Abstract
Zirconia crowns are employed in pediatric dentistry for the complete restoration of anterior and posterior deciduous teeth. They are considered the best option due to their esthetic appeal, high strength, biocompatibility, and resistance to wear and corrosion. This study aims to evaluate the physico-chemical, cytological, and microbial properties of zirconia crowns to determine their biocompatibility, safety for surrounding tissues, and effectiveness in preventing microbial influence on tooth tissue based on their biofilm deposition potential. XRD measurements were conducted to confirm the crown composition. For the microbiological examination, a quantitative assessment of the adhesion capacity of the analyzed strains and the formation of a mixed biofilm was performed using a Zeiss Cell Observer SD confocal microscope. This study used a mixed biofilm containing Streptococcus mutans (ATCC 25175), Lactobacillus rhamnosus (ATCC 9595), Candida albicans (ATCC 90028), and Candida albicans (ATCC 10231) to simulate the oral environment and the possible dynamics created between different types of microorganisms. A direct contact method was used to assess cytotoxic properties. The zirconia crown biomaterial shows a low ability to adhere to specific microorganisms, with L. rhamnosus predominating, indicating low clinical potential for causing inflammation of the tissues surrounding the crown. The cytotoxic properties of the biomaterial were found to be at level 2, indicating moderate cytotoxicity. Their biggest flaws are price and the need for passive fitting, which involves aggressive grinding; this is a potential limitation when it occurs in children, as their cooperation with the treatment can be difficult to guarantee.
1. Introduction
Zirconia crowns address the growing esthetic demands in modern pediatric dentistry. These prefabricated crowns provide complete coverage for both anterior and posterior deciduous teeth, with their convenience being especially evident in the anterior dental arch. They come in the form of kits, containing several sizes dedicated to the restoration of specific primary teeth. They are made of crystalline zirconium dioxide, which is a polymorph of three forms—monoclinic, tetragonal, and cubic. Pure zirconia is in the monoclinic phase and stays in that phase up to 1170° [1]. The indications to use zirconia crowns are the presence of early childhood caries (ECC), severe hard tooth tissue destruction [2,3,4,5,6,7,8,9], post-traumatic tooth damage [10,11], developmental tooth disorders [12], and teeth restoration after pulpal therapy [13,14,15,16,17]. The advantages of zirconia include its good esthetics, high strength, biocompatibility, high wear, and corrosion resistance [13]. It can be used in patients with Ni- Cr allergy or sensitivity. Its disadvantages include its high costs [18]. The use of zirconia crowns involves the necessity for proper tooth preparation, which increases the risk of pulp exposure and requires sufficient cooperation from the pediatric patient [5]. Zirconia crowns cannot be cut and crimped, resulting in the tooth requiring adaptation to the crown, not the other way around [19]. The preparation must be appropriate so that it provides a passive fit [20]. An alternative method of restoration of anterior damaged teeth is the strip crown method or the use of pre-veneered preformed metal crowns (PVPCs) [21]. Based on a study by Walia et al., the retention of zirconia crowns was 100% 6 months after placement, while that of PVPMCs was 95%, and that of the strip crown method was only 78% [22]. Based on Abdurahman et al., the survival rate of zirconia crowns can be found to be 90% in the absence of pulp therapy and 76% for teeth restored with zirconia crowns after pulp therapy. The mean survival time was 39.5 months [23]. In pediatric dentistry, due to the frequent difficulty in guaranteeing cooperation associated with the age of the patient, practitioners strive to use such methods that are associated with a long-term survival rate [22,23,24,25,26,27]. Another consideration is the effect of the zirconia crown material on the tissues surrounding the tooth. Studies show no significant inflammation in the surrounding gingival tissues. In comparison to stainless steel crowns (SSCs), the inflammation rate for this material was significantly lower [28,29]. Gingival health was also better around the zirconia crown compared to that when PVPMCs were used. The study by Esquivel-Upshaw JF et al. also showed no increased tissue abrasion of the teeth opposite the zirconia crowns compared to that observed with steel crowns or control enamel tissue. Nevertheless, many experts have drawn attention to the possible risk factors associated with the use of permanent restorations, such as prefabricated zirconia crowns, in the oral environment. The purpose of this study is to evaluate some of the properties of the material from which prefabricated zirconia crowns are made. The first aspect is the physico-chemical composition of the crowns and their potential effect on the surrounding tissues. These effects are not only related to the functional properties of the crown itself; they are also related to the wear products that can be observed in the tissues. The second issue is the microbiological aspect of the zirconia crown material and its possible inflammatory response. Lastly, the cytotoxic aspect of the zirconia biomaterial is evaluated.
2. Materials and Methods
The subject of the study is NuSmile® zirconia crowns (Houston, TX, USA), used in pediatric dentistry. The crowns are used for the restoration of both anterior and posterior primary teeth (see Figure 1). The crowns are distributed in classic, professional, anterior, and lateral kits, containing different sets of crowns. Each tooth can be restored by a crown available from one of six sizes and two thicknesses—light and extra light. To improve and help adjust the crown size during clinical procedures, NuSmile® introduced try-in crowns, which allow practitioners to avoid the unnecessary sterilization of incorrectly scaled crowns coming into contact with the patient’s saliva and blood at the point during which the size is selected. It is also possible to purchase a package containing 1 crown of a certain size. According to the safety data sheet, the composition of the Nu Smile zirconia crown is 88–96% Zirco\nium oxide, 4–6% yttrium oxide (Y2O3), 5% hafnium oxide (HfO2), 2–5% organic binder, and 1–4% pigment (63 NuSmile®). The tests were performed on crown number B1L (second primary incisor size 1).
Figure 1.
NuSmile® zirconia crowns, size B1L (Houston, USA), used in pediatric dentistry: (A) frontal view; (B) lateral view.
This study aims to analyze the composition and properties of the zirconia crown biomaterial, its potential cytotoxicity, and its ability to form a bacterial biofilm, which is essential for maintaining oral health. The test samples were sterilized in a Vacuklav 24B+ autoclave (Melag, Berlin, Germany) using the steam method, in accordance with European standard EN 13060 [30], at a temperature of 121 °C.
2.1. Physico-Chemical Assay
The structure of zirconia crowns was investigated based on X-Ray Powder Diffraction (XRPD) patterns recorded in a 2θ range of 10–90° using X’Pert Pro PANalytical X-ray diffractometer (Malvern Panalytical Ltd., Mavern, UK) equipped with Ni-filtered Cu Kα1 radiation (Kα1: 1.54060 Å). The obtained patterns were analyzed by Match! software version 3.11.1.183 (Crystal Impact, Bonn, Germany).
