Nanoformulations Loaded with Phytochemicals for Combating Wound Infections and Promoting Wound Healing: Current Applications and Innovations
Abstract
1. Introduction
2. Phytochemicals with Wound Healing and Antimicrobial Efficacy
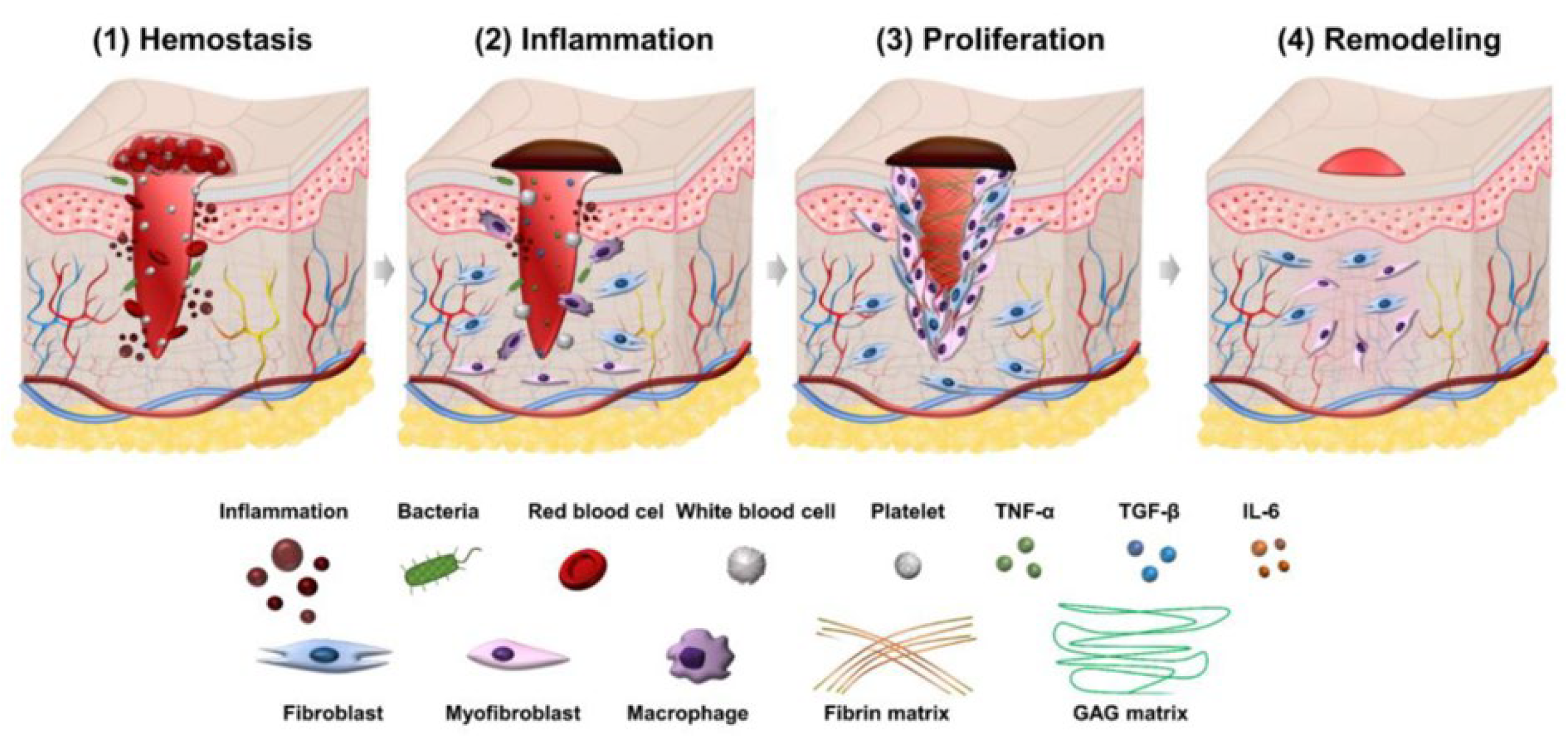
3. Wound Healing Phases
4. Factors Affecting Wound Healing Process
4.1. Local Factors Influencing Wound Healing
4.1.1. Oxygenation
4.1.2. Infections
4.2. Systemic Factors That Influence Healing
4.2.1. Age
4.2.2. Sex Hormones
4.2.3. Stress
4.2.4. Diabetes and Other Autoimmune Diseases
4.2.5. Obesity
4.3. Medications
4.3.1. Glucocorticoid Steroids
4.3.2. Non-Steroidal Anti-Inflammatory Drugs
4.3.3. Alcohol Consumption
4.3.4. Smoking
4.3.5. Nutrition
5. Nanoformulations for Wound Healing
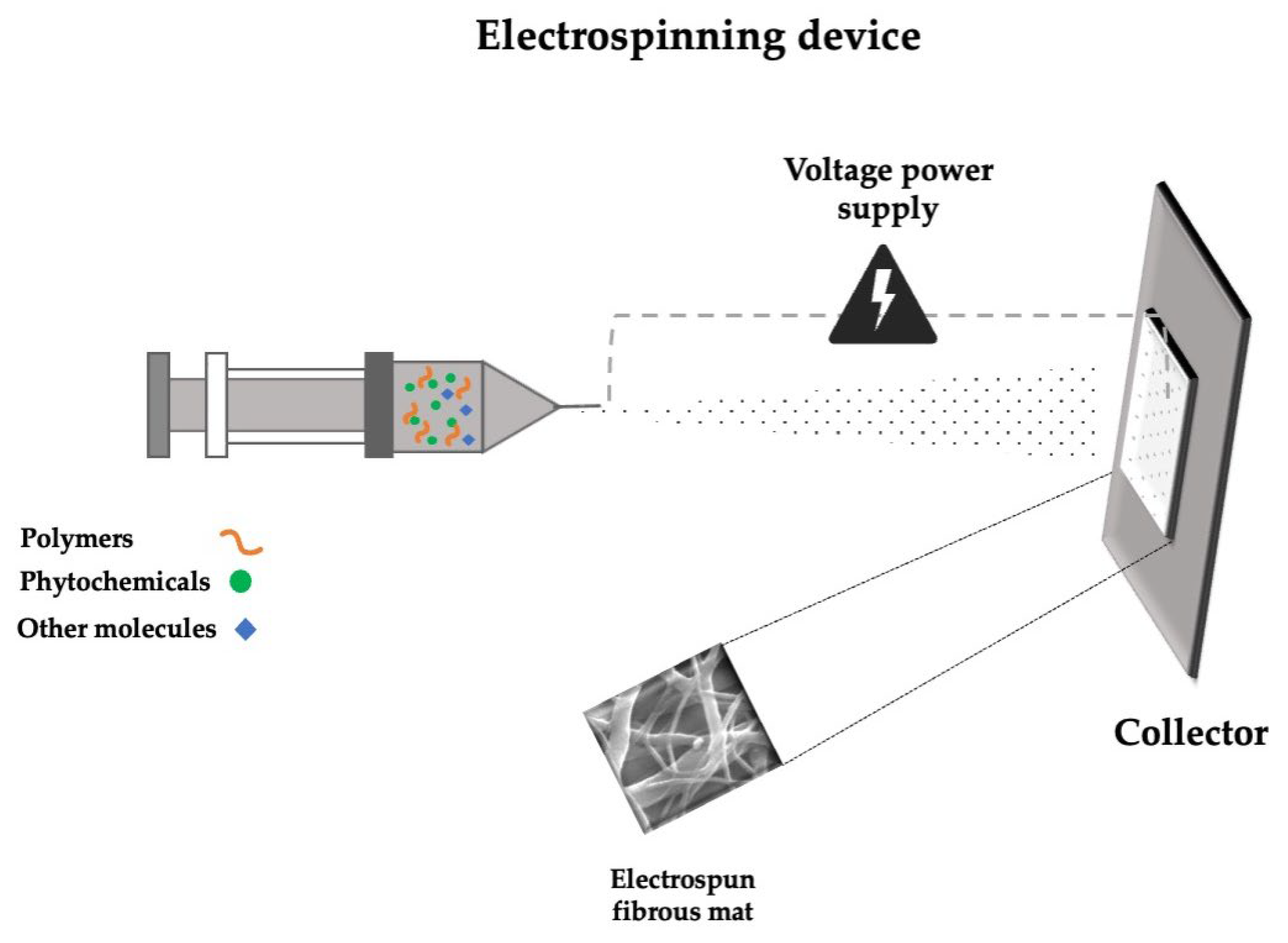
5.1. Electrospun Nanofibers Loaded with Phytochemicals
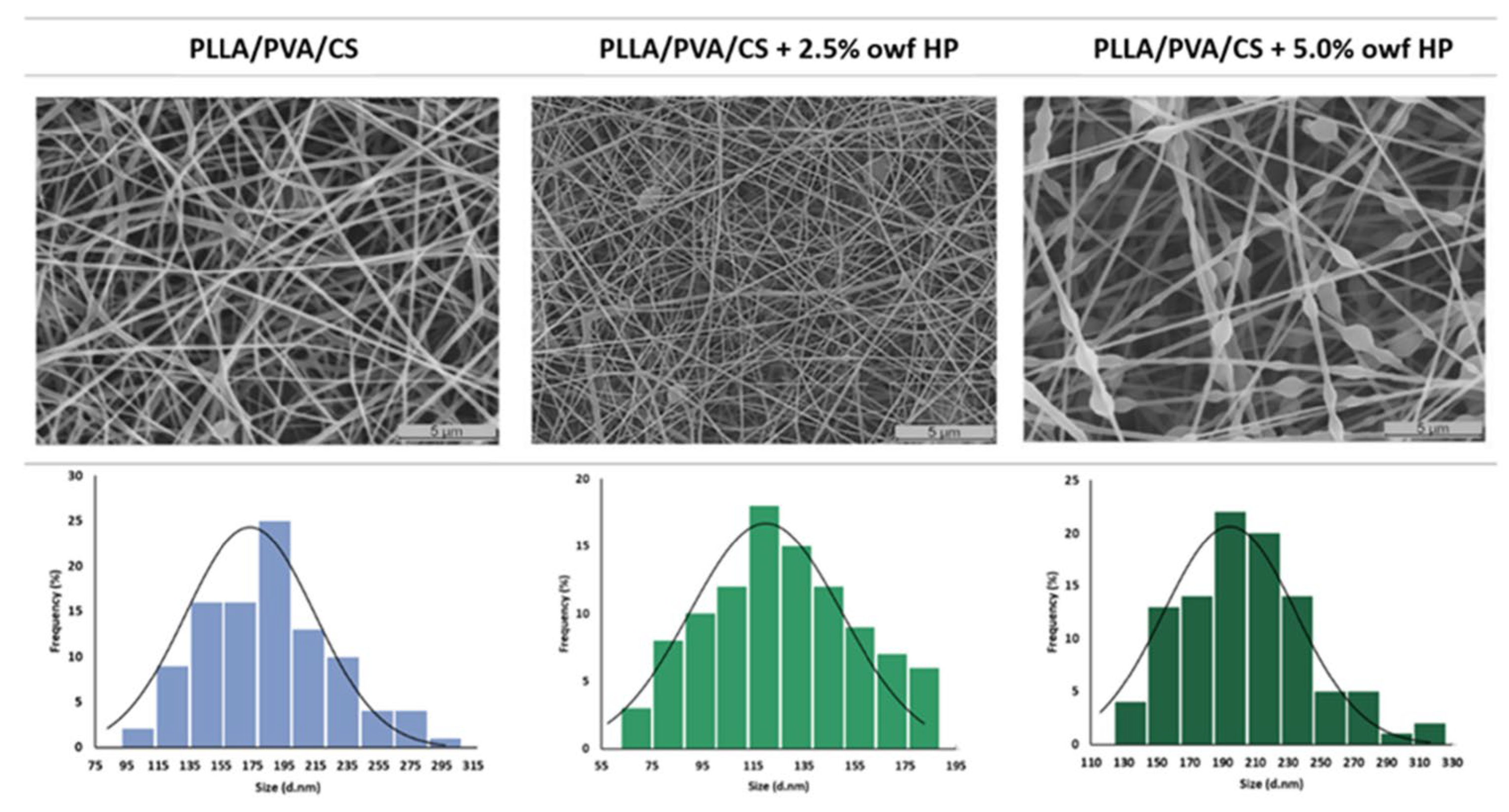

| Polymers Used for the Wound Dressings | Phytochemical/Plant/Extract | In Vivo Models | Ref. |
|---|---|---|---|
| PLLA/PVA/CS | Hypericum perforatum L. | No | [76] |
| PVA | Flaxseed extract (Linum usitatissimum) | No | [14] |
| Chitosan (CS) | Baicalein (Scutellaria baicalensis Georgi) | No | [85] |
| PVA/CS | Royal Jelly | No | [86] |
| PVA/CS | Thymus vulgaris, Zingiber officinale | Yes | [87] |
| CS/PVA-PCL bilayer | Pomegranate flower extract | Yes | [88] |
| PVA/Alginic Acid (ALG) | Lawsonia inermis, Scrophularia striata | Yes | [102] |
| PVA/DA-functionalized Alginate (Alg-DA) | Chlorogenic acid | Yes | [89] |
| PCL/PVA-CS | Tridax procumbens L. extract | Yes | [90] |
| PCL-Silk Sericin/CS-Alginate Hydrogel | 10-Hydroxydecanoic acid (10-HDA) | Yes | [91] |
| Cellulose (Cel)/Pectin (Pec)/Soy Protein Isolate (SPI) | Pomegranate peel extract | Yes | [92] |
| PCL/Collagen | Malva sylvestris extract | Yes | [93] |
| PCL | Curcumin (Curcuma longa), Berberine chloride | Yes | [94] |
| PCL-CS | Oryzanol (Oryza sativa) | Yes | [95] |
| PCL/Cellulose Acetate (CA) | Rhamnus prinoides leaf extract | Yes | [96] |
| PCL/Collagen | Propolis extract | Yes | [97] |
| Pectin Polysaccharides (PP)/CS/PVA | Dihydromyricetin (Hovenia dulcis, Ampelopsis grossedentata) | Yes | [98] |
| Sodium Alginate (SA)/Gelatin (Gel) with PAN top layer | Matricaria chamomilla L. extract, silver sulfadiazine (AgSD) | Yes | [99] |
| PLGA/PMMA/Collagen/Glycine | Syzygium cumini leaf extract | Yes | [100] |
| PLGA | Propolis extract | Yes | [101] |
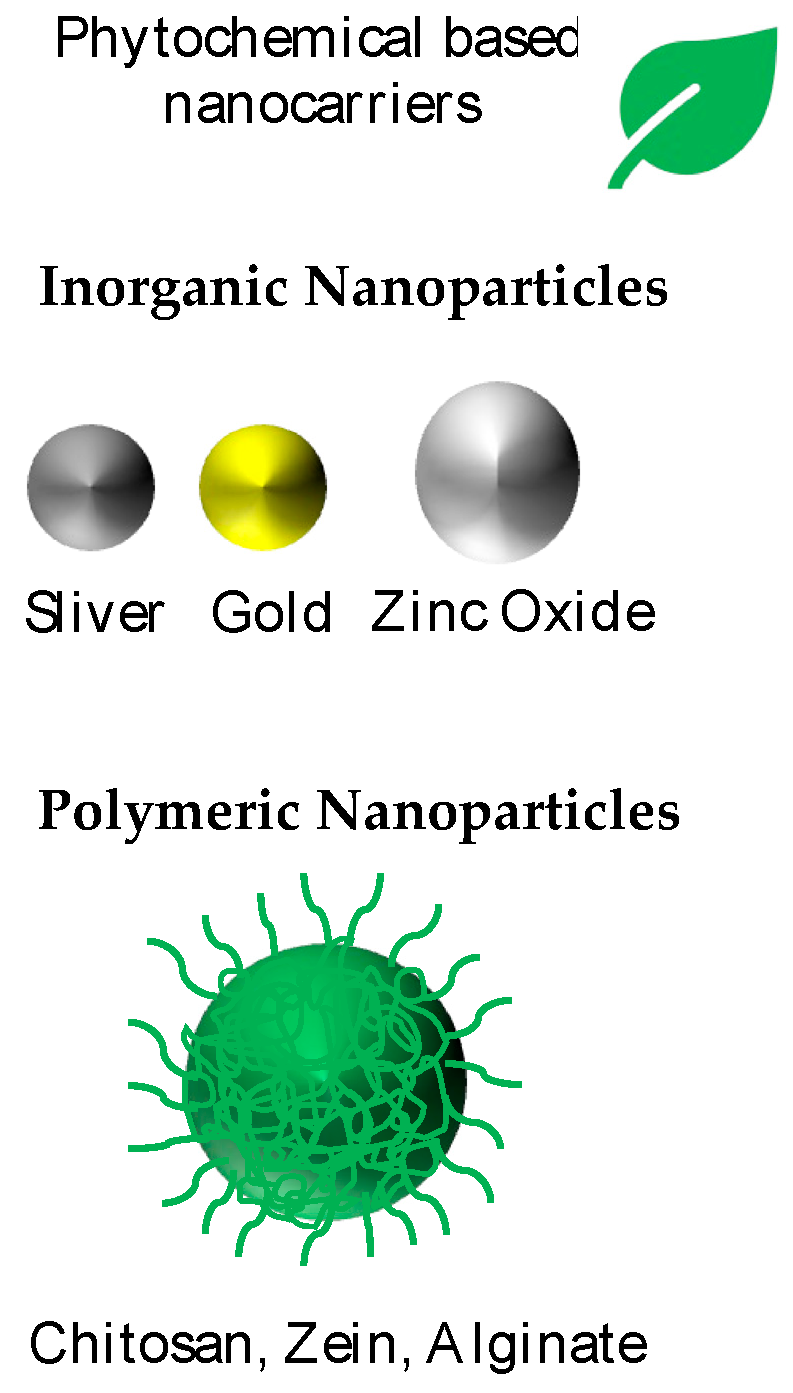
5.2. Nanoparticles Loaded with Phytochemicals
5.2.1. Inorganic Nanocarriers
5.2.2. Polymeric Nanocarriers
5.2.3. Other Nanoformulations Loaded with Phytochemicals and Extracts
| Type of Nanocarriers | Materials | Phytochemical/Plant/Extract | Animal Models | Ref. |
|---|---|---|---|---|
| Nanoparticles | Aptamer functionalized mesoporous silica-coated UCNPs | Curcumin (Cur) | Yes | [103] |
| Nanoparticles | Hydrogel with zinc oxide | Gliricidia sepium | Yes | [104] |
| Nanoparticles | Chitosan-nano zinc oxide | Sericin | Yes | [105] |
| Nanoparticles | Bioreduced silver nanoparticles | Juglans regia L. pellicle extract | Yes | [106] |
| Nanoparticles/scaffolds | Silver nanoparticles (AgNPs) in chitosan/alginic acid scaffolds | Acer tegmentosum extract | Yes | [107] |
| Nanoparticles | Hordein/chitosan nanoparticles | Quercetin | Yes | [108] |
| Nanoparticles | Zein nanoparticles | Moringa oleifera | Yes | [109] |
| Carbon quantum dots | Zein-based electrospun membrane | Thea sinensis | N/A | [110] |
