Abstract
Money Art is a growing contemporary practice where artists transform banknotes into unique visual works. While conceptually powerful, these artworks present significant conservation challenges due to their fragile substrates and complex material compositions. This study investigates the degradation behaviour of UniPosca acrylic markers applied on zero-euro banknotes, drawing on the techniques of artist RichardHTT, and explores bio-based protective strategies suitable for their preservation. Laboratory samples were prepared to replicate the original artwork and subjected to accelerated ageing. A multi-analytical approach was employed, including multispectral imaging, Fourier trasform infrared (FTIR) and Raman spectroscopy, and scanning electron microscopy (SEM-EDS) colorimetric analysis. Thickness and adhesion properties were assessed with contact micrometry and peel tests, while wettability was evaluated through static contact angle measurements. Four biopolymer coatings, chitosan and chitosan–nanocellulose films with varying CNC concentrations, were evaluated for their transparency, mechanical stability, and compatibility with the substrate. Results showed that painted areas, especially those with blue and black pigments, experienced marked degradation, while, after coating application, samples demonstrated improved chromatic stability, hydrophobicity, and adhesion. Importantly, all coatings were fully removable via enzymatic cleaning with α-amylase, confirming their reversibility. This research highlights the potential of chitosan-based biocomposites as conservation materials for non-traditional artworks and contributes to developing tailored, reversible strategies for contemporary art preservation.
1. Introduction
Art and money have always maintained a complex and layered relationship, oscillating between the concepts of intrinsic and economic value and those of social and institutional critique [1,2,3]. In contemporary times, this relationship has materialised into an artistic practice known as Money Art, characterised by the direct use of monetary supports as expressive media. This trend fits into a broader artistic exploration aimed at questioning the symbolic value of money, transforming it into a unique and unrepeatable work of art [4]. The phenomenon of Money Art manifests through various artistic strategies: from Banksy’s famous altered banknotes, which subvert the imagery of the British monarchy, to Andy Warhol’s autographed banknotes and the works of contemporary artists such as RichardHTT, who intervenes on zero-euro banknotes using UniPosca markers. Posca markers, manufactured by Uni Mitsubishi Pencil since the early 1980s, are widely recognised for their vivid colours, versatility, and ability to adhere to a wide variety of surfaces. They are composed of water-based pigment paints, free from alcohols and solvents, delivered through polypropylene barrels, with nibs made from acrylic and stainless-steel mixing balls to homogenise the paint [5].
While these gestures are deeply rooted in conceptual and aesthetic frameworks, they raise crucial conservation issues. The materials and supports involved are unconventional and often inherently unstable, making these artworks vulnerable to rapid deterioration processes [6,7,8]. The conservation of contemporary art represents an increasingly complex and urgent field of inquiry. This is due not only to the extreme diversity of materials used but also to the ephemeral or even deliberately degradable nature of many contemporary artistic expressions [9,10,11,12]. Unlike historical artworks, for which well-established treatment protocols are available, modern pieces often require tailor-made solutions. These include managing chemically unstable industrial materials, dealing with mixtures of incompatible substances, and respecting the artist’s intention, even when degradation is a deliberate part of the work [13]. According to Cesare Brandi’s restoration theory, any conservation effort must preserve the historical and artistic values of a work. However, in contemporary art, the notion of “age value” is often secondary to principles such as reversibility and fidelity to the artist’s intent, principles which Brandi himself laid out, though in a different context [14]. As Muriel Verbeeck points out, Brandi’s theory still offers a useful theoretical framework for contemporary conservation, provided it is reinterpreted to address new material and conceptual challenges [15].