The crowns were also subjected to surface topography measurements using Leica DCM8 (Leica Microsystems, Wetzlar, Germany), a 3D surface metrology microscope based on confocal microscopy. The measurements determined parameters describing surface roughness (Sa, Sq, Sv). The values obtained were obtained for a small homogeneous measurement area of the same size (700 × 900 µm) for the Sa and Sq parameters and for a measurement line of 800 µm for the Ra and Rq parameters, with a 20× magnification confocal objective. Measurements were taken in five test areas on two sides of the crown: anterior and posterior. In addition, the wetting angle values of the zirconia surfaces were measured using the deposited droplet method using an OEG Surftense goniometer (OEG GmbH, Frankfurt, Germany) to determine the surface properties related to the absorption of water and other substances, as well as biofilm [31].
2.2. Microbiological Assay
The representative strains selected for this study were obtained from the American Collection of Type Cultures: Streptococcus mutans (ATCC 25175), Lactobacillus rhamnosus (ATCC 9595), Candida albicans (ATCC 90028), and Candida albicans (ATCC 10231) [31,32,33]. Zirconia crowns were used as the dental material, specifically two crowns of size B1L (second primary incisor size 1). The test samples were sterilized in a Vacuklav 24B+ autoclave (Melag, Germany) using the steam method, in accordance with European standard EN 13060, at a temperature of 121 °C.
A quantitative assessment was performed to evaluate the adhesion capacity of the analyzed microbial strains and their ability to form a mixed biofilm on the selected dental material. Microbial suspensions were prepared from fresh cultures and adjusted to a McFarland standard of 0.5, corresponding to 1.5 × 108 CFU/mL for bacterial strains and 1.5 × 10⁶ CFU/mL for fungal strains. Brain Heart Infusion Broth (BHI) supplemented with 5% sucrose (Biomaxima, Lublin, Poland) served as the growth medium.
Two microbial mixtures were prepared by combining the strains in the following volumes: (i) 400 µL of Candida albicans ATCC 90028, 400 µL of Streptococcus mutans ATCC 25175, and 200 µL of Lactobacillus rhamnosus ATCC 9595; (ii) 400 µL of C. albicans ATCC 10231, 400 µL of S. mutans ATCC 25175, and 200 µL of L. rhamnosus ATCC 9595. Sterile dental crowns were then immersed into the prepared microbial suspensions.
The samples were incubated for 24 h at 37 °C under aerobic conditions with elevated CO2. Following incubation, the crowns were rinsed three times with sterile saline and subsequently vortexed for one minute in 1 mL of 0.5% saponin solution (Sigma-Aldrich, Poznań, Poland) to detach adherent microorganisms. The resulting suspensions were quantitatively cultured on BHI agar (Biomaxima, Lublin, Poland). After incubation, colony-forming units per milliliter (CFU/mL) were enumerated to determine the extent of microbial adhesion and biofilm formation.
The CFU/mL value was determined using the following ratio:
CFU/mL = average number of colonies × inverse of dilution × 10
The test was carried out in 2 repetitions.
For confocal microscopy analysis, a microbial suspension at a McFarland standard of 0.5 was prepared from fresh cultures of Streptococcus mutans, Lactobacillus rhamnosus, and Candida albicans ATCC 90028. A 1 mL mixed suspension, composed of 400 µL C. albicans, 400 µL S. mutans, and 200 µL L. rhamnosus, was incubated with the biomaterial for 24 h at 37 °C under aerobic conditions with elevated CO2 levels.
Following incubation, the biomaterial was rinsed with sterile saline and stained using the LIVE/DEAD BacLight Viability Kit (Invitrogen, Life Technologies Corporation, Oregon, USA). After a 30 min incubation period with the staining solution, the samples were washed and prepared for imaging. To immobilize the samples during microscopy, they were mounted on silicone paste and covered with a No. 1.5 coverslip.
Initial imaging was conducted using a Zeiss Cell Observer SD confocal microscope. Further high-resolution imaging was performed using a Leica SP8 confocal microscope equipped with an HC Fluotar L 25× water immersion objective (numerical aperture [NA] 0.95) and an HC PL Apo 10× objective (NA 0.40). The confocal aperture was set to 1 Airy unit. The LIVE stain (Syto9) and DEAD stain (propidium iodide) were both excited using a 488 nm laser, with fluorescence emissions detected in the 493–539 nm range. Additionally, reflected light from a 561 nm laser was collected to visualize the coronal surface of the material.
Multiple representative regions of each sample were imaged in Z-stacks, with optical section thickness ranging from 60 to 450 µm and a section interval of 2–5 µm. Image noise was minimized using a 3 × 3 median filter. Image processing and three-dimensional visualizations were performed using Imaris software (ver. 10.2; Oxford Instruments, Singapore), with live cells displayed in green, dead cells in red, and the coronal surface in blue.
2.3. Histology Assay
A B1L zirconia crown (second primary incisor, size 1) was selected for use in the study. The sample was sterilized using steam autoclaving in a Vacuklav 24B+ autoclave (Melag, Germany), in compliance with European Standard EN 13060, at 121 °C. Cytotoxicity testing was conducted via the direct contact method, following the guidelines outlined in ISO 10993-12 [34] and ISO 10993-5 [35], which govern the biological evaluation of medical devices.
Direct contact cytotoxicity assays were performed using the Balb/3T3 normal mouse fibroblast cell line (clone A31; ATCC® CCL-163™, Manassas, VA, USA), a widely recognized model for the in vitro assessment of biomaterial cytotoxicity, as specified in EN ISO 10993-5:2009. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Lonza, Basel, Switzerland) containing 4.5 g/L glucose and 25 mM HEPES, supplemented with 1% L-glutamine, penicillin-streptomycin (Sigma-Aldrich®, St. Louis, MO, USA), and 10% calf serum (Sigma-Aldrich®). Cultures were maintained under standard conditions (37 °C, 5% CO2, and constant humidity) using a HERAcell CO2 150i incubator (Thermo Scientific, Waltham, MA, USA).
Cells were trypsinized using 0.25% Trypsin-EDTA (Sigma-Aldrich®), resuspended in complete medium, and seeded in 6-well culture plates (TPP, Trasadingen, Switzerland) at a density of 1.5 × 105 cells per well. After 24 h of incubation, the sterilized zirconia crown was introduced into the wells. Following a further 24-h incubation period in direct contact with the test material, cell morphology was evaluated under an inverted phase-contrast microscope (CKX53, Olympus, Tokyo, Japan). Observations focused on cells beneath and adjacent to the test material, as well as in unaffected regions of the well.
Control wells contained cells cultured under identical conditions but without exposure to the test material. Cytotoxicity was assessed based on morphological changes, according to the classification system described in ISO 10993-5. The degree of cytotoxicity was graded on a 0–4 scale, where higher values indicated greater morphological alterations and cytotoxic effects (see Table 1).