| Nanocomposite | Chitosan and alginate hydrogel with nanoemulsions | Urtica dioica | N/A | [111] |
| Nanogels | Chitosan based nanogels | Perovskia abrotanoides | Yes | [112] |
| Nanoparticles | Silver-modified chitosan nanoparticles | Lupeol | Yes | [113] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okur, M.E.; Bülbül, E.Ö.; Mutlu, G.; Eleftherıadou, K.; Karantas, I.D.; Okur, N.Ü.; Siafaka, P.I. An Updated Review for the Diabetic Wound Healing Systems. Curr. Drug Targets 2021, 23, 393–419. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements Affecting Wound Healing Time: An Evidence Based Analysis. Wound Repair Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, F.; James, A.; Khan, S.; Haider, S.; Ullah, S.; Darwish, G.; Taqvi, S.A.H.R.; Ali, R.; Younas, Q.; Rehman, A. Multidrug-Resistant Pathogens in Wound Infections: A Systematic Review. Cureus 2024, 16, e58760. [Google Scholar] [CrossRef]
- Fajardo, J.B.; Vianna, M.H.; Polo, A.B.; Cordeiro Comitre, M.R.; de Oliveira, D.A.; Ferreira, T.G.; de Oliveira Lemos, A.S.; Souza, T.d.F.; Campos, L.M.; de Lima Paula, P.; et al. Insights into the Bioactive Potential of the Amazonian Species Acmella Oleracea Leaves Extract: A Focus on Wound Healing Applications. J. Ethnopharmacol. 2025, 337, 118866. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The Therapeutic Wound Healing Bioactivities of Various Medicinal Plants. Life 2023, 13, 317. [Google Scholar] [CrossRef]
- Khumaidi, A.; Murwanti, R.; Damayanti, E.; Hertiani, T. Empirical Use, Phytochemical, and Pharmacological Effects in Wound Healing Activities of Compounds in Diospyros Leaves: A Review of Traditional Medicine for Potential New Plant-Derived Drugs. J. Ethnopharmacol. 2025, 337, 118966. [Google Scholar] [CrossRef]
- Zain, M.; Nayab, S.; Rashid, Z.; Aleem, A.; Raza, H.; Yousif, M.D. Biosynthesis and in Vivo Wound Healing Abilities of Dactyloctenium Aegyptium-Mediated Silver Nanoparticles Used as Hydrogel Dressing. Process Biochem. 2024, 147, 31–38. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of Phytochemicals and Its Enhancement by Drug Delivery Systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Teli, D.; Satasia, R.; Patel, V.; Nair, R.; Khatri, R.; Gala, D.; Balar, P.C.; Patel, K.; Sharma, A.; Vadodariya, P.; et al. Nature Meets Technology: Harnessing Nanotechnology to Unleash the Power of Phytochemicals. Clin. Tradit. Med. Pharmacol. 2024, 5, 200139. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Majeed, K.R.; Altemimi, A.B.; Hassan, A.M.; Qadir, S.A.; Saeed, M.N.; Faraj, A.M.; Salih, T.H.; Abd Al-Manhel, A.J.; Najm, M.A.A.; et al. Nanotechnology-Based Herbal Medicine: Preparation, Synthesis, and Applications in Food and Medicine. J. Agric. Food Res. 2025, 19, 101661. [Google Scholar] [CrossRef]
- Khan, F.; Mondal, B.; Bairagi, B.; Mandal, S.; Mandal, D.; Nath, D. Fabrication of Chitosan/PEO/Rosmarinic Acid Based Nanofibrous Mat for Diabetic Burn Wound Healing and Its Anti-Bacterial Efficacy in Mice. Int. J. Biol. Macromol. 2025, 301, 140416. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.G.; Nageh, H.; Abdalla, M.S.; Abdo, S.M.; Amer, A.A.; Loutfy, S.A.; Alsalme, A.; Cornu, D.; Bechelany, M.; Barhoum, A. Enhanced Wound Healing with Flaxseed Extract-Loaded Polyvinyl Alcohol Nanofibrous Scaffolds: Phytochemical Composition, Antioxidant Activity, and Antimicrobial Properties. J. Sci. Adv. Mater. Devices 2025, 10, 100862. [Google Scholar] [CrossRef]