In the specific case of Money Art, conservation challenges stem from the inherent nature of the monetary substrates. While banknotes and coins are engineered to withstand wear from circulation, they are not designed to support long-term artistic applications. The paper used for banknotes, primarily composed of cotton fibers, is often treated with heavy-metal-rich inks and various organic additives, which hinder the stable adhesion of pigments and markers, especially those based on water. As recent studies have shown, the application of such materials on non-absorbent surfaces like watermark paper or polymeric substrates can lead to abrasion, flaking, and chromatic alteration over time [16,17]. Moreover, the multilayered structure of banknotes, including security threads and metallic pigments, further complicates the physical and chemical stability of the artwork over time. Morphological and spectroscopic analyses of fibers recovered from banknote production waste have revealed the presence of metals such as aluminium, iron, and nickel, which can interfere with the durability of subsequently applied materials [18,19]. These properties pose significant challenges for the conservation of Money Art and demand targeted approaches to ensure the long-term stability of the artistic materials employed. Another major concern involves the interaction between artist-applied materials, particularly acrylic-based media, and environmental factors such as humidity, light exposure, and airborne pollutants [20,21,22]. Numerous studies have demonstrated that volatile organic compounds (VOCs), often released by conservation materials like adhesives, coatings, or display case polymers, can compromise the chemical and chromatic stability of acrylic films [23,24]. The diffusion of VOCs and moisture into the polymer matrix can promote the formation of hydrogen bonds and initiate chemical reactions that alter the polymer structure, leading to internal stress, plasticisation, and progressive deterioration [25]. These effects are especially critical for water-based acrylic formulations, which are widely used in commercial markers such as UniPosca. Their relatively low glass transition temperature (Tg) makes them sensitive to temperature fluctuations and promotes increased tackiness, dust accumulation, and the potential migration of additives under display or storage conditions. Additionally, photodegradation triggered by UV exposure may result in the breakdown of chromophores, binder oxidation, and discolouration of pigments, further compromising visual integrity [26,27,28].
While protective coatings are commonly employed in preventive conservation to enhance resistance to chemical and physical stressors, their application to contemporary art remains largely confined to urban artworks and outdoor murals [29,30,31,32,33,34,35,36,37]. In contrast, applying such treatments to works like Money Art, characterised by unconventional, fragile substrates such as banknotes, raises additional concerns. Other strategies include coatings based on pullulan, pectin, or starch derivatives, which have been evaluated for food packaging and are now being considered for conservation contexts due to their barrier properties and environmental compatibility [38]. Additionally, layered coatings incorporating CeO2 nanoparticles and chitosan have been proposed as UV-protective, transparent barriers suitable for light-sensitive materials [39]. Among the most promising candidates is chitosan, a natural cationic polysaccharide that forms transparent, biocompatible films with intrinsic antimicrobial properties [40]. However, pure chitosan films often exhibit limited mechanical resistance and are susceptible to humidity-induced deformation. For this reason, recent studies have focused on hybrid chitosan-based systems, combining chitosan with cellulose nanocrystals (CNCs) or cellulose nanofibrils (CNFs) to enhance dimensional stability and tensile strength. Camargos et al. (2022) [41] demonstrated the efficacy of such nanocomposites in preserving paper-based substrates under accelerated ageing, while maintaining transparency and reversibility. Recent studies have also demonstrated innovative uses of chitosan-based coatings for the conservation of modern materials. Macchia et al. (2024) [42] applied a chitosan coating to protect modified artworks against graffiti, highlighting its transparency, reversibility, and minimal aesthetic impact. Furthermore, chitosan’s known antimicrobial and film-forming properties have made it a focus of recent research as a sustainable conservation material [43,44,45,46]. Nonetheless, pure chitosan films tend to lack mechanical strength and dimensional stability [47,48,49,50]. For this reason, blending chitosan with structurally supportive materials such as nanocellulose has become an area of active investigation. Nanocellulose contributes tensile reinforcement and enhances film integrity, forming hybrid coatings that combine the bioactivity of chitosan with superior mechanical properties [51,52,53,54,55,56,57].
While numerous studies have identified biological, mechanical, chemical, thermal, and environmental factors as primary contributors to banknote deterioration during circulation [16,17], the present investigation focuses specifically on the stability of the applied pictorial layer. By assessing its behaviour under accelerated environmental conditions, this study addresses conservation issues from a material and stratigraphic perspective, rather than limiting the analysis to the banknote substrate as a passive vector of degradation. Consequently, this study aims to systematically investigate the conservation challenges associated with Money Art, focusing on the chemical degradation of the materials involved and evaluating the potential of chitosan-based protective coatings to mitigate this process. Simulated samples were created using UniPosca markers applied to zero-euro banknotes and subjected to accelerated ageing under different environmental conditions. Four types of chitosan-based films, either pure or reinforced with nanocellulose at varying concentrations, were tested to assess their mechanical integrity, transparency, and compatibility with the substrate. Analytical techniques including multispectral imaging, Raman and Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM-EDS), and spectrocolorimetry were employed to evaluate material degradation and the performance of these film-forming conservation treatments.
2. Materials and Methods
2.1. Original Artwork and Experimental Design
2.1.1. Preliminary Analysis of the Original Artwork
Before the laboratory phase, a representative artwork by the artist RichardHTT, realised as part of the “Money Art” movement on a zero-euro souvenir banknote, was analysed to define its material composition and to address the design of the laboratory samples. The artwork presents multiple chromatic applications realised using UniPosca acrylic paint markers.