Table 1.
Cytotoxicity degree of the test material in direct contact (PN-EN ISO 10993-5:2009).
3. Results
3.1. Physico-Chemical Assay Results
The adhesion of biological materials is determined by the physico-chemical properties of the substrate, particularly its topography and wettability, but also by the chemical composition of the substrate, which determines both the nature and quality of the adhesive force.
Due to the shape of the crown, which is related to the surfaces in direct contact with the enamel, the physico-chemical properties were studied for the anterior and posterior surfaces. Due to the change in curvature of the surfaces tested, differences of up to 50% in the roughness values measured both along the line (Ra, Rq) and for the surface (Sa, Sq, Sz) can be observed from the results shown in the table (Table 2).

Table 2.
Comparison of the surface roughness values, the linear roughness (PN-EN ISO values and the contact angle values for zirconium crowns.
A measure of the wettability of a surface is the wetting angle θ, the angle between the wetted surface of the solid and the tangent to the surface of the wetting liquid droplet, derived from its point of contact with the surface of the solid. From the results shown in Table 2, it can be seen that these coronae are characterized by a water contact angle value of less than 90, and the surface is considered hydrophilic.
Surface roughness significantly influences the contact angle and wettability of a surface. The impact of roughness depends on whether the droplet wets the surface ridges or if air pockets remain between the droplet and the surface.
Figure 2 shows images of the surface topography of the Y-ZrO2 ceramic with respect to the measurement surfaces: anterior and posterior. The results show the microporosity of each surface. In addition, the anterior surface of the crown is characterized by numerous scratches and pits, which also modify the surface roughness and waviness values. It is in these areas that the crown is most likely to come into contact with the enamel of the natural tooth, which interacts with it in the occlusion. As a result of this contact, the hardness of the crown must have a lower hardness value and a higher abrasion value than that of the tooth in order to avoid abrasion of the natural tooth.
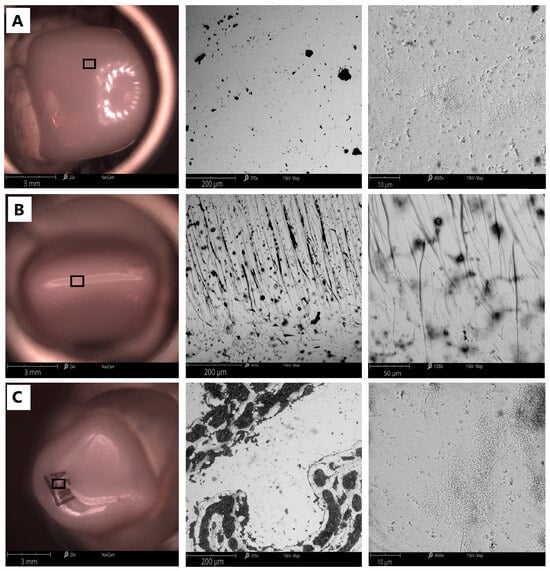
Figure 2.
Comparison of the surface topography of a dental crown obtained with the Phenom ProX Desktop SEM: (A) anterior side; (B) top of crown; (C) posterior side; resolution 200 µm (middle), 10 µm (right side).
This microporosity of the zirconia ceramic can also be seen in the surface images shown in the figure (Figure 3), obtained at a magnification of ×4500. Using energy-dispersive X-ray diffraction analysis (EDS), which the Phenon ProX microscope (ThermoFisher Scientific, Wlatham, MA, USA) is equipped with, the surface content of the elements Y, Hf, Zr and O, of which these crowns are composed, was analyzed. Comparing the two surfaces, it can be seen that the highly polished surface (anterior side) has a higher concentration of Zr, Hf and Y ions. The findings from our analysis of the elemental composition of the surface are in accordance with the results obtained by X-ray diffraction (Figure 4).
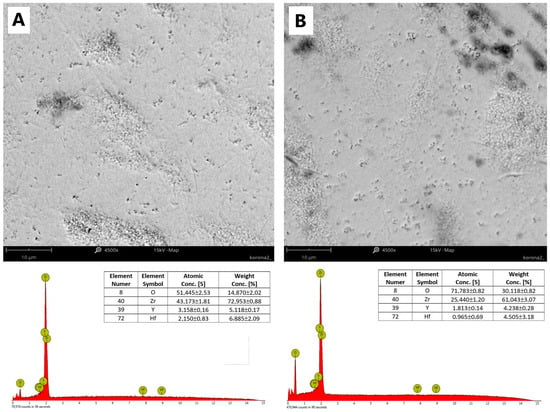
Figure 3.
Comparison of anterior and posterior coronal surfaces (×4500) and results of energy-dispersive X-ray diffraction analysis conducted with the Phenom ProX microscope for (A), the anterior side, and (B), the posterior side.
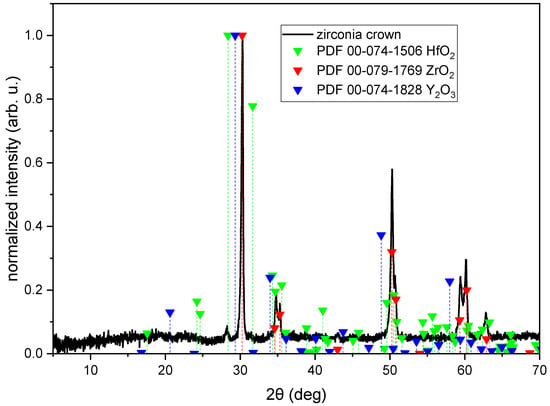
Figure 4.
XRD diagram of zirconia crown (black line) with reference patterns of HfO2 (red green triangles), ZrO2 (red triangles), and Y2O3 (blue triangles).
Figure 4 shows an XRD diagram of a zirconia crown measured in a 5–70° 2θ range with marked positions of ZrO2, HfO2, and Y2O3. It is clearly visible that the crown is composed mostly of ZrO2. There is also a HfO2 phase visible (peak at 2θ = 28.2°). In the case of Y2O3, there are bumps in the position of the most intense peaks (2θ = 29.3°, 34°, and 48.8°). According to the Match! Software, the composition of the crown is ZrO2—91.2%; HfO2—6.7%; and Y2O3—2.1%.
3.2. Microbiological Assay Results
Figure 5 presents the final results of the quantitative culture of the microbial suspension desorbed from the material’s surface. Table 3 and Figure 6 summarize the obtained values of colony-forming units for the mixed microbial suspension used.
