The Application of Local Doxycycline Gel for the Nonsurgical Treatment of Peri-Implant Diseases: A Systematic Review of the Literature
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- In vivo studies on humans, any design, excluding case reports;
- Studies describing the local application of doxycycline gel as monotherapy or in conjunction with other therapies;
- Studies describing the local application of doxycycline gel for the nonsurgical treatment of peri-implant diseases, such as peri-implantitis or peri-implant mucositis;
- English, German, Italian, French, or Spanish language;
- Any follow-up.
2.2. Search Strategy
2.3. Selection Process
2.4. Risk of Bias and Quality of Evidence Appraisal
2.5. Data Synthesis and Analysis
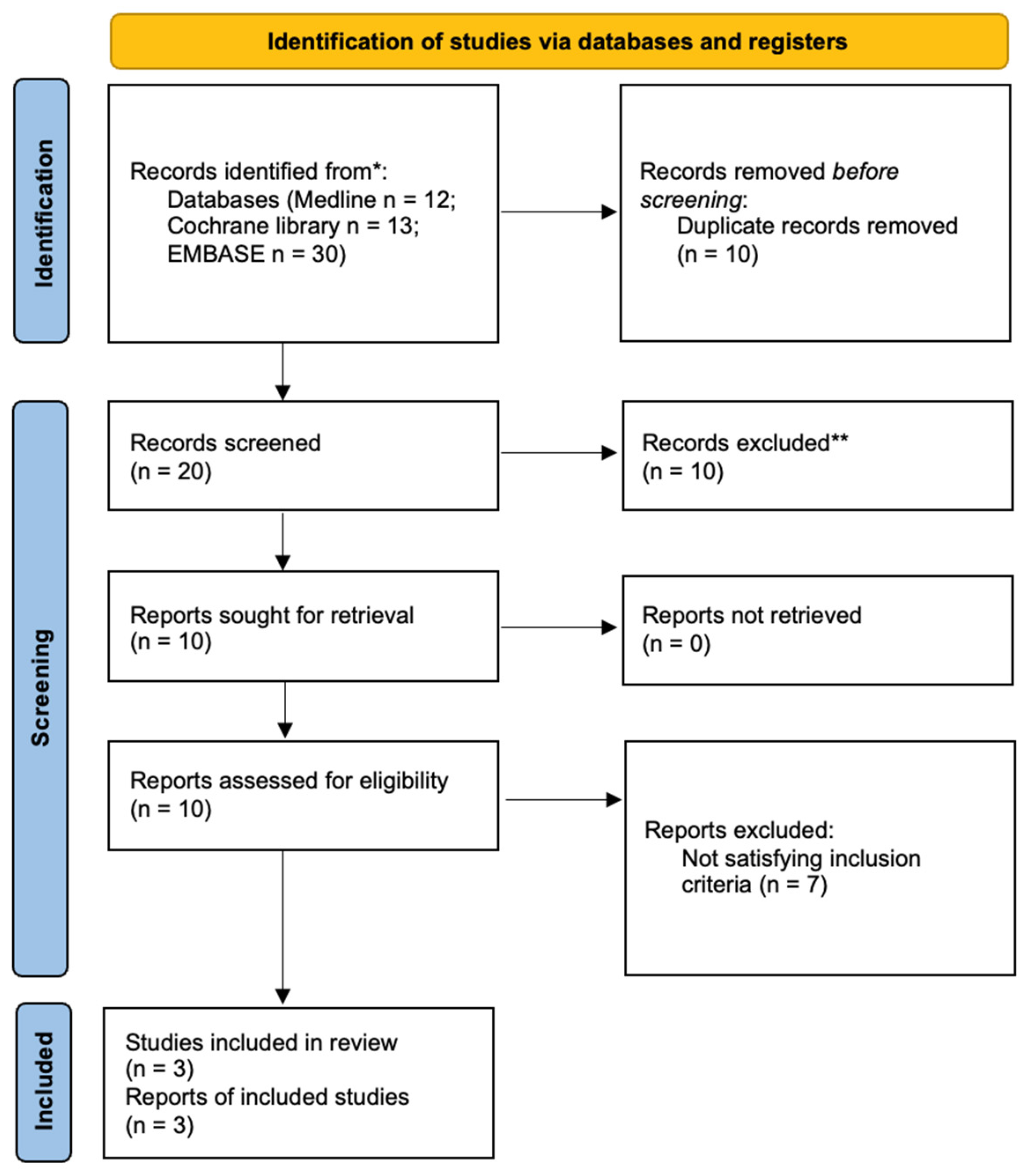
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| MeSH Terms | Free-Text Search | N° Articles Found | |
|---|---|---|---|
| Intervention | exp doxycycline AND peri-implantitis | doxycycline AND (peri-implantitis OR perimplantitis OR periimplantitis OR “peri-implant mucositis” OR “peri-implant disease” OR “peri-implant diseases”) | 12 |
| MeSH Terms | Free-Text Search | N° Articles Found | |
|---|---|---|---|
| Intervention | exp doxycycline AND peri-implantitis | doxycycline AND (peri-implantitis OR perimplantitis OR periimplantitis OR “peri-implant mucositis” OR “peri-implant disease” OR “peri-implant diseases”) | 13 |
| MeSH Terms | Free-Text Search | N° Articles Found | |
|---|---|---|---|
| Intervention | ‘doxycycline’/exp OR ‘doxycycline’ AND periimplantitis | doxycycline AND (peri-implantitis OR perimplantitis OR periimplantitis OR “peri-implant mucositis” OR “peri-implant disease” OR “peri-implant diseases”) | 30 |
References
- Heitz-Mayfield, L.J.A. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Hoedke, D.; Valles, C.; Vilarrasa, J.; Jepsen, S.; Pascual La Rocca, A. Efficacy of professionally administered chemical agents as an adjunctive treatment to sub-marginal instrumentation during the therapy of peri-implant mucositis. J. Clin. Periodontol. 2023, 50, 146–160. [Google Scholar] [CrossRef]
- Renvert, S.; Lessem, J.; Dahlén, G.; Lindahl, C.; Svensson, M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J. Clin. Periodontol. 2006, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Doxycycline. Available online: https://medlineplus.gov/druginfo/meds/a682063.html (accessed on 18 February 2025).
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Hamlet, S.; Ivanovski, S. Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and Doxycycline—A prospective 3-year cohort study. Clin. Oral Implant. Res. 2018, 29, 583–591. [Google Scholar] [CrossRef]
- Büchter, A.; Kleinheinz, J.; Meyer, U.; Joos, U. Treatment of severe peri-implant bone loss using autogenous bone and a bioabsorbable polymer that delivered doxycycline (AtridoxTM). Br. J. Oral Maxillofac. Surg. 2004, 42, 454–456. [Google Scholar] [CrossRef]
