for an undesirable flavour reminiscent of buttermilk. It is noted that other fermentation products such as acetic acid, ethanol, formic acid, and CO
2 are also produced. LAB were detected in almost every fresh-cut product [
24]. As demonstrated by Nissen et al. in 1996 [
49], a significant increase (log
10 5–6) in the number of LAB was detected in the analysed product after storage at low temperatures in a vacuum. This indicates that spoilage can be induced by LAB in environments devoid of oxygen.
In the work by Jiang et al. [
29], a comparative analysis was conducted on the effects of vacuum packaging and modified atmosphere packaging (MAP) on fresh-cut potatoes. Their study revealed that vacuum packaging resulted in higher germ counts during storage at 4 °C when compared to MAP, which indicates that the application of vacuum packaging by itself is inadequate to extend the shelf life of fresh-cut potatoes, even when stored at low temperatures. It is therefore reasonable to combine vacuum packaging and low-temperature storage with other antimicrobial effects in order to increase the shelf life of fresh products.
An investigation was conducted by Buick and Damoglou in 1987 [
50] to ascertain the impact of vacuum packaging on the microbial spoilage of ready-to-use carrot slices and the shelf life of the product. The microbial development on vacuum-packaged carrots was found to be slower than that of the non-vacuum-packaged material. The predominant organisms present were
Leuconostoc spp. in the vacuum packs, as opposed to
Erwinia spp. in the aerobic ones. The application of vacuum packaging to sliced carrots resulted in a substantial extension of the shelf life of the product when stored at 4 °C, with the shelf life being extended from 5 to 8 days.
In the study conducted by Kim et al. [
51], the impact of storage under oxygen-free conditions on bacterial communities present in strawberry puree was analysed. The findings indicated that the application of vacuum resulted in an extended lag phase or the absence of growth for anaerobic bacteria, despite the fact that vacuum conditions are known to foster anaerobic conditions. Anaerobic organisms are typically characterised by their ability to thrive in the absence of oxygen. However, it has been demonstrated that sudden pressure changes (from atmospheric pressure to vacuum) can physically stress or rupture bacterial cells, and anaerobes are not specifically adapted to handle such rapid or extreme shifts in external pressure. Furthermore, the growth and metabolism of anaerobic bacteria are contingent on the specific gas composition of the environment. The process of removing all gases by means of a vacuum results in the deprivation of gas components that are essential to the metabolism of anaerobic microorganisms [
52]. In the case of vacuum-processed puree, minimal alterations in yeast and mould populations were observed during storage [
51]. Moulds are strictly aerobic microorganisms, and their growth is contingent on the oxygen availability in the environment [
53].
In our study the dominant microbes of the sweet potatoes proved to be LAB, which have the potential to induce organoleptic alterations in stored products as a result of their capacity to produce organic acids, thereby influencing the quality attributes of the sweet potato samples. In contrast to our observations, Jiang et al. [
29] reported that the predominant bacteria identified from vacuum-packaged fresh-cut potatoes were fermentative Gram-negative bacteria.
In addition to LAB, fungi and
Enterobacteriaceae, which were among the microorganisms detected, can also be problematic from a quality point of view.
Enterobacteriaceae may be present in the natural microbiota of certain foods or can be introduced as a result of (post-)process contamination.
Enterobacteriaceae have been shown to cause food spoilage in a broad range of food products, including milk, dairy products, meat, poultry, fish, seafood, fruits, vegetables, and other foods. The growth and metabolic activity of
Enterobacteriaceae in foodstuffs have been demonstrated to result in a range of undesirable effects, including off-flavours, odours, colour defects, and other organoleptic deviations. Such changes may be attributed to the enzymatic breakdown of proteins or lipids, the production of volatile components, and the generation of gas. Genera such as
Erwinia,
Brennaria, and
Pectobacterium are predominantly associated with plants; as a consequence, they are often present in the foodstuffs of vegetable or plant origin. Soft rot is a prevalent form of vegetable and fruit spoilage that is primarily triggered by the actions of
Enterobacteriaceae. These bacteria are responsible for the decomposition of pectins, resulting in a distinctive mushy texture, accompanied by a foul odour and a water-soaked appearance [
54]. Furthermore, members of the
Enterobacteriaceae family may present a food safety risk [
55] if inappropriate kitchen techniques are employed during the production of food made from ready-to-cook sweet potato.
Erturk and Picha [
56] demonstrated in their study that the microbiota of fresh-cut sweet potato slices was dominated by mesophiles, followed by psychrotrophs and fungi initially and during storage. In our study, we found that no growth was detected in samples stored at 5 °C, so the presence of psychrotrophic microbes is not significant, and they do not contribute to any possible degradation processes. However, during storage, the colony counts of the groups detected usually increased, but as there are no limits for these groups, it is difficult to assess their microbiological status in terms of shelf life. The technological and hygiene criterion of Regulation (EC) No. 2073/2005 [
57] pertains solely to the determination of the number of
E. coli in chopped or cut fruit and vegetables, allowing between 100 and 1000 per 1 g sample. Nevertheless, Decree 4/1998 of the Hungarian Ministry of Health [
58] also establishes a threshold for the number of moulds, specifying a range between three and four orders of magnitude. Moulds were very rare in our samples, and anaerobic storage reduced their numbers below the detection limit, with yeasts becoming the dominant fungi.