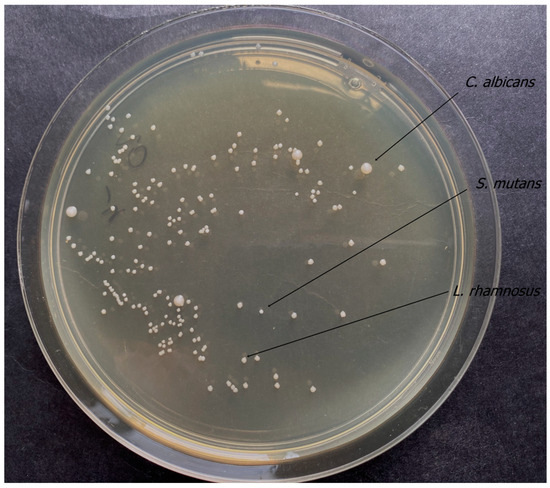
Figure 5.
Results of quantitative culture of microbial suspension.

Table 3.
Values of colony-forming units for the mixed microbial suspension.
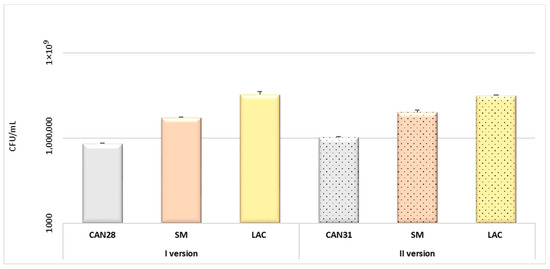
Figure 6.
Evaluation of biofilm Candida–Streptococcus–Lactobacillus formation. Degree of adhesion of microorganisms to the crown surface: version I—3-species biofilm C. albicans ATCC 90028 (CAN28), S. mutans ATCC 25175 (SM), L. rhamnosus ATCC 9595 (LAC)); version II—3-species biofilm (C. albicans ATCC 10231 (CAN31), S. mutans ATCC 25175, L. rhamnosus ATCC 9595).
Figure 7 and Figure 8 present a confocal microscopy visualization of the crown material surface using the laser reflection technique. Figure 7 shows examples of the three-dimensional rendering of different areas of the untreated crown surface, while Figure 8 shows the material and patches of the biofilm that formed on it.
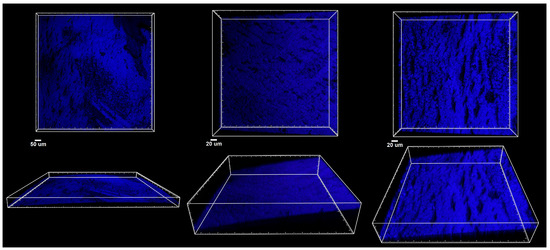
Figure 7.
Confocal microscopy imaging of zirconia crown. Top panel: XY projections of crown surface (blue); bottom panel: side XZ views of the same areas indicating the curvature of the material.
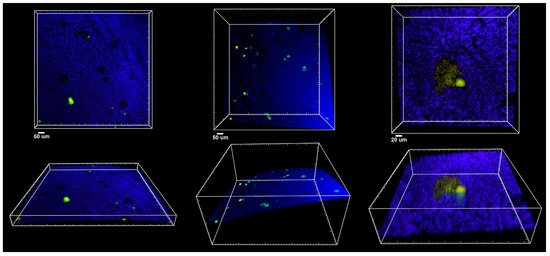
Figure 8.
Confocal microscopy imaging of zirconia crown material after exposure to biofilm-forming microorganisms. Top panel: XY projections of crown surface (blue); bottom panel: side XZ views of the same areas indicating the curvature of the material covered with LIVE/DEAD stained microorganisms (green to yellow).
The conclusions of the study were that the colony-forming unit values for both C. albicans 90028 and C. albicans 10231 were similar. Furthermore, the highest CFU/mL values for the mixed microbial suspensions used were shown for L. rhamnosus. Based on confocal microscopy, a small number of bacterial flaps were observed on the surface of the biomaterial. C. albicans cells were absent.
3.3. Histology Assay Results
Balb/3T3 cells showed abnormal changes in morphology under the sample after contact with zirconia crown material. These changes involved the inhibition of the growth of the culture; the shape of most of the cells was spherical. In the rest of the well and at the edge of the sample, the morphology of the cells was normal. The cytotoxicity of the material was assessed as moderate. Table 4 and Figure 9 summarize the results of the histological examination.

Table 4.
Cytotoxicity degree of the test material in the direct contact (PN-EN ISO 10993-5:2009).
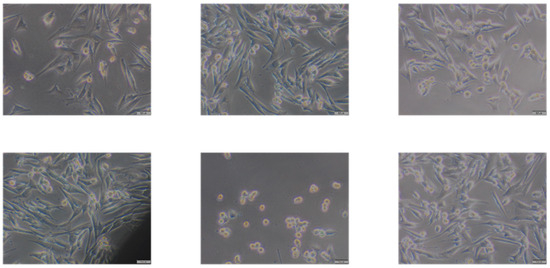
Figure 9.
Morphological image of Balb/3T3 fibroblasts after 24 H incubation with dental crown, from left to right: control culture without contact with tested material; 1 cm from the edge of the specimen; more than 1 cm away from the specimen; at the edge of the sample; under the specimen; further away from the specimen. Magnification 100×.
The material supplied for testing (crown fragment) is characterized by moderate cytotoxicity. According to the above-mentioned standard, cytotoxic material has a grade 2 rating at least.