RichardHTT is a contemporary artist whose visual language is both recognisable and systematically reproducible, characterised by the exclusive use of UniPosca acrylic markers on zero-euro banknotes. His works combine standardised materials with a wide chromatic range and a stratified structure that is consistently repeatable. These features make them particularly suitable for the modelling of degradation phenomena in a controlled setting. Moreover, the use of UniPosca markers offers a significant comparative advantage in conservation research. Their water-based acrylic formulation is chemically and functionally analogous to the paints commonly employed in contemporary artworks on various supports, including canvas, wood, and paper. Studying their behaviour under accelerated ageing and protective treatments therefore provides valuable insights that extend beyond the specific context of Money Art and contribute to broader strategies for the conservation of acrylic-based modern artworks.
Through multispectral imaging (VIS, UVF, IR), specific behaviours of the chromatic areas under different lighting conditions were investigated, including fluorescence phenomena and infrared absorption patterns. Thickness measurements of banknote and pictorial layer were performed with a TESA μ-HITE measuring station with a nominal precision of ±0.01 mm. The analytical data collected from the original artwork were used to address the preparation of the laboratory samples and the applied analytical investigations.
2.1.2. Preparation of the Laboratory Samples
The laboratory samples were designed to reproduce the chromatic configuration and material characteristics of the original artwork by RichardHTT. Each sample consisted of a zero-euro souvenir banknote (dimensions: 135 mm × 74 mm), divided into four horizontal rows, each containing two adjacent colour fields (approximately 20 mm × 20 mm per field), separated by unpainted control areas. The chromatic areas were obtained using UniPosca water-based acrylic paint markers, directly applied with their integrated nibs. To broaden the range of test cases beyond those found in the original artwork by RichardHTT, eight colours were selected: black, red, light blue, blue, white, yellow, green, and pink (Figure 1). The colour was applied in two uniform passes with the marker held at a 45° angle, maintaining consistent manual pressure to ensure a controlled and self-regulated paint flow, as determined by the internal valve mechanism of the Posca pens. All samples were left to dry at ambient conditions (25 ± 2 °C, 45 ± 5% RH) for one week. No forced drying or heat sources were used. The final dry film exhibited the typical matte, opaque appearance consistent with Posca paint properties. Subsequently, each chromatic row was treated with a different protective coating, and the entire set of samples was subjected to accelerated ageing protocols to assess material degradation and the performance of the coatings under controlled conditions. In Figure 1 is reported the scheme of laboratory samples.

Figure 1.
Schematic representation of the laboratory sample and the areas treated with the different protective coatings. The left half of the banknote remained unaged, while the right half was subjected to artificial ageing. Each coating formulation is marked with a dashed coloured line, as indicated in the legend.
2.1.3. Application of Protective Coatings
Four protective coatings were formulated using chitosan and cellulose nanocrystals (CNC), both of which are biocompatible, renewable polysaccharides widely studied for their film-forming, mechanical, and barrier properties [54,55,56,57]. To synthesise the nanocomposite, chitosan was dissolved in an aqueous solution, as described by Sakai Y. et al. (2001) [57], while nanocellulose was produced using a ball mill and subsequently dispersed in water at a concentration of 0.7%, as reported by Mao H. et al. (2019) [56]. The coatings were prepared by mixing chitosan with three different concentrations of CNC to investigate the influence of filler content on coating performance. A preservative (2.5% w/w sodium benzoate) and UV stabiliser (Tinuvin 292, 1–1.5% w/w) were added to all formulations. Nanocellulose was produced via a top-down approach using a Retsch PM 100 planetary ball mill, operating at 500 rpm for 30 min with 5 mm diameter grinding balls [58]. Chitosan and sodium benzoate were purchased from Sigma-Aldrich (Sigma-Aldrich Corporation, St. Louis, MO, USA), while Tinuvin was supplied by CTS Conservation (CTS srl, Altavilla Vicentina, Italy).
The four resulting coatings were as follows:
- Chitosan (CS);
- Chitosan with 1.5% nanocellulose dispersion (CS/CNC1.5);
- Chitosan with 3% nanocellulose dispersion (CS/CNC3);
- Chitosan with 6% nanocellulose dispersion (CS/CNC6).
Each coating was applied by brush in a uniform layer over the surface of the painted experimental banknotes. After application, the samples were dried at ambient conditions (22 ± 2 °C, 45 ± 5% RH) for 7 days.