In the case of sweet potato samples, the data obtained during microbiological tests were found to be significantly heterogeneous. This discrepancy may be attributable to the fact that, in the case of the three parallel samples, one of the tested sweet potato cubes exhibited a significantly lower microbial count than the other two. As demonstrated in
Figure 2, the sample’s standard deviation exhibited a range of up to three orders of magnitude, as evidenced by the data from Covington for anaerobic bacteria counts and lactic acid bacteria. It has been demonstrated that significant deviations may be attributable to a number of factors, including, but not limited to, the heterogeneous distribution of microbes, sampling or dilution errors, plating or colony-counting errors, contamination, or even real variability in microbial load [
59,
60,
61,
62].
In the case of the Covington (dark-orange-fleshed sweet potato) variety, the treatments resulted in a reduction in lactic acid bacteria during storage, leading to a slight increase in pH values when treated with apple pomace and chokeberry pomace extracts. It has also been demonstrated that this variety underwent a decline in LAB following treatment with ascorbic acid and citric acid. However, a decrease in pH values was observed in response to the application of the organic acids. In the case of the Bonita (white-fleshed sweet potato) variety, it was also observed that the treatments reduced the lactic acid bacteria, but not to the same extent, resulting in minimal changes in pH values. Consequently, the findings of the treatment studies indicate that citric acid treatment is the most efficacious procedure; thus, we recommend its utilisation to maintain microbiological stability and extend the shelf life of sweet potato varieties. However, for the Bonita (white) variety, a combination of citric acid and ascorbic acid would be advantageous in order to achieve a more effective impact.
The phenomenon that organic acids have a stronger antimicrobial effect than fruit pomace extracts can be attributed to different factors. Organic acids (e.g., acetic, lactic, citric, or malic acid) are typically in a pure or highly concentrated state, thereby ensuring consistent and potent antimicrobial activity. Conversely, fruit pomace extracts comprise a multifaceted array of compounds, including fibres, flavonoids, polyphenols, and sugars, as well as negligible quantities of organic acids [
63,
64]. Consequently, the concentration of active antimicrobial substances is considerably lower and more variable. The mechanism of action of organic acids involves the disruption of microbial cell membranes and the reduction in the pH level. Organic acids are capable of penetrating cells in an undissociated state, thereby acidifying the cytoplasm [
65,
66]. These mechanisms have been shown to be direct and efficient. However, it is important to note that pomace extracts may rely more on flavonoids and phenolic acids, which have weaker and often species-specific antimicrobial activity [
67]. These compounds usually act via extracellular enzyme inhibition, antiadhesion activity, membrane destabilisation and/or permeabilisation, and the chelation of metal ions [
68]. While pomace extracts may contain a variety of bioactive compounds, the lack of synergy or the presence of certain compounds, such as sugars or enzymes, may reduce their antimicrobial effectiveness [
69,
70]. It is evident that organic acids have a substantial impact on the pH of the medium, resulting in the creation of an environment that is conducive to the inhibition of bacterial growth. It is noteworthy that pomace extracts may not adequately acidify the environment unless they are concentrated or supplemented. In the case of Golden Delicious apple pomace extract, successful antimicrobial and antioxidant activity was found [
71]. It was concluded that the antioxidant activity was provided by compounds belonging to the flavonoid group of apple pomace. The pomace extract showed good inhibitory activity against
Staphylococcus aureus and
Escherichia coli. Phloridzin and phloretin have the potential to be used as natural alternatives to synthetic antioxidants and antimicrobial agents. Also, in the case of apple pomace, a high percentage of inhibition was shown against
S. enterica,
S. aureus, and
E. coli, and a high correlation was shown between the total phenolic content and the percentage of bacterial inhibition of pomace extracts [
72]. Similar results were reported for chokeberry pomace extract against food-borne strains of
S. aureus,
E. coli, and
Streptococcus pyogenes. Furthermore, both aqueous and ethanolic extracts were highly effective against pathogens even at low (5%) concentrations [
73].
In the case of analytical measurements, a comparative analysis of the treated samples and the corresponding control samples was conducted. The sweet potato cubes were subjected to a surface colour measurement, refraction (water-soluble dry matter content), and a pH measurement. Subsequently, extracts were prepared from the ground samples, enabling the total polyphenol content and antioxidant capacity to be quantified.
The findings of this study demonstrate that the antimicrobial properties of fruit pomace are less pronounced than those exhibited by organic acids. However, it would be advantageous to investigate the potential of utilising fruit pomace in conjunction with acids. This approach has the potential to enhance the efficacy of the utilisation of pomace in food production. The combination of organic acids with fruit pomace extracts may result in a synergistic antimicrobial effect, whereby the combined impact of the mixture may exceed the sum of the individual components’ actions. The combination can attack microbes through multiple mechanisms, making it harder for them to adapt or resist. The employment of both agents may facilitate reduced concentrations of each microbe, thereby minimising the potential for adverse sensory or toxic effects, a matter of particular significance in the context of food applications. Furthermore, beyond its role in preservation, pomace provides fibre, antioxidants, and phytochemicals, thereby enhancing the nutritional value of food or supplements. The combination of natural by-products, such as pomace, with organic acids is in alignment with consumer demand for natural, clean-label food preservatives. Moreover, it adds value to agricultural waste, thus contributing to the principles of a circular economy.