4. Discussion
The human oral cavity offers a unique environment for microorganisms to form complex biofilms. It hosts several hundred species of microorganisms, including bacteria, fungi, protozoa, and viruses [36]. The first stage of biofilm formation in the oral cavity is for free-living microorganisms to adhere to the surface and cover it with an acquired membrane (pellicule) [37]. This includes both natural structures (enamel, dentin, mucosal epithelium) and restorative or prosthetic materials (implants, dental fillings, crowns) [37]. Through simple chemical bonds (van der Waals forces, electrostatic and Lewis electron forces), gravitational forces, and surface tension, microorganisms begin to adhere to the membrane surface. Bacteria bind to the membrane within the first few hours after brushing teeth. Priority is given to “early colonizers”, which include bacteria of the genus Streptococcus, Eikenella, Streptococcus, Neisseria, and Haemophilus. “Late colonizers” attach to pioneer bacteria to form coaggregates. Such abilities are attributed to bacteria of the genera Eubacterium, Actinomyces, Prevotella, Porphyromonas, and Fusobacterium [38,39,40,41]. A biofilm is an organized cluster of bacteria and fungi, characterized by numerous synergistic and antagonistic interactions that create microbial interdependencies and confer resistance to external factors [42,43]. In the oral cavity, biofilm plays a significant role in the development of caries, gingivitis, and periodontitis [44]. Although biofilm formation on dental biomaterials may appear harmless, it poses risks similar to those of periodontal disease and peri-implantitis [39,45]. Biofilm formation around dental fillings can lead to secondary caries [39]. Therefore, there is a significant need for innovative materials that minimize bacterial adhesion to their surfaces [32]. Developing artificial dental materials with reduced bacterial adhesion is crucial for maintaining oral health [40]. Zirconium (zirconium oxide) was introduced by Martin Heinrich Klaproth in 1789. It is distinguished by its lack of cytotoxicity and anti-adhesive properties regarding bacteria [46]. The aim of our study was the formation of three species of biofilms on the surfaces of zirconia crowns. We investigated the in vitro adhesion abilities of Streptococcus mutans, Candida albicans and Lactobacillus rhamnosus. S. mutans was chosen in our study because it is commonly associated with dental caries and periodontitis [41]. Moreover, placing the crown in the mouth creates a new niche for the adhesion of S. mutans, among other microorganisms, which plays a crucial role in the development of secondary caries on the cemented tooth. In the long term, this may impact the clinical success of the restored tooth [33]. Candida albicans is often isolated along with S. mutans from carious lesions in children with severe early childhood caries (ECC) [47]. The Lactobacillus (Lactobacillus casei, L. rhamnosus, L. acidophilus) species, including lactic acid bacilli, in addition to the caries pathogens described above, are considered to be associated with the ECC process. These bacteria are pioneering microorganisms in the progression of the carious process, especially in dentin. Compared to our study performed on stainless steel crowns [32], where S. mutans showed no adhesion to SSC steel crowns, in the case of zirconia crowns, S. mutans adhered at a level of 5.25 × 106–8 × 106 CFU/mL. The difference in our results may have to do with the different surfaces of the material and also the biofilm model. The biofilm on steel crowns was used in a single-species model. Single-species biofilms are typically used to evaluate adhesion to dental materials [48]. In the current study, we created a three-species Streptococcus–Candida–Lactobacillus biofilm to replicate conditions more similar to those in the oral cavity. The susceptibility of in vitro biomaterial surfaces to adhesion is quite different in a multispecies environment than for a single species [49]. C. albicans and S. mutans show synergistic behavior in mixed biofilms [41]. In the presence of sugar, Candida can form a complex biofilm and attach to S. mutans; thanks to carbohydrates, with the use of glucosyltransferase B, they form sticky glucans, which form a scaffold to S. mutans. In the natural oral environment, these microorganisms affect each other’s properties, amplifying their negative effects on the tissues that create the oral environment [50]. S. oralis and C. albicans combined form stronger biofilms than either microorganism alone. An enhancement of C. albicans invasiveness and promotion of bacterial biomass proliferation were observed in the presence of S. mutans [44,51]. In our study, the observation was that both strains of C. albicans used in the biofilm showed a similar level of cell adhesion to the surface of zirconia crowns at 6.25 × 106–1.05 × 106. Both quantitative assessment of the adhesion ability of the strains analyzed, the formation of a mixed biofilm and the confocal microscopy results showed that the microorganism with the highest adherence is L. rhamnosus at a level of 3.4 × 107 ± 9.89 × 106 CFU/mL for version I and 3.15 × 107 ± 1.41 × 106 for version II. L. rhamnosus has the ability to interfere with caries-forming biofilm formation by various processes, such as through the formation of biosurfactants and a reduction in biofilm acidity [52,53]. Figure 6, Figure 7 and Figure 8 provide the results of the microbiological tests, while Table 2 shows the exact values of the degree of adhesion of the different microbial strains. L. rhamnosus GG demineralizes both enamel and dentin. In its mixed form with S. mutans, it induces similar or even increased demineralization compared to the action of S. mutans alone [53]. Aouame A. et al. evaluated the adhesion of S. aureus to the surface of tooth fragments and stainless steel fragments. The study showed that the teeth used in the study had a rougher surface and were more prone to biofilm formation, as opposed to stainless steel, which is smooth [38].
The process of biofilm formation on a surface is related to its nature and properties, such as porosity or roughness, which determine the degree of surface development. One way of influencing this parameter is the type of initial and final treatment to which the material is subjected. In the case of ceramics, the processes that strongly influence the quality of this surface are surface glazing and subsequent polishing. Both processes aim to obtain a smooth surface, which improves both the physical and functional properties of the material, such as the elimination of irregularities and fine scratches in the material, but also influences the biological properties of the surface. A smooth and polished surface is characterized by a low surface energy, which has the effect of reducing the formation and accumulation of plaque, which slows the initiation and development of caries and periodontal disease [54]. Plaque adheres in greater amounts when the surface of the material is uneven. Grooves and other surface defects lead to an increase in potential surface area for seeding and are sites that favor microbial niche formation. Biofilm primarily develops in surface depressions through the irreversible attachment of pioneer planktonic bacteria [38].
On the other hand, the reduced surface roughness associated with glazing and polishing improves the mechanical and service properties of the material. The reduction in surface roughness, and therefore the reduction in roughness value, is aimed at obtaining a homogeneous material that would be able to transmit loads uniformly throughout its volume without the development of stress concentrations that could lead to the initiation and propagation of microcracks, which is very unfavorable for ceramics due to the brittle nature of the material and its catastrophic failure. Greater surface roughness leads to greater microbial colonization because it provides shelter from shear forces, particularly during initial attachment [40].
In a study by Tu et al., the strongest adhesion ability to resin material was shown by S. mutans and Actinomyces viscosus. In contrast, the adhesion of S. mutans and A. viscosus to glass–ionomer material was lower than that to resin material. At the same time, there was no significant difference in the number of both S. mutans and A. viscosus bacteria adhering to glass–ionomer material [55]. Zirconia crowns used in pediatric dentistry, like any material used in the oral environment, will be exposed to various factors and stimuli. According to Murali et al., who compared stainless steel crowns, preformed zirconia crowns used in pediatric dentistry to preserve the tissue of posterior primary teeth show a similar success rate. However, zirconia crowns show slightly poorer retention and marginal integrity, and, according to their findings, accumulate more plaque on their surface. According to this study, both methods are an excellent way to preserve tissue; the only significant difference is esthetics [56]. Agraval et al. compared SSC to zirconia crowns. Both crowns were bonded with glass–ionomer luting cement, with a GC gold label. Zirconia crowns have also been shown to develop microcracks under occlusal forces. In the case of gingival tissues, both types of crowns initially cause soft tissue inflammation with slightly increased severity in the case of zirconia crowns, which is associated with more aggressive grinding [57]. In clinical studies, many authors have shown a definite advantage of zirconia crowns over strip crowns. Singh et al. conducted studies at 3, 6, 9, and 12 months on zirconia, SSC, and strip crowns. They found that the polished, smooth surfaces of zirconia crowns may contribute to less plaque buildup and thus less gingival irritation [58]. A similar study was conducted by Nikhil et al. The team restored teeth in a 4-year-old boy with zirconia crowns. After a 30-month follow-up, the crowns showed normal functioning, including no discoloration, no loss of filling, and no plaque accumulation, unlike composite strip crowns [59]. Mathew’s team measured the degree of adhesion of S. mutans bacteria to zirconia and stainless steel crowns in a clinical study and compared the degree of plaque accumulation and gingival inflammation over a 1-year period in children. S. mutans adhered more to SSCs compared to zirconia crowns in all control studies [29]. Mohamed A. Wakwak evaluated the adhesion of S. mutans and Lactobacillus to zirconia and SSCs in primary molars. A swab was taken from the occlusal and buccal surfaces. The researchers found that zirconia crowns performed better than SSCs in terms of preventing plaque adhesion [60].