- Zare, I.; Zahed Nasab, S.; Rahi, A.; Ghaee, A.; Koohkhezri, M.; Ramezani Farani, M.; Madadi Gholipour, H.; Atabaki, A.H.; Hamblin, M.R.; Mostafavi, E.; et al. Antimicrobial Carbon Materials-Based Quantum Dots: From Synthesis Strategies to Antibacterial Properties for Diagnostic and Therapeutic Applications in Wound Healing. Coord. Chem. Rev. 2025, 522, 216211. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Jeya Rajkumar, R.S.; Nadar MSA, M.; Mosae Selvakumar, P. Phytochemicals as a Potential Source for Anti-Microbial, Anti-Oxidant and Wound Healing—A Review. MOJ Biorg. Org. Chem. 2018, 2, 61–70. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in Wound Healing. Adv. Wound Care 2016, 5, 230–241. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeni, S.A.; Negm, W.A. The Wound Healing Effect of Botanicals and Pure Natural Substances Used in in Vivo Models. Inflammopharmacology 2023, 31, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.T.; Al-Mahdy, D.A.; El Fishawy, A.M.; Salama, A.; Al-Karmalawy, A.A.; Otify, A.M. Phytochemical Investigation, Role in Wound Healing Process and Cytotoxicity of Sphagneticola Trilobata: In Vitro, in Vivo and in Silico Approach. J. Ethnopharmacol. 2025, 342, 119394. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Raja, K.; Vasanth, K.; Khusro, A.; Sadhasivam, S.; Sahibzada, M.U.K.; Gawwad, M.R.A.; Al Farraj, D.A.; Elshikh, M.S. Phytochemical Screening and in Vitro Antibacterial, Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Wound Healing Attributes of Senna Auriculata (L.) Roxb. Leaves. Arab. J. Chem. 2021, 14, 103345. [Google Scholar] [CrossRef]
- Anwar, M.A.; El Gedaily, R.A.; Salama, A.; Aboulthana, W.M.; Kandil, Z.A.; Abdel-dayem, S.I.A. Phytochemical Analysis and Wound Healing Properties of Malva Parviflora L. Ethanolic Extract. J. Ethnopharmacol. 2025, 337, 118983. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-Based Therapy for Wound Healing. Nanomaterials 2022, 12, 618. [Google Scholar] [CrossRef]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Özcan Bülbül, E.; Okur, M.E.; Üstündağ Okur, N.; Siafaka, P.I. Traditional and Advanced Wound Dressings: Physical Characterization and Desirable Properties for Wound Healing. In Natural Polymers in Wound Healing and Repair; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–50. [Google Scholar]
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized Tissues for Wound Healing: Towards Closing the Gap Between Scaffold Design and Effective Extracellular Matrix Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef]
- Talebi, M.; Ghale, R.A.; Asl, R.M.; Tabandeh, F. Advancements in Characterization and Preclinical Applications of Hyaluronic Acid-Based Biomaterials for Wound Healing: A Review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100706. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-Epithelialization of Adult Skin Wounds: Cellular Mechanisms and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Kanwal, S.; Sivaramakrishnan, R.; Katelakha, K. Therapeutic Potential of Microalgae-Derived Natural Compounds in Diabetic Wound Healing: A Comprehensive Review. Heliyon 2025, 11, e42723. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous Dressings of Modified Chitosan with Poly(2-Hydroxyethyl Acrylate) for Topical Wound Delivery of Levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Tandara, A.A.; Mustoe, T.A. Oxygen in Wound Healing—More than a Nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef]