- Ardila, C.M.; Granada, M.I.; Guzmán, I.C. Antibiotic resistance of subgingival species in chronic periodontitis patients. J. Periodontal Res. 2010, 45, 557–563. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Book Series, C.; Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration®: London, UK, 2008. [Google Scholar]
- Latronico, M.; Camurati, A.; Currarino, F.; Giargia, M. Non surgical mechanical/pharmacological therapy of peri-implantitis: One year results. Dent. Cadmos. 2022, 90, 358–366. [Google Scholar] [CrossRef]
- Mensi, M.; Cochis, A.; Sordillo, A.; Uberti, F.; Rimondini, L. Biofilm removal and bacterial re-colonization inhibition of a novel erythritol/chlorhexidine air-polishing powder on titanium disks. Materials 2018, 11, 1510. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Lang, N.P.; Cortellini, P.; Suvan, J.E.; Eickholz, P.; Fourmousis, I.; Topoll, H.; Vangsted, T.; Wallkamm, B. Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy. J. Clin. Periodontol. 2012, 39, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Dannewitz, B.; Lippert, K.; Lang, N.P.; Tonetti, M.S.; Eickholz, P. Supportive periodontal therapy of furcation sites: Non-surgical instrumentation with or without topical doxycycline. J. Clin. Periodontol. 2009, 36, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Kim, T.S.; Bürklin, T.; Schacher, B.; Renggli, H.H.; Schaecken, M.T.; Holle, R.; Kübler, A.; Ratka-Krüger, P. Non-surgical periodontal therapy with adjunctive topical doxycycline: A double-blind randomized controlled multicenter study (I). Study design and clinical results. J. Clin. Periodontol. 2002, 29, 108–117. [Google Scholar] [CrossRef]
- Ratka-Krüger, P.; Schacher, B.; Bürklin, T.; Böddinghaus, B.; Holle, R.; Renggli, H.H.; Eickholz, P.; Kim, T. Non-Surgical Periodontal Therapy With Adjunctive Topical Doxycycline: A Double-Masked, Randomized, Controlled Multicenter Study. II. Microbiological Results. J. Periodontol. 2005, 76, 66–74. [Google Scholar] [CrossRef]
- Klein, M.O.; Al-Nawas, B. For which clinical indications in dental implantology is the use of bone substitute materials scientifically substantiated?: Systematic review, consensus statements and recommendations of the 1st DGI Consensus Conference in September 2010, Aerzen, Germany. Eur. J. Oral Implantol. 2011, 4, 11–29. [Google Scholar]
- Aimetti, M.; Baima, G.; Aliyeva, N.; Lorenzetti, V.; Citterio, F.; Franco, F.; Di Scipio, F.; Berta, G.N.; Romano, F. Influence of locally delivered doxycycline on the clinical and molecular inflammatory status of intrabony defects prior to periodontal regeneration: A double-blind randomized controlled trial. J. Periodontal Res. 2023, 58, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.V.C.; Nociti, F., Jr.; Moura, L.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontology 2000 2021, 86, 231–240. [Google Scholar] [CrossRef]