Elizabeth JA et al. studied 21 children aged 4 to 7 years with bilateral decayed molars, who were divided into two groups: one group had zirconia crowns and the other had SSCs. After 1 and 3 months, plaque samples were taken from the gingival fissures, buccal mucosa, and cuspid surfaces of the teeth. To assess gingival health, the gingival index (GI) and plaque index (PI) were measured. Adherence of bacteria (S. mutans) to zirconia crowns was lower compared to that with SSCs, as demonstrated by their better gingival health and oral hygiene [61]
The team of Alaki et al. compared zirconia crowns with strip crowns in terms of gingival health, plaque accumulation, recurrent caries, failure of fillings, and abrasion of opposing teeth at 3, 6, and 12 months in 120 patients. They observed that teeth covered with zirconium crowns exhibited better gingival health, less bleeding, and reduced plaque accumulation. None of the teeth with zirconia crowns developed recurrent caries throughout the follow-up period. In contrast, 6.7% of teeth covered with composite resin strip crowns developed secondary caries during the 12-month observation period [60,61]. T. Walia et al. sampled 129 anterior primary teeth, comparing resin composite strip crowns and pre-veneered stainless steel crowns to zirconia crowns. The conclusion of their 6-month observations suggest that zirconia crowns used in anterior teeth show the lowest gingival inflammation rate compared to other restoration methods and show the lowest retention failure [60,62]. Ideally, dental prosthetic materials should closely mimic natural tooth structures [48]. The main advantage of zirconia crowns is their esthetics [13]. Zirconia crowns made of zirconium oxide are durable and color-matched to tooth tissue [38]. Thanks to these properties, they are rated as an alternative to SSCs [55].
In the studies published in the literature, the range of crown surface roughness values is characterized by a large spread in the results obtained, in (0.02–1.9) µm [63,64,65,66], mainly due to the different measurement ranges. The larger the surface area, the more varied and higher the values of the roughness parameters. The use of recent measurement techniques, such as confocal microscopy or AFM [63], makes it possible to obtain values that characterize a very small area, making the roughness results independent of the waviness of the surface. The results of the surface roughness (Sa) values (Table 2) are 29.18 nm for the anterior side and 66.05 nm for the posterior side, and the Ra parameters (measured for the test section) are 40 nm and 50 nm, respectively. These values characterize materials of roughness class N2, i.e., materials with very low roughness values. These results are also confirmed by microscopic observations (Figure 3) in which the material is homogeneous; only at a magnification of ×4500 is porosity visible, with pores no larger than a single nanometer.
Surface properties can significantly influence bacterial adhesion. Materials with high surface roughness and low hydrophobicity are more prone to plaque accumulation compared to those with low surface roughness and high hydrophobicity [43]. Bacteria tend to adhere most effectively to slightly hydrophobic or hydrophilic surfaces. The hydrophobicity or hydrophilicity of a surface correlates with the wettability of the surface, often expressed as the wetting angle (θ) of a water droplet on the substrate. In addition, bacteria tend to adhere best to surfaces with moderate wettability. The studies carried out show that, despite the differences in the structure and topography of the crown surface, the wetting angle values for each side are less than 90°, and are 66.57 ± 2.6° for the anterior surface and 75.95 ± 3.6° for the posterior surface. Ceramic is therefore a material characterized by high wettability, which increases its water adsorption capacity and the adhesion of different types of cells. In vitro studies suggest that, in general, water wetting angles in the range of 40° to 130° appear to lead to the greatest bacterial adhesion.
The XRD measurements confirmed the composition of the crown according to the manufacturer’s information. No other phases were visible, while the ratio of zirconium, hafnium, and yttrium oxide was appropriate. In clinical practice, it is important to remember that a preformed zirconia crown cannot be crimped. It is the tooth that must be adapted to the crown, not the other way around. The fit should be passive; subgingival preparation feather edge and bleeding control are also required. Proper preparation affects bacterial adhesion and reduces the risk of creating a niche for plaque.
Another crucial aspect is the biocompatibility of restorative materials in the oral environment. It is essential that they do not cause toxic reactions in the host, both in the short and long term [38].
According to Figure 9, zirconia crown material in close contact with the fibroblast cell shows changes in the morphology of the cells with the tested material (rounded cells, shrunken cells, stunted growth of the culture) and is characterized by moderate cytotoxicity, which must be taken into account when used in clinical practice.
5. Conclusions
Zirconia crown material exerts a moderate biological impact on surrounding tissues. Due to its classification as having medium cytotoxicity, its use in patients with significant systemic burdens or chronic immunosuppression should be approached with caution. Comparable to stainless steel crowns commonly utilized in pediatric dentistry, zirconia crowns offer full coronal coverage and are associated with a low risk of clinical failure.
The material also demonstrates minimal biofilm adhesion, which reduces the risk of gingival inflammation. However, as with all restorative materials, maintenance of proper oral hygiene is essential to ensuring long-term clinical success. Zirconia crowns are indicated in various clinical scenarios, including extensive loss of dental tissue due to caries, traumatic injury, or non-carious lesions.
A primary advantage of zirconia crowns is their superior esthetic outcome. Nevertheless, their placement necessitates substantial tooth preparation and passive crown adaptation, often requiring general anesthesia in pediatric patients. In situations where anesthesia poses a risk or is declined by caregivers, stainless steel crowns may offer a more practical and cost-effective alternative.
Ultimately, the selection of the appropriate restorative method should be individualized, taking into account clinical indications as well as the expectations and preferences of both the patient and their caregivers.
Author Contributions
Conceptualization, K.S., R.J.W. and M.D.; methodology, K.S., M.P., J.N., A.R., G.C., Ł.D., A.N. and A.W.; software, M.P., A.R., Ł.D. and A.W.; validation, K.S., A.N., R.J.W., A.W. and M.D.; formal analysis, K.S.; investigation, K.S., M.P., J.N., A.R., G.C., A.N., Ł.D., A.W. and R.J.W.; resources, K.S., M.P. and A.R.; data curation, K.S., J.N., A.R., Ł.D. and A.W.; writing—original draft preparation, K.S., M.P. and A.R.; writing—review and editing, A.N., R.J.W., A.W. and M.D.; visualization, K.S. and Ł.D.; supervision, A.N., R.J.W. and M.D.; project administration, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a subsidy from Wroclaw Medical University, number SUBZ.B180.24.058.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ajayakumar, L.P.; Chowdhary, N.; Reddy, V.R.; Chowdhary, R. Use of Restorative Full Crowns Made with Zirconia in Children: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2020, 13, 551–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Planells Del Pozo, P.; Fuks, A. Zirconia crowns-an esthetic and resistant restorative alternative for ECC affected primary teeth. J. Clin. Pediatr. Dent. 2014, 38, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Salami, A.; Walia, T.; Bashiri, R. Comparison of parental satisfaction with three tooth-colored full-coronal restorations in primary maxillary incisors. J. Clin. Pediatr. Dent. 2015, 39, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, D.M.; Wells, M.H.; Scarbecz, M.; Donaldson, M. Clinical evaluation and parental satisfaction with pediatric zirconia anterior crowns. Pediatr. Dent. 2016, 38, 192–198. [Google Scholar]
- Ashima, G.; Bhatia, S.K.; Gauba, K.; Mittal, H.C. Zirconia crowns for rehabilitation of decayed primary incisors: An Esthetic alternative. J. Clin. Pediatr. Dent. 2014, 39, 18–22. [Google Scholar] [CrossRef]
- Cohn, C. Zirconia-prefabricated crowns for pediatric patients with primary dentition: Technique and cementation for esthetic outcomes. Comp. Cont. Educ. Dent. 2016, 37, 554–558. [Google Scholar]
- Schmoeckel, J.; Gorseta, K.; Splieth, C.H.; Juric, H. How to intervene in the caries process: Early childhood caries—A systematic review. Caries Res. 2020, 54, 102–112. [Google Scholar] [CrossRef]
- An, S.Y.; Shim, Y.S.; Park, S.Y. Aesthetic rehabilitation in maxillary anterior tooth with early childhood caries using ZIRKIZ® crown: Long-term follow-up. Indian J. Sci. Technol. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Karaca, S.; Ozbay, G.; Kargul, B. Primary zirconia crown restorations for children with early childhood caries. Acta Stomatol. Croat. 2013, 47, 64–71. [Google Scholar] [CrossRef]
- Kara, N.B.; Yilmaz, Y. Assessment of oral hygiene and periodontal health around posterior primary molars after their restoration with various crown types. Int. J. Paediatr. Dent. 2013, 24, 303–313. [Google Scholar] [CrossRef]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.H.; Han, C.H.; Kim, S. Influence of internal-gap width and cement type on the retentive force of zirconia copings in pullout testing. J. Dent. 2012, 40, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A. Esthetic zirconia crown in pedodontics. Int. J. Pedod. Rehabil. 2017, 2, 31–33. [Google Scholar] [CrossRef]
- Seminario, A.L.; Garcia, M.; Spiekerman, C.; Rajanbabu, P.; Donly, K.J.; Harbert, P. Survival of zirconia crowns in primary maxillary incisors at 12-, 24- and 36-month follow-up. Pediatr. Dent. 2019, 41, 385–390. [Google Scholar]
- Yanover, L.; Tickotsky, N.; Waggoner, W.; Kupietzky, A.; Moskovitz, M. Zirconia crown performance in primary maxillary anterior teeth: A retrospective photographic and radiographic cohort study. Eur. Arch. Paediatr. Dent. 2020, 22, 417–423. [Google Scholar] [CrossRef]
- Donly, K.J.; Sasa, I.; Contreras, C.I.; Mendez, M.J.C. Prospective randomized clinical trial of primary molar crowns: 36-month results. Am. J. Dent. 2020, 33, 165–168. [Google Scholar]
- Lee, J.H. Guided tooth preparation for a pediatric zirconia crown. J. Am. Dent. Assoc. 2018, 149, 202–208. [Google Scholar] [CrossRef]
- Sztyler, K.; Wiglusz, R.J.; Dobrzynski, M. Review on Preformed Crowns in Pediatric Dentistry—The Composition and Application. Materials 2022, 15, 2081. [Google Scholar] [CrossRef]
- Lee, J.-H. Case Report Guided tooth preparation for a pediatric zirconia crown. Orig. Contrib. Case Rep. 2018, 149, P202–P208.E2. [Google Scholar] [CrossRef]
- Ayad, M.F.; Maghrabi, A.A.; Rosenstiel, S.F. Assessment of convergence angles of tooth preparations for complete crowns among dental students. J. Dent. 2005, 33, 633–638. [Google Scholar] [CrossRef]
- Alrashdi, M.; Ardoin, J.; Liu, J.A. Zirconia crowns for children: A systematic review. Int. J. Paediatr. Dent. 2021, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Walia, T.; Salami, A.A.; Bashiri, R.; Hamoodi, O.M.; Rashid, F. A randomised controlled trial of three aesthetic full-coronal restorations in primary maxillary teeth. Eur. J. Paediatr. Dent. 2014, 15, 113–118. [Google Scholar] [PubMed]
- Alhissan, A.S.; Pani, S.C. Factors Influencing the Survival of Preformed Zirconia Crowns in Children Treated under General Anesthesia. Int. J. Dent. 2021, 2021, 5515383. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- A Townsend, J.; Knoell, P.; Yu, Q.; Zhang, J.-F.; Wang, Y.; Zhu, H.; Beattie, S.; Xu, X. In vitro fracture resistance of three commercially available zirconia crowns for primary molars. Pediatr. Dent. 2014, 36, 125–129. [Google Scholar]
- Choi, J.-W.; Bae, I.-H.; Noh, T.-H.; Ju, S.-W.; Lee, T.-K.; Ahn, J.-S.; Jeong, T.-S.; Huh, J.-B. Wear of primary teeth caused by opposed all ceramic or stainless steel crowns. J. Adv. Prosthodont. 2016, 8, 43–52. [Google Scholar] [CrossRef]
- Donly, K.J.; Sasa, I.; Contreras, C.I.; Mendez, M.J.C. Prospective randomized clinical trial of primary molar crowns: 24-month results. Pediatr. Dent. 2018, 40, 253–258. [Google Scholar]
- Mathew, M.G.; Samuel, S.R.; Soni, A.J.; Roopa, K.B. Evaluation of adhesion of Streptococcus mutans, plaque accumulation on zirconia and stainless steel crowns, and surrounding gingival inflammation in primary molars: Randomized controlled trial. Clin. Oral Investig. 2020, 24, 3275–3280. [Google Scholar] [CrossRef]
- EN 13060; Small Steam Sterilizers. Available online: https://standards.iteh.ai/catalog/standards/cen/c427dd54-2927-4833-a160-f6730b5cec2f/en-13060-2014a1-2018?srsltid=AfmBOoqfWSU1pH5dZi1m8ivPxxVW8xUGsm7PekWTdO805w_MbbNdMUHq (accessed on 7 May 2025).