- Sen, C.K. Wound Healing Essentials: Let There Be Oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and Molecular Roles of Reactive Oxygen Species in Wound Healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental Biofilms: Difficult Therapeutic Targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and Wound Healing: From Bench to Bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE Pathogens in the Environment: Antibiotic Resistance Status, Community-Acquired Infection and Risk to Human Health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Maitz, J.; Merlino, J.; Rizzo, S.; McKew, G.; Maitz, P. Burn Wound Infections Microbiome and Novel Approaches Using Therapeutic Microorganisms in Burn Wound Infection Control. Adv. Drug Deliv. Rev. 2023, 196, 114769. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. N. Am. 2020, 100, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, M.A.; Hadi, H. Aging and Wound Healing of the Skin: A Review of Clinical and Pathophysiological Hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex Hormones and Mucosal Wound Healing. Brain Behav. Immun. 2009, 23, 629–635. [Google Scholar] [CrossRef]
- Hardman, M.J.; Ashcroft, G.S. Estrogen, Not Intrinsic Aging, Is the Major Regulator of Delayed Human Wound Healing in the Elderly. Genome Biol. 2008, 9, R80. [Google Scholar] [CrossRef]
- Christian, L.M.; Graham, J.E.; Padgett, D.A.; Glaser, R.; Kiecolt-Glaser, J.K. Stress and Wound Healing. Neuroimmunomodulation 2006, 13, 337–346. [Google Scholar] [CrossRef]
- Ebrecht, M.; Hextall, J.; Kirtley, L.-G.; Taylor, A.; Dyson, M.; Weinman, J. Perceived Stress and Cortisol Levels Predict Speed of Wound Healing in Healthy Male Adults. Psychoneuroendocrinology 2004, 29, 798–809. [Google Scholar] [CrossRef]
- Gouin, J.-P.; Kiecolt-Glaser, J.K. The Impact of Psychological Stress on Wound Healing: Methods and Mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81–93. [Google Scholar] [CrossRef]
- Hosnyak, R.; Thornton, D. The Impact of Occupation on Wound Healing: A Literature Review on the Holistic Approach of Wound Management in High-Risk Patient Groups. Wounds UK 2017, 3, 40–47. [Google Scholar]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic Foot Ulcers. J. Am. Acad. Dermatol. 2014, 70, 1.e1–1.e18. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Kirloskar, K.M.; Dekker, P.K.; Kiene, J.; Zhou, S.; Bekeny, J.C.; Rogers, A.; Zolper, E.G.; Fan, K.L.; Evans, K.K.; Benedict, C.D.; et al. The Relationship Between Autoimmune Disease and Disease-Modifying Antirheumatic Drugs on Wound Healing. Adv. Wound Care 2022, 11, 650–656. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-Derived Targeted Therapy for Wound Healing: Beyond Classical Strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef]
- Cotterell, A.; Griffin, M.; Downer, M.A.; Parker, J.B.; Wan, D.; Longaker, M.T. Understanding Wound Healing in Obesity. World J. Exp. Med. 2024, 14, 86898. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Schacke, H. Mechanisms Involved in the Side Effects of Glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef]
- Hofman, D.; Moore, K.; Cooper, R.; Eagle, M.; Cooper, S. Use of Topical Corticosteroids on Chronic Leg Ulcers. J. Wound Care 2007, 16, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Koga, T.; Ohba, M.; Okuno, T.; Koike, M.; Murakami, A.; Matsuda, A.; Yokomizo, T. Non-Steroidal Anti-Inflammatory Drug Delays Corneal Wound Healing by Reducing Production of 12-Hydroxyheptadecatrienoic Acid, a Ligand for Leukotriene B4 Receptor 2. Sci. Rep. 2017, 7, 13267. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological Control of Inflammation in Wound Healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef]
- Silva, M.L.S.; de J. Inês, E.; da S. Souza, A.B.; dos S. Dias, V.M.; Guimaraes, C.M.; Menezes, E.R.; Barbosa, L.G.; Del Carmen M. Alves, M.; Teixeira, M.C.A.; Soares, N.M. Association between Strongyloides Stercoralis Infection and Cortisol Secretion in Alcoholic Patients. Acta Trop. 2016, 154, 133–138. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P. A Recent Perspective on Alcohol, Immunity, and Host Defense. Alcohol. Clin. Exp. Res. 2009, 33, 220–232. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; da Matta, S.L.P.; Gonçalves, R.V. Effect of a High-Fat Diet and Alcohol on Cutaneous Repair: A Systematic Review of Murine Experimental Models. PLoS ONE 2017, 12, e0176240. [Google Scholar] [CrossRef]
- Lipa, K.; Zając, N.; Owczarek, W.; Ciechanowicz, P.; Szymańska, E.; Walecka, I. Does Smoking Affect Your Skin? Adv. Dermatol. Allergol. 2021, 38, 371–376. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Browning, K.K. Smoking, Chronic Wound Healing, and Implications for Evidence-Based Practice. J. Wound Ostomy Cont. Nurs. 2014, 41, 415–423. [Google Scholar] [CrossRef]
- Sadler, R.A.; Shoveller, A.K.; Shandilya, U.K.; Charchoglyan, A.; Wagter-Lesperance, L.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. Beyond the Coagulation Cascade: Vitamin K and Its Multifaceted Impact on Human and Domesticated Animal Health. Curr. Issues Mol. Biol. 2024, 46, 7001–7031. [Google Scholar] [CrossRef]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Hobson, R. Vitamin E and Wound Healing: An Evidence-based Review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef]
- Ju, M.; Kim, Y.; Seo, K.W. Role of Nutrition in Wound Healing and Nutritional Recommendations for Promotion of Wound Healing: A Narrative Review. Ann. Clin. Nutr. Metab. 2023, 15, 67–71. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Xu, C.; Zhang, Q.; Ren, H.; Huang, X.; He, C.; Ma, J.; Wang, Z. Metabolic Reprogramming in Skin Wound Healing. Burns Trauma 2024, 12, tkad047. [Google Scholar] [CrossRef]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Emulsion Electrospinning of PLLA/PVA/Chitosan with Hypericum Perforatum L. as an Antibacterial Nanofibrous Wound Dressing. Gels 2023, 9, 353. [Google Scholar] [CrossRef]
- He, F.-L.; Deng, X.; Zhou, Y.-Q.; Zhang, T.-D.; Liu, Y.-L.; Ye, Y.-J.; Yin, D.-C. Controlled Release of Antibiotics from Poly-ε-Caprolactone/Polyethylene Glycol Wound Dressing Fabricated by Direct-Writing Melt Electrospinning. Polym. Adv. Technol. 2019, 30, 425–434. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Qian, D.; Chen, L.; Mo, X.; Wang, L.; Wang, Y.; Cui, W. Electrospun Fibrous Sponge via Short Fiber for Mimicking 3D ECM. J. Nanobiotechnol. 2021, 19, 131. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Cheng, Y.; Chang, Y.; Ko, Y.; Liu, C.J. Sustained Release of Levofloxacin from Thermosensitive Chitosan-based Hydrogel for the Treatment of Postoperative Endophthalmitis. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 8–13. [Google Scholar] [CrossRef]
- Ay Şenyiğit, Z.; Coşkunmeriç, N.; Çağlar, E.Ş.; Öztürk, İ.; Atlıhan Gündoğdu, E.; Siafaka, P.I.; Üstündağ Okur, N. Chitosan-Bovine Serum Albumin-Carbopol 940 Nanogels for Mupirocin Dermal Delivery: Ex-Vivo Permeation and Evaluation of Cellular Binding Capacity via Radiolabeling. Pharm. Dev. Technol. 2021, 26, 852–866. [Google Scholar] [CrossRef]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.-A.; Sarmento, B.; Hirvonen, J.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual Chitosan/Albumin-Coated Alginate/Dextran Sulfate Nanoparticles for Enhanced Oral Delivery of Insulin. J. Control. Release 2016, 232, 29–41. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun Chitosan/Polycaprolactone-Hyaluronic Acid Bilayered Scaffold for Potential Wound Healing Applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Brianezi, S.F.S.; Castro, K.C.; Piazza, R.D.; Melo, M.d.S.F.; Pereira, R.M.; Marques, R.F.C.; Campos, M.G.N. Preparation and Characterization of Chitosan/MPEG-PCL Blended Membranes for Wound Dressing and Controlled Gentamicin Release. Mater. Res. 2018, 21, e20170951. [Google Scholar] [CrossRef]
- Song, Y.; Han, N.; Guo, Z.; Li, H.; Guo, M.; Dou, M.; Ye, J.; Peng, Z.; Lu, X.; Li, M.; et al. Baicalein-Modified Chitosan Nanofiber Membranes with Antioxidant and Antibacterial Activities for Chronic Wound Healing. Int. J. Biol. Macromol. 2024, 279, 134902. [Google Scholar] [CrossRef]
- Yu, H.; Chen, D.; Lu, W.; Zhang, C.; Wang, H.; Peng, Z.; Jiang, H.; Xiao, C. Characterization of Polyvinyl Alcohol/Chitosan Nanofibers Loaded with Royal Jelly by Blending Electrospinning for Potential Wound Dressings. Int. J. Biol. Macromol. 2025, 307, 141977. [Google Scholar] [CrossRef]
- Maleki, H.; Doostan, M.; Khoshnevisan, K.; Baharifar, H.; Maleki, S.A.; Fatahi, M.A. Zingiber Officinale and Thymus Vulgaris Extracts Co-Loaded Polyvinyl Alcohol and Chitosan Electrospun Nanofibers for Tackling Infection and Wound Healing Promotion. Heliyon 2024, 10, e23719. [Google Scholar] [CrossRef]
- Karamat-Iradmousa, M.; Karimi, H.; Mahboubi, A.; Rabbani, S.; Kamalinejad, M.; Haeri, A. Bi-Layered Nanofibers Loaded with Pomegranate Flowers Extract as a Novel Wound Dressing: Fabrication, Characterization, and in Vivo Healing Promotion. Ind. Crop. Prod. 2023, 202, 117042. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Yin, X.; Xue, M.; Zhao, X.; Zheng, R.; Qiu, J.; Zhu, Z. Chlorogenic Acid-Assisted Dopamine-sodium Alginate Composite Nanofiber Membranes for Promoting Wound Healing. Carbohydr. Polym. 2025, 354, 123298. [Google Scholar] [CrossRef]
- Cheriyan, S.; Shin, H.; Razack, S.A.; Kang, M.; Boopathi, T.S.; Kang, H.W.; Mani, K. In-Vitro and in-Vivo Studies of Tridax Procumbens Leaf Extract Incorporated Bilayer Polycaprolactone/Polyvinyl Alcohol-Chitosan Electrospun Nanofiber for Wound Dressing Application. Int. J. Biol. Macromol. 2025, 299, 139920. [Google Scholar] [CrossRef]
- Nazemoroaia, M.; Bagheri, F.; Mirahmadi-Zare, S.Z.; Eslami-kaliji, F.; Derakhshan, A. Asymmetric Natural Wound Dressing Based on Porous Chitosan-Alginate Hydrogel/Electrospun PCL-Silk Sericin Loaded by 10-HDA for Skin Wound Healing: In Vitro and in Vivo Studies. Int. J. Pharm. 2025, 668, 124976. [Google Scholar] [CrossRef]
- Mirhaj, M.; Varshosaz, J.; Nasab, P.M.; Al-Musawi, M.H.; Almajidi, Y.Q.; Shahriari-Khalaji, M.; Tavakoli, M.; Alizadeh, M.; Sharifianjazi, F.; Mehrjoo, M.; et al. A Double-Layer Cellulose/Pectin-Soy Protein Isolate-Pomegranate Peel Extract Micro/Nanofiber Dressing for Acceleration of Wound Healing. Int. J. Biol. Macromol. 2024, 255, 128198. [Google Scholar] [CrossRef]
- Hajati Ziabari, A.; Ebrahimi, S.; Jafari, K.; Doodmani, S.M.; Natouri, O.; Nobakht, A.; Mouseli, S. Bilayer Nanofibers Loaded with Malva Sylvestris Extract for Enhanced Wound Healing Applications. J. Drug Deliv. Sci. Technol. 2024, 93, 105373. [Google Scholar] [CrossRef]
- Kandaswamy, K.; Prasad Panda, S.; Subramanian, R.; Khan, H.; Rafi Shaik, M.; Althaf Hussain, S.; Guru, A.; Arockiaraj, J. Synergistic Berberine Chloride and Curcumin-Loaded Nanofiber Therapies against Methicillin-Resistant Staphylococcus Aureus Infection: Augmented Immune and Inflammatory Responses in Zebrafish Wound Healing. Int. Immunopharmacol. 2024, 140, 112856. [Google Scholar] [CrossRef]
- Li, W.; He, J.; Chen, Q.; Bao, F.; Huo, Y.; Deng, J.; Lin, Q.; Luo, F. Enhancement of Oryzanol Application by Constructing Modified β-CD Inclusion Complex and Polycaprolactone-Chitosan Electrospun Fiber Membranes: Perspectives on Wound Dressings and Grape Preservation. Food Chem. 2025, 473, 143025. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Xiangnan, Y.; Tan, S.; Zhao, H.; Jhatial, A.K. Rhamnus Prinoides Leaf Extract Loaded Polycaprolactone-Cellulose Acetate Nanofibrous Scaffold as Potential Wound Dressing: An in Vitro Study. Int. J. Biol. Macromol. 2024, 279, 134934. [Google Scholar] [CrossRef]
- Doodmani, S.M.; Bagheri, A.; Natouri, O.; Nobakht, A.; Saghebasl, S. Electrospinning-Netting of Spider-Inspired Polycaprolactone/Collagen Nanofiber-Nets Incorporated with Propolis Extract for Enhanced Wound Healing Applications. Int. J. Biol. Macromol. 2024, 267, 131452. [Google Scholar] [CrossRef]
- Guo, H.; Ran, W.; Jin, X.; Huang, Y.; Long, F.; Xiao, Y.; Gan, R.-Y.; Wu, Y.; Gao, H. Development of Pectin/Chitosan-Based Electrospun Biomimetic Nanofiber Membranes Loaded with Dihydromyricetin Inclusion Complexes for Wound Healing Application. Int. J. Biol. Macromol. 2024, 278, 134526. [Google Scholar] [CrossRef]
- Moshfeghi, T.; Najmoddin, N.; Arkan, E.; Hosseinzadeh, L. A Multifunctional Polyacrylonitrile Fibers/Alginate-Based Hydrogel Loaded with Chamomile Extract and Silver Sulfadiazine for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2024, 279, 135425. [Google Scholar] [CrossRef]
- Abdelazim, E.B.; Abed, T.; Goher, S.S.; Alya, S.H.; El-Nashar, H.A.S.; EL-Moslamy, S.H.; El-Fakharany, E.M.; Abdul-Baki, E.A.; Shakweer, M.M.; Eissa, N.G.; et al. In Vitro and in Vivo Studies of Syzygium Cumini -Loaded Electrospun PLGA/PMMA/Collagen Nanofibers for Accelerating Topical Wound Healing. RSC Adv. 2024, 14, 101–117. [Google Scholar] [CrossRef]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef]
- Akbarpour, A.; Rahimnejad, M.; Sadeghi-Aghbash, M.; Feizi, F. Bioactive Nanofibrous Mats Constructs: Separate Efficacy of Lawsonia Inermis and Scrophularia Striata Extracts in PVA/Alginate Matrices for Enhanced Wound Healing. Int. J. Biol. Macromol. 2024, 277, 134545. [Google Scholar] [CrossRef]
- Ni, C.; Li, X.; Jiang, H.; Gui, S.; Yin, H.; Nie, X. A Targeted and Synergetic Nano-Delivery System against Pseudomonas Aeruginosa Infection for Promoting Wound Healing. Mater. Today Bio 2025, 31, 101470. [Google Scholar] [CrossRef]
- Wafaey, A.A.; El-Hawary, S.S.; El Raey, M.A.; Abdelrahman, S.S.; Ali, A.M.; Montaser, A.S.; Abdelhameed, M.F.; Kirollos, F.N. Gliricidia Sepium (Jacq.) Kunth. Ex. Walp. Leaves-Derived Biogenic Nanohydrogel Accelerates Diabetic Wound Healing in Rats over 21 Days. Burns 2025, 51, 107368. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Chowdhury, T.; Islam, M.H.; Nuruzzaman, M. Development of Nano ZnO Loaded Chitosan-Sericin Nano-Biocomposite- a Rapid Homeostasis and Effective Wound Healing Agent. Mater. Today Chem. 2024, 42, 102446. [Google Scholar] [CrossRef]