- Yu, X.L.; Chan, Y.; Zhuang, L.; Lai, H.C.; Lang, N.P.; Keung Leung, W.; Watt, R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral Implant. Res. 2019, 30, 760–776. [Google Scholar] [CrossRef] [PubMed]
- Neely, A.L.; Thompson, T.N.; Gupta, V.; Kinaia, B. Successful Management of Peri-Implantitis Using a Titanium Brush and a Doxycycline-Saline Slurry for Surface Detoxification With Guided Bone Regeneration: A 5-Year Follow-Up. Clin. Adv. Periodontics. 2020, 10, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.A.; Oliveira Giorgetti Bossolan, A.P.; de Rezende Duek, E.A.; Sallum, E.A.; Nociti, F.H.; Casati, M.Z.; Sallum, A.W. Treatment of peri-implantitis using nonsurgical debridement with bioresorbable nanospheres for controlled release of doxycycline: Case report. Compend. Contin. Educ. Dent. 2012, 33, E145–E149. [Google Scholar] [PubMed]
- da Silva, R.V.C.; Almeida, A.B.; de Assis, R.I.F.; Santos, E.J.D.; Rozendo, D.M.M.; Sallum, A.W. Treatment of peri-implantitis using nonsurgical debridement combined with bioresorbable doxycycline nanospheres: A case report with 3-year follow-up. Gen. Dent. 2024, 72, 70–73. [Google Scholar]
- Stein, J.M.; Conrads, G.; Abdelbary, M.M.H.; Yekta-Michael, S.S.; Buttler, P.; Glock, J.; Sadvandi, G.; Kaufmann, R.; Apel, C. Antimicrobial efficiency and cytocompatibility of different decontamination methods on titanium and zirconium surfaces. Clin. Oral Implant. Res. 2023, 34, 20–32. [Google Scholar] [CrossRef]
- Patianna, G.; Valente, N.A.; D’addona, A.; Andreana, S. In vitro evaluation of controlled-release 14% doxycycline gel for decontamination of machined and sandblasted acid-etched implants. J. Periodontol. 2018, 89, 325–330. [Google Scholar] [CrossRef]
| Study Authors | Year | Objective | Sample | Methodology | Probe | Follow-Up | Group 1 | Group 2 | Results | Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Büchter et al. [9] | 2004 | Compare mechanical debridement with and without doxycycline (Atridox™ 1) application for peri-implantitis treatment | 28 patients (48 peri-implant defects) | Control group: mechanical debridement with plastic hand instruments | PCP 11 periodontal probe (Hu-Friedy, Chicago, IL, USA), 3–3–2–3 mm calibration, 0.4 mm diameter | 18 weeks | Mechanical debridement with plastic hand instruments | Mechanical debridement + controlled-release topical doxycycline (Atridox™ 1, 8.5% doxycycline), one application | Greater reduction in PPD (1.15 mm vs. 0.56 mm) and BOP (0.27 vs. 0.13) in doxycycline group compared to debridement alone | Treatment with debridement and doxycycline showed better results than debridement alone | Limited duration (18 weeks), small sample size |
| Mensi et al. [13] | 2017 | Evaluate the efficacy of a non-surgical protocol (MAINST) for acute peri-implantitis | 15 patients (27 implants) | MAINST: decontamination with air-polishing (erythritol and chlorhexidine) and 14% topical doxycycline application (Ligosan® 2) | PCP-UNC 15 periodontal probe (Hu-Friedy, Chicago, IL, USA) | 12 months | MAINST protocol: Decontamination with erythritol powder and chlorhexidine, 14% topical doxycycline application (Ligosan® 2), one application | Not applicable | Significant reduction in BOP (from 98.5% to 4.5%) and PPD (from 7.89 mm to 3.16 mm) between baseline and 12 months | MAINST protocol effective up to 12 months with significant reduction in clinical parameters | Absence of control group, small sample size |
| Latronico et al. [14] | 2022 | Evaluate the efficacy of a non-surgical protocol for peri-implantitis using 14% doxycycline gel (Ligosan® 2) | 26 patients (49 implants) | Supra/subgingival debridement with dedicated tools, biofilm removal, doxycycline gel application (Ligosan® 2) | Not specified | 12 months | Non-surgical treatment with debridement and 14% doxycycline (Ligosan® 2), one application | Not applicable | Average PPD reduction from 6.7 mm to 4.6 mm; BoP reduced from 100% to 29% | Protocol effective in reducing PPD and BoP, potentially lowering the need for surgery. | Small sample size, inability to evaluate the role of individual protocol components. |
| Classification | Organism | Mean Minimal Inhibitory Concentration (µg/mL) |
|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | 1.6 |
| Streptococcus pyogenes | 0.39 | |
| Streptococcus pneumoniae | 0.2 | |
| Viridans group streptococci | 0.39 | |
| Gram-negative bacteria | Neisseria gonorrhoeae | 0.39 |
| Neisseria meningitidis | 1.6 | |
| Haemophilus influenzae | 1.6 | |
| Legionella pneumophila | 1.0 | |
| Bacteroides fragilis | 0.1–8 | |
| Mycoplasma/chlamydia | Mycoplasma pneumoniae | 1.6 |
| Ureaplasma urealyticum | 0.13 | |
| Chlamydia spp. | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbella, S.; Vendrame, A.; Tedeschi, L.; Ashurko, I.; Francetti, L. The Application of Local Doxycycline Gel for the Nonsurgical Treatment of Peri-Implant Diseases: A Systematic Review of the Literature. Appl. Sci. 2025, 15, 5357. https://doi.org/10.3390/app15105357
Corbella S, Vendrame A, Tedeschi L, Ashurko I, Francetti L. The Application of Local Doxycycline Gel for the Nonsurgical Treatment of Peri-Implant Diseases: A Systematic Review of the Literature. Applied Sciences. 2025; 15(10):5357. https://doi.org/10.3390/app15105357
Chicago/Turabian StyleCorbella, Stefano, Alex Vendrame, Lucia Tedeschi, Igor Ashurko, and Luca Francetti. 2025. "The Application of Local Doxycycline Gel for the Nonsurgical Treatment of Peri-Implant Diseases: A Systematic Review of the Literature" Applied Sciences 15, no. 10: 5357. https://doi.org/10.3390/app15105357
APA StyleCorbella, S., Vendrame, A., Tedeschi, L., Ashurko, I., & Francetti, L. (2025). The Application of Local Doxycycline Gel for the Nonsurgical Treatment of Peri-Implant Diseases: A Systematic Review of the Literature. Applied Sciences, 15(10), 5357. https://doi.org/10.3390/app15105357