2.1.4. Artificial Ageing
Artificial ageing was performed in accordance with the UNI EN ISO 16474-2:2014 standard, which provides guidelines for simulating natural weathering through exposure to a xenon arc light source [59]. The experimental setup used a Q-SUN Xe-1 test chamber (Q-Lab Corporation, Westlake, OH, USA) equipped with an air-cooled xenon lamp and borosilicate inner and outer filters. Samples were exposed to alternating cycles of light and humidity for a total of 96 h. The exposure parameters were set as follows:
- Irradiance: 60 W/m2 (at 340 nm);
- Black panel temperature: 38 ± 3 °C;
- Relative humidity: 50 ± 5%;
- Light/dark cycle: 102 min light, 18 min dark with condensation.
All samples were positioned horizontally at 250 mm from the lamp and rotated periodically to ensure uniform exposure. The goal of this phase was to simulate the photochemical and physical degradation typically associated with environmental exposure of artworks.
2.1.5. Coating Removal Tests
Following the application of the coatings and the analytical assessments of their performance, cleaning tests were conducted to evaluate the removability of the chitosan-based films. A bio-enzymatic approach was employed using α-amylase, an enzyme capable of hydrolysing the glycosidic bonds within the chitosan matrix. The selection of α-amylase was based on its proven efficacy in degrading chitosan under mild, non-invasive conditions, leading to the formation of chitooligosaccharides and glucosamine [60,61,62]. The enzymatic cleaning solution was prepared according to the protocol described by Tortora et al. (2020) [63], dissolving α-amylase at 1 wt% in deionised water and thickening it with 5 wt% Klucel® G (reagents were purchased from I.M.A.R. Italia S.r.l., Rome, Italy). This formulation enabled localised application with extended contact time, facilitating a controlled and reversible removal process. The hydrolysis was carried out at neutral to mildly acidic pH (4.5–5.0) and moderate temperatures (50–55 °C), which are recognised in the literature as optimal for both enzyme activity and chitosan solubility [60,64].
2.2. Analytical Investigations
All analyses were carried out on three replica laboratory banknotes for each condition (untreated, treated with different protective coatings, before and after artificial ageing) to ensure the reproducibility of results. The spectra and images presented are representative of phenomena observed consistently across all analysed samples. For quantitative measurements, the data were statistically processed and presented with their respective standard deviation values, as reported in the corresponding tables and figures, while for spectroscopic analyses (FTIR, Raman) and imaging, the most representative results were selected after verification of inter-sample reproducibility.
2.2.1. Multispectral Imaging
Multispectral imaging was employed to systematically document the condition of the banknote and its constituent materials, assess degradation phenomena, and evaluate variations following the protective treatment. The banknote was examined using different wavelengths under the following imaging modalities: visible radiation (VIS), ultraviolet fluorescence (UVF), and infrared reflectography (IR960). Analyses were performed on the entire banknote before and after the artificial ageing process. Imaging was conducted using a CANON EOS M50 full-spectrum camera equipped with an EF-M 18–150 mm f/3.5–6.3 IS STM lens (Canon Inc., Tokyo, Japan). Ultraviolet fluorescence was captured utilizing a HOYA UV-IR filter (Hoya Corporation, Tokyo, Japan), with an additional B+W Yellow 495 F-PRO MRC 022 filter (Schneider Kreuznach, Bad Kreuznach, Germany) to eliminate the blue component of the UV lamp (UVF-G). Infrared reflectography images were obtained using a high-pass filter at 960 nm. The light sources included a 365 nm UV source filtered at 400 nm and a 950 nm IR illuminator. All images were white-balanced using a standardised colour-check reference target to ensure comparability across the imaging modalities.
2.2.2. X-Ray Fluorescence Spectroscopy (EDXRF)
Portable energy-dispersive X-ray fluorescence (EDXRF) spectroscopy was performed to investigate the elemental composition of both the original artwork and the laboratory samples (Figure 2). The analysis aimed to identify characteristic inorganic elements present in the UniPosca marker pigments and in the banknote substrate. Measurements were conducted in situ using the portable EDXRF spectrometer HITACHI X-MET8000 Expert equipped with an Rh X-ray tube and a Si-PIN detector (Hitachi High-Tech Corporation, Tokyo, Japan). The acquisition parameters were set to 45 kV, 40 μA, live time 60 s.

Figure 2.
EDXRF spots of measurements performed on the original artwork.
2.2.3. Optical Microscopy
Optical microscopy was employed to examine the morphological characteristics of the painted surfaces, compare non-aged and aged areