Lymph Node Involvement Prediction Using Machine Learning: Analysis of Prostatic Nodule, Prostatic Gland, and Periprostatic Adipose Tissue (PPAT)
Abstract
1. Introduction
2. Materials and Methods
- -
- Lymph node positive status (n = 35): at least one lymph node showed metastatic involvement.
- -
- Lymph node negative status (n = 50): all examined lymph nodes were free of metastases.
2.1. Magnetic Resonance Imaging
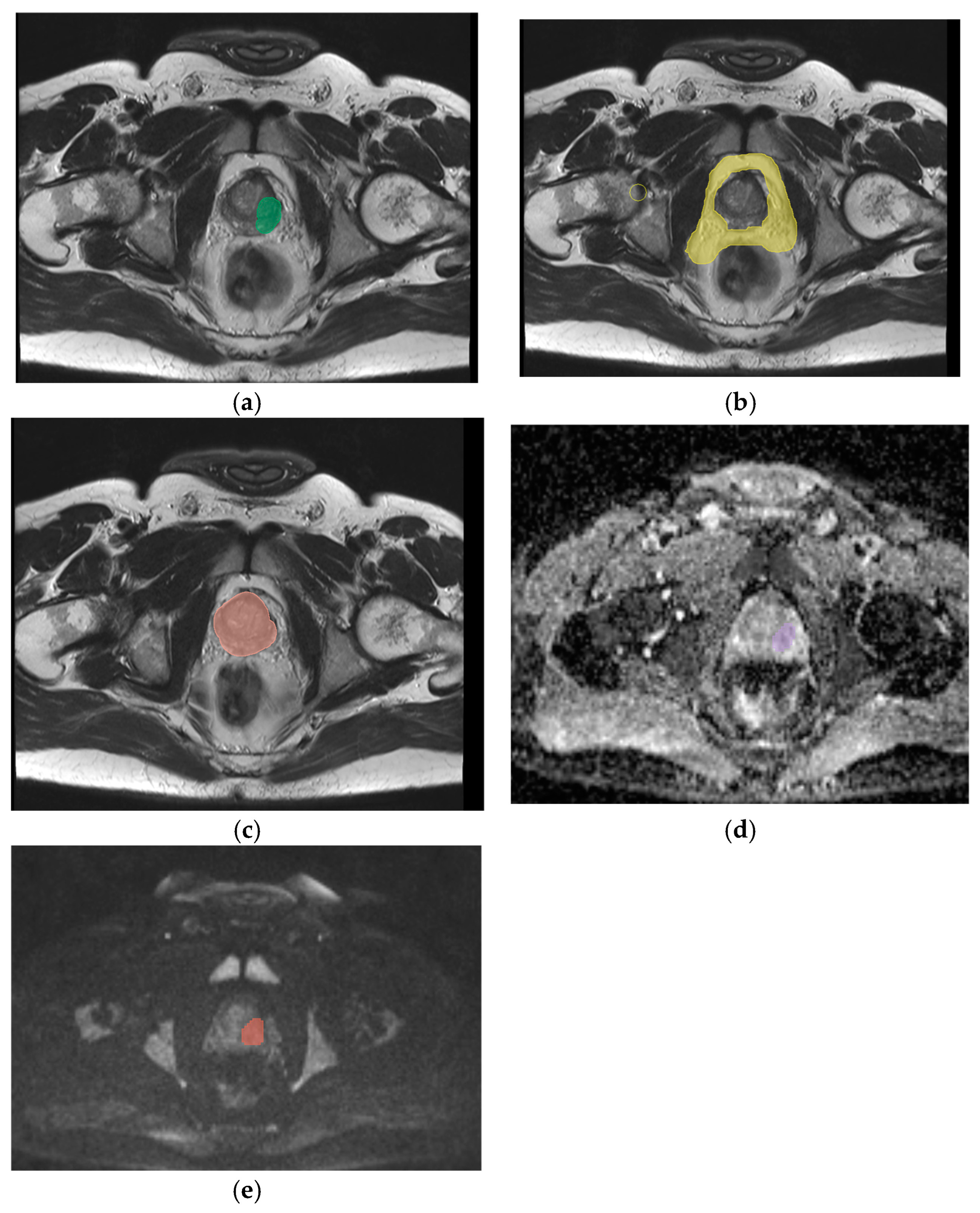
2.2. Segmentation, Feature Extraction and Selection
- ○
- Shape Features: Describing geometric properties of the region of interest (ROI), including surface area, total volume, maximum diameter, elongation, sphericity, and surface-to-volume ratio;
- ○
- First-Order Statistics: Histogram-based features representing the distribution of voxel intensities in the ROI, including metrics such as energy, entropy, mean, interquartile range, skewness, kurtosis, and uniformity;
- ○
- Second-Order Textural Features: Capturing the statistical interrelationships between neighboring voxels. These include:
- -
- Gray-Level Co-Occurrence Matrix (GLCM): Analyzes spatial gray-level intensity distributions within a 3D image;
- -
- Gray-Level Run-Length Matrix (GLRLM): Quantifies contiguous voxels with the same gray-level value in multiple directions;
- -
- Gray-Level Size Zone Matrix (GLSZM): Measures zones of connected voxels with identical gray-level intensity in a 3D space;
- -
- Gray-Tone Difference Matrix (NGTDM): Evaluates differences between voxel intensity and the average intensity of neighboring voxels within a set distance;
- -
- Gray-Level Dependence Matrix (GLDM): Assesses the degree of dependence between neighboring voxels at varying distances.
2.3. Radiomics Analysis and Model Development
3. Results
- -
- DWI nodule features: Accuracy of 67% and AUC of 0.83;
- -
- T2-weighted PPAT features: Accuracy of 78% and AUC of 0.86;
- -
- T2-weighted whole gland features: Accuracy of 78% and AUC of 0.97.
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V.; Davis, B.J.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; Horwitz, E.M.; et al. Prostate Cancer, Version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, V.; Ruzgas, T.; Milonas, D. Mortality Rate Estimation Models for Patients with Prostate Cancer Diagnosis. Balt. J. Mod. Comput. 2022, 10, 170–184. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Calabrese, A.; Santucci, D.; Landi, R.; Zobel, B.B.; Faiella, E.; de Felice, C. Radiomics MRI for lymph node status prediction in breast cancer patients: The state of art. J. Cancer Res. Clin. Oncol. 2021, 147, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gandaglia, G.; Fossati, N.; Suardi, N.; Moschini, M.; Cucchiara, V.; Bianchi, M.; Damiano, R.; Schiavina, R.; Shariat, S.F.; et al. Pelvic Lymph Node Dissection in Prostate Cancer: Indications, Extent and Tailored Approaches. Urol. J. 2017, 84, 9–19. [Google Scholar] [CrossRef]
- Preisser, F.; van den Bergh, R.C.N.; Gandaglia, G.; Ost, P.; Surcel, C.I.; Sooriakumaran, P.; Montorsi, F.; Graefen, M.; van der Poel, H.; de la Taille, A.; et al. Effect of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D’Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-Institutional Study. J. Urol. 2020, 203, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanya, S.V.G.; Shetty, D.K.; Patil, V.; Hameed, B.M.Z.; Paul, R.; Smriti, K.; Naik, N.; Somani, B.K. The role of data science in healthcare advancements: Applications, benefits, and future prospects. Ir. J. Med Sci. 2021, 191, 1473–1483. [Google Scholar] [CrossRef]
- Santucci, D.; Faiella, E.; Cordelli, E.; Sicilia, R.; de Felice, C.; Zobel, B.B.; Iannello, G.; Soda, P. 3T MRI-Radiomic Approach to Predict for Lymph Node Status in Breast Cancer Patients. Cancers 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberini, V.; Laudicella, R.; Balma, M.; Nicolotti, D.G.; Buschiazzo, A.; Grimaldi, S.; Lorenzon, L.; Bianchi, A.; Peano, S.; Bartolotta, T.V.; et al. Radiomics and artificial intelligence in prostate cancer: New tools for molecular hybrid imaging and theragnostics. Eur. Radiol. Exp. 2022, 6, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faiella, E.; Santucci, D.; Calabrese, A.; Russo, F.; Vadalà, G.; Zobel, B.B.; Soda, P.; Iannello, G.; de Felice, C.; Denaro, V. Artificial Intelligence in Bone Metastases: An MRI and CT Imaging Review. Int. J. Environ. Res. Public Health 2022, 19, 1880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santucci, D.; Faiella, E.; Calabrese, A.; Zobel, B.B.; Ascione, A.; Cerbelli, B.; Iannello, G.; Soda, P.; de Felice, C. On the Additional Information Provided by 3T-MRI ADC in Predicting Tumor Cellularity and Microscopic Behavior. Cancers 2021, 13, 5167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, J.; Huan, Y.; Wang, H.; Zhao, H.; Ge, Y.; Chang, Y.; Liu, Y. Diffusion-weighted imaging in normal prostate and differential diagnosis of prostate diseases. Abdom. Imaging 2008, 33, 724–728. [Google Scholar] [CrossRef]
- Iglesias, Á.S.; Macías, V.M.; Peris, A.P.; Fuster-Matanzo, A.; Infante, A.N.; Soria, R.M.; Bataller, F.B.; Pomar, M.D.; Meléndez, C.C.; Huertas, R.Y.; et al. Prostate Region-Wise Imaging Biomarker Profiles for Risk Stratification and Biochemical Recurrence Prediction. Cancers 2023, 15, 4163. [Google Scholar] [CrossRef] [PubMed]
- Vertulli, D.; Santucci, D.; Esperto, F.; Beomonte Zobel, B.; Grasso, R.F.; Faiella, E. Impact of adipose tissue distribution on prostate cancer recurrence after radical prostatectomy. Actas Urol. Esp. 2023, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Vaccarino, F.; Ragone, R.; D’amone, G.; Cirimele, V.; Piccolo, C.L.; Vertulli, D.; Grasso, R.F.; Zobel, B.B.; Santucci, D. Can Machine Learning Models Detect and Predict Lymph Node Involvement in Prostate Cancer? A Comprehensive Systematic Review. J. Clin. Med. 2023, 12, 7032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faiella, E.; Vergantino, E.; Vaccarino, F.; Bruno, A.; Perillo, G.; Grasso, R.F.; Zobel, B.B.; Santucci, D. A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features. Cancers 2024, 16, 1697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, S.; Choyke, P.L.; Eberhardt, S.C.; Oto, A.; Tempany, C.M.; Turkbey, B.; Rosenkrantz, A.B. The Current State of MR Imaging–targeted Biopsy Techniques for Detection of Prostate Cancer. Radiology 2017, 285, 343–356. [Google Scholar] [CrossRef]
- Chen, M.; Dang, H.-D.; Wang, J.-Y.; Zhou, C.; Li, S.-Y.; Wang, W.-C.; Zhao, W.-F.; Yang, Z.-H.; Zhong, C.-Y.; Li, G.-Z. Prostate cancer detection: Comparison of t2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol. 2008, 49, 602–610. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Choi, J.; Gage, K.; Feuerlein, S.; Pow-Sang, J.M.; Gillies, R.; Balagurunathan, Y. Radiological semantics discriminate clinically significant grade prostate cancer. Cancer Imaging 2019, 19, 81. [Google Scholar] [CrossRef]
- Chiacchio, G.; Castellani, D.; Nedbal, C.; De Stefano, V.; Brocca, C.; Tramanzoli, P.; Galosi, A.B.; da Silva, R.D.; Teoh, J.Y.-C.; Tiong, H.Y.; et al. Radiomics vs radiologist in prostate cancer. Results from a systematic review. World J. Urol. 2023, 41, 709–724. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef]
- Cheung, D.C.; Fleshner, N.; Sengupta, S.; Woon, D. A narrative review of pelvic lymph node dissection in prostate cancer. Transl. Androl. Urol. 2020, 9, 3049–3055. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Wu, J.; Zhang, Y.; Wang, X.; Zhang, X.; Wang, X. Utility of diffusion weighted imaging-based radiomics nomogram to predict pelvic lymph nodes metastasis in prostate cancer. BMC Med. Imaging 2022, 22, 190. [Google Scholar] [CrossRef]
- Kufel, J.; Paszkiewicz, I.; Bielówka, M.; Bartnikowska, W.; Janik, M.; Stencel, M.; Czogalik, Ł.; Gruszczyńska, K.; Mielcarska, S. Will ChatGPT pass the Polish specialty exam in radiology and diagnostic imaging? Insights into strengths and limitations. Pol. J. Radiol. 2023, 88, e430–e434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caglic, I.; Barrett, T. Diffusion-weighted imaging (DWI) in lymph node staging for prostate cancer. Transl. Androl. Urol. 2018, 7, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Bedrikovetski, S.; Dudi-Venkata, N.N.; Maicas, G.; Kroon, H.M.; Seow, W.; Carneiro, G.; Moore, J.W.; Sammour, T. Artificial intelligence for the diagnosis of lymph node metastases in patients with abdominopelvic malignancy: A systematic review and meta-analysis. Artif. Intell. Med. 2021, 113, 102022. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; de Cobelli, O.; Musi, G.; del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in prostate cancer: An up-to-date review. Ther. Adv. Urol. 2022, 14, 175628722211090. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Czarniecki, M.; Mehralivand, S.; Stoyanova, R.; Choyke, P.L.; Harmon, S.; Turkbey, B. Radiomics and radiogenomics of prostate cancer. Abdom. Imaging 2019, 44, 2021–2029. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jian, T.; Chi, C.; Liang, Y.; Liang, X.; Yu, Y.; Jiang, F.; Lu, J. Machine Learning-Based Models Enhance the Prediction of Prostate Cancer. Front. Oncol. 2022, 12, 941349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lay, N.S.; Tsehay, Y.; Greer, M.D.; Turkbey, B.; Kwak, J.T.; Choyke, P.L.; Pinto, P.; Wood, B.J.; Summers, R.M. Detection of prostate cancer in multiparametric MRI using random forest with instance weighting. J. Med. Imaging 2017, 4, 024506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, H.; Wang, Y.; Zhang, D.; Liu, B.; Zhou, H.; Wang, S. Periprostatic Adipose Tissue: A New Perspective for Diagnosing and Treating Prostate Cancer. J. Cancer 2024, 15, 204–217. [Google Scholar] [CrossRef]
- Passos, G.R.; Ghezzi, A.C.; Antunes, E.; de Oliveira, M.G.; Mónica, F.Z. The Role of Periprostatic Adipose Tissue on Prostate Function in Vascular-Related Disorders. Front. Pharmacol. 2021, 12, 626155. [Google Scholar] [CrossRef]
- Toren, P.; Venkateswaran, V. Periprostatic Adipose Tissue and Prostate Cancer Progression: New Insights into the Tumor Microenvironment. Clin. Genitourin. Cancer 2014, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sacca, P.A.; Calvo, J.C. Periprostatic Adipose Tissue Microenvironment: Metabolic and Hormonal Pathways During Prostate Cancer Progression. Front. Endocrinol. 2022, 13, 863027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, R.; Monteiro, C.; Catalán, V.; Hu, P.; Cunha, V.; Rodríguez, A.; Gómez-Ambrosi, J.; Fraga, A.; Príncipe, P.; Lobato, C.; et al. Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Med. 2012, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Shahait, M.; Usamentiaga, R.; Tong, Y.; Sandberg, A.; Lee, D.I.; Udupa, J.K.; Torigian, D.A. Periprostatic Adipose Tissue MRI Radiomics-Derived Features Associated with Clinically Significant Prostate Cancer. J. Endourol. 2023, 37, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Karaarslan, E.; Güner, A.L.; Sağlıcan, Y.; Tuna, M.B.; Kural, A.R. Comparing the Diagnostic Performance of Multiparametric Prostate MRI Versus 68Ga-PSMA PET-CT in the Evaluation Lymph Node Involvement and Extraprostatic Extension. Acad. Radiol. 2022, 29, 698–704. [Google Scholar] [CrossRef]
- Saha, A.; Kolonin, M.G.; DiGiovanni, J. Obesity and prostate cancer—Microenvironmental roles of adipose tissue. Nat. Rev. Urol. 2023, 20, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hou, M.; Wang, J.; Song, D.; Niu, Y. Interpretable machine learning model for predicting clinically significant prostate cancer: Integrating intratumoral and peritumoral radiomics with clinical and metabolic features. BMC Med. Imaging 2024, 24, 353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santucci, D.; Ragone, R.; Vergantino, E.; Vaccarino, F.; Esperto, F.; Prata, F.; Scarpa, R.M.; Papalia, R.; Zobel, B.B.; Grasso, F.R.; et al. Comparison between Three Radiomics Models and Clinical Nomograms for Prediction of Lymph Node Involvement in PCa Patients Combining Clinical and Radiomic Features. Cancers 2024, 16, 2731. [Google Scholar] [CrossRef] [PubMed]

| Category | Number |
|---|---|
| Patients with positive LNS | 35 |
| Patients with negative LNS | 50 |
| PSA (ng/mL) (Median, range) | 4.5 |
| Gleason Grade (Median) | 7 |
| Tumor target Zone Peripheral | 33 |
| Tumor target Zone Transition | 12 |
| Disease Grade (TNM) | T3 (16) |
| T2 (10) | |
| T3.5 (19) |
| Seq | Accuracy | AUC | Precision | Recall | F1-Score | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|
| ADC | 78% | 0.722 | 1.000 | 0.333 | 0.500 | 100 | 33 |
| DWI | 67% | 0.83 | 0.50 | 0.33 | 0.40 | 100 | 33 |
| T2 nod | 78% | 0.78 | 0.50 | 0.33 | 0.40 | 100 | 33 |
| all nodule seq | 88 | 50 | 87 | 1 | 93 | 100 | 50 |
| T2 PPAT | 78% | 0.86 | 1.000 | 0.33 | 0.50 | 100 | 33 |
| T2 gland | 78% | 0.972 | 1.000 | 0.333 | 0.500 | 100 | 33 |
| ALL mask | 88.89% | 1 | 1.000 | 0.67 | 0.80 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiella, E.; D’amone, G.; Ragone, R.; Pileri, M.; Vergantino, E.; Zobel, B.B.; Grasso, R.F.; Santucci, D. Lymph Node Involvement Prediction Using Machine Learning: Analysis of Prostatic Nodule, Prostatic Gland, and Periprostatic Adipose Tissue (PPAT). Appl. Sci. 2025, 15, 5426. https://doi.org/10.3390/app15105426
Faiella E, D’amone G, Ragone R, Pileri M, Vergantino E, Zobel BB, Grasso RF, Santucci D. Lymph Node Involvement Prediction Using Machine Learning: Analysis of Prostatic Nodule, Prostatic Gland, and Periprostatic Adipose Tissue (PPAT). Applied Sciences. 2025; 15(10):5426. https://doi.org/10.3390/app15105426
Chicago/Turabian StyleFaiella, Eliodoro, Giulia D’amone, Raffaele Ragone, Matteo Pileri, Elva Vergantino, Bruno Beomonte Zobel, Rosario Francesco Grasso, and Domiziana Santucci. 2025. "Lymph Node Involvement Prediction Using Machine Learning: Analysis of Prostatic Nodule, Prostatic Gland, and Periprostatic Adipose Tissue (PPAT)" Applied Sciences 15, no. 10: 5426. https://doi.org/10.3390/app15105426
APA StyleFaiella, E., D’amone, G., Ragone, R., Pileri, M., Vergantino, E., Zobel, B. B., Grasso, R. F., & Santucci, D. (2025). Lymph Node Involvement Prediction Using Machine Learning: Analysis of Prostatic Nodule, Prostatic Gland, and Periprostatic Adipose Tissue (PPAT). Applied Sciences, 15(10), 5426. https://doi.org/10.3390/app15105426