- Al-Nadaf, A.H.; Awadallah, A.; Thiab, S. Superior Rat Wound-Healing Activity of Green Synthesized Silver Nanoparticles from Acetonitrile Extract of Juglans Regia L: Pellicle and Leaves. Heliyon 2024, 10, e24473. [Google Scholar] [CrossRef]
- Li, Z.; Saravanakumar, K.; Yao, L.; Kim, Y.; Choi, S.Y.; Yoo, G.; Keon, K.; Lee, C.-M.; Youn, B.; Lee, D.; et al. Acer Tegmentosum Extract-Mediated Silver Nanoparticles Loaded Chitosan/Alginic Acid Scaffolds Enhance Healing of E. Coli-Infected Wounds. Int. J. Biol. Macromol. 2024, 267, 131389. [Google Scholar] [CrossRef]
- Liang, T.; Lu, C.; Zhao, M.; Cao, X.; Hao, J.; Zhang, X.; Fu, H.; Cao, Q.; Li, L.; Jiang, J. Multifunctional Quercetin-Hordein-Chitosan Nanoparticles: A Non-Antibiotic Strategy for Accelerated Wound Healing. Int. J. Biol. Macromol. 2025, 305, 140943. [Google Scholar] [CrossRef]
- Mamgain, A.; Kenwat, R.; Paliwal, R. Biopolymer Zein Nanoparticles Loaded with Moringa Oleifera Extract for Improved Wound Healing Activity: Development, Qbd Based Optimization and in Vivo Study. Int. J. Biol. Macromol. 2024, 263, 130314. [Google Scholar] [CrossRef]
- Dong, Z.; Yin, J.; Zhou, X.; Li, S.; Fu, Z.; Liu, P.; Shen, L.; Shi, W. Natural and Biocompatible Dressing Unit Based on Tea Carbon Dots Modified Core-Shell Electrospun Fiber for Diabetic Wound Disinfection and Healing. Colloids Surf. B Biointerfaces 2023, 226, 113325. [Google Scholar] [CrossRef]
- Salehi, P.; Heidarizadeh, F.; Motamedi, H.; Makvandi, P. Chitosan/Alginate Hydrogel Adorned with Nanoemulsions for Potential Wound Healing: Fabrication, Characterization, and Biocompatibility Evaluation. Alex. Eng. J. 2025, 117, 45–52. [Google Scholar] [CrossRef]
- Fakhraei, F.; Marouzi, S.; Yazdian-Robati, R.; Kalalinia, F.; Hashemi, M. Formulation and In Vivo Evaluation of Perovskia Abrotanoides Kar. Essential Oil Nanoemulsion Incorporated Chitosan Gel as a Novel Paradigm in Promoting Wound Healing. Environ. Technol. Innov. 2025, 37, 104001. [Google Scholar] [CrossRef]
- Chu, W.; Wang, P.; Ma, Z.; Peng, L.; Guo, C.; Fu, Y.; Ding, L. Lupeol-Loaded Chitosan-Ag+ Nanoparticle/Sericin Hydrogel Accelerates Wound Healing and Effectively Inhibits Bacterial Infection. Int. J. Biol. Macromol. 2023, 243, 125310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siafaka, P.I.; Miliotou, A.N.; Okur, M.E.; Karaotmarlı Güven, G.; Karantas, I.D.; Üstündağ Okur, N. Nanoformulations Loaded with Phytochemicals for Combating Wound Infections and Promoting Wound Healing: Current Applications and Innovations. Appl. Sci. 2025, 15, 5413. https://doi.org/10.3390/app15105413
Siafaka PI, Miliotou AN, Okur ME, Karaotmarlı Güven G, Karantas ID, Üstündağ Okur N. Nanoformulations Loaded with Phytochemicals for Combating Wound Infections and Promoting Wound Healing: Current Applications and Innovations. Applied Sciences. 2025; 15(10):5413. https://doi.org/10.3390/app15105413
Chicago/Turabian StyleSiafaka, Panoraia I., Androulla N. Miliotou, Mehmet Evren Okur, Gökçe Karaotmarlı Güven, Ioannis D. Karantas, and Neslihan Üstündağ Okur. 2025. "Nanoformulations Loaded with Phytochemicals for Combating Wound Infections and Promoting Wound Healing: Current Applications and Innovations" Applied Sciences 15, no. 10: 5413. https://doi.org/10.3390/app15105413
APA StyleSiafaka, P. I., Miliotou, A. N., Okur, M. E., Karaotmarlı Güven, G., Karantas, I. D., & Üstündağ Okur, N. (2025). Nanoformulations Loaded with Phytochemicals for Combating Wound Infections and Promoting Wound Healing: Current Applications and Innovations. Applied Sciences, 15(10), 5413. https://doi.org/10.3390/app15105413