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization. Vitr. Tests BioMed Res. Int. 2019, 2019, 9130806. [Google Scholar] [CrossRef]
- Sztyler, K.; Pajączkowska, M.; Nowicka, J.; Rusak, A.; Chodaczek, G.; Nikodem, A.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M. Evaluation of the microbial, cytotoxic and physico-chemical properties of the stainless steel crowns used in pediatric dentistry. Acta Bioeng. Biomech. 2022, 24, 127–137. [Google Scholar] [CrossRef]
- Reśliński, A.; Mikucka, A.; Szmytkowski, J.; Głowacka, K.; Szczęsny, W.; Gospodarek, E.; Dąbrowiecki, S. Biofilm Detection on the Surface of Hernia Mesh Implants. Adv. Clin. Exp. Med. 2010, 19, 685–690. [Google Scholar]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; Rosas-García, M.L.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J. Microbiol. Immunol. Infect. 2020, 54, 1038–1047. [Google Scholar] [CrossRef]
- El Aouame, A.; El Quars, F.; Bentahar, Z.; Zerouali, K.; Sidqui, M. In Vitro Evaluation of Bacterial Adhesion to Dental and Stainless-Steel Surfaces. Open J. Med. Microbiol. 2021, 11, 176–197. [Google Scholar] [CrossRef]
- Gheorghe, D.C.; Niculescu, A.G.; Bîrcă, A.C.; Grumezescu, A.M. Biomaterials for the Prevention of Oral Candidiasis Development. Pharmaceutics 2021, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Bigos, P.; Czerwińska, R.; Pajączkowska, M.; JNowicka, J. Mixed Oral Biofilm. Postępy Mikrobiol. Adv. Microbiol. 2021, 60, 47–58. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.B. Oral Biofilms. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 643–652. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.; Cury, A.A.D.B.; Jenkinson, H.F.; Nobbs, A.H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol. Oral Microbiol. 2017, 32, 60–73. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Deng, S.; Wang, Y.; Lin, X.; Yang, Z. Adhesive Ability of Different Oral Pathogens to Various Dental Materials: An In Vitro Study. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9595067. [Google Scholar] [CrossRef]
- Huffines, J.T.; Scoffield, J.A. Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci. Rep. 2020, 10, 19661. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Pfeifer, C.S.; Khajotia, S.; Ferracane, J.L. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J. Dent. Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Deng, D.M.; Kraneveld, E.A.; Manders, E.M.M.; Cury, A.A.D.B.; Cate, J.M.T.; Crielaard, W. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 2008, 53, 755–764. [Google Scholar] [CrossRef]
- Diaz, P.I.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: Linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014, 4, 101. [Google Scholar] [CrossRef]
- Salehi, B.; Kregiel, D.; Mahady, G.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Management of Streptococcus mutans-Candida spp. Oral Biofilms’ Infections: Paving the Way for Effective Clinical Interventions. J. Clin. Med. 2020, 9, 517. [Google Scholar] [CrossRef]
- Lin, Y.-T.J.; Chou, C.-C.; Hsu, C.-Y.S. Effects of Lactobacillus Casei Shirota Intake on Caries Risk in Children. J. Dent. Sci. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Schwendicke, F.; Dörfer, C.; Kneist, S.; Meyer-Lueckel, H.; Paris, S. Cariogenic effects of probiotic lactobacillus rhamnosus GG in a dental biofilm model. Caries Res. 2014, 48, 186–192. [Google Scholar] [CrossRef]
- Murali, G.; Mungara, J.; Vijayakumar, P.; Keerthi, T.; Kothimbakkam, S.S.K.; Priya, A.S. Clinical Evaluation of Pediatric Posterior Zirconia and Stainless Steel Crowns: A Comparative Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.I.; Hajwal, S.I.; Ab-Ghani, Z. Bacterial Adhesion on Zirconia, Lithium Desilicated and Gold Crowns-In Vivo Study. Adv. Dent. Oral Health 2016, 1, 555574. [Google Scholar] [CrossRef]
- Gupta, S.; Khanduja, R.; Agrawal, R.; Singhal, M.; Kaushik, M. Clinical Evaluation of Stainless Steel Crown versus Zirconia Crown in Primary Molars: An In Vivo Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.; Sahoo, S.S.; Singh, C.; Mukhopadhyaya, I.; Joshi, A. Effect of pre-fabricated strip, zirconia and stainless steel crowns in primary molars. Bioinformation 2023, 19, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, D.K.R.; H, K.S.; Savitha, S.; Allwin, A.; Nandan, S. Clinical Evaluation of Zirconia and Stainless-Steel crowns in Primary Molars—A Randomized Control Trial: Original Research. Int. J. Pedod. Rehabil. 2022, 7, 30–38. [Google Scholar] [CrossRef]
- Wakwak, M.A.; Bayoumy, S.Y.; Barakat, I.F.; Tawfik, A. Assessment of microbial adhesion to zirconia and stainless-steel crowns in primary molars. Al-Azhar J. Dent. Sci. 2019, 2, 165–169. [Google Scholar]
- Paulindraraj, S.; Ramkumar, H.; Jayakaran, T.G.; Dakshinamurthy, S.; Elizabeth, J.A.; Manoharan, R. Evaluation of Streptococcus mutans Colonization and Oral Hygiene Status in Primary Molars Restored with Two Different Crowns: A Randomized Clinical Trial. Int. J. Clin. Pediatr. Dent. 2023, 16 (Suppl. S2), S183–S189. [Google Scholar] [CrossRef]
- Alaki, S.M.; Abdulhadi, B.S.; AbdElBaki, M.A.; Alamoudi, N.M. Comparing zirconia to anterior strip crowns in primary anterior teeth in children: A randomized clinical trial. BMC Oral Health 2020, 20, 313. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Konieczny, B.; Siarkiewicz, P.; Leniart, A.; Lukomska-Szymanska, M.; Skrzypek, S.; Lapinska, B. Surface Characterization of Current Dental Ceramics Using Scanning Electron Microscopic and Atomic Force Microscopic Techniques. Coatings 2022, 12, 1122. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 08, 571–579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rani, V.; Mittal, S.; Sukhija, U. An In vitro Evaluation to Compare the Surface Roughness of Glazed, Reglazed and Chair Side Polished Surfaces of Dental Porcelain. Contemp. Clin. Dent. 2021, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials-Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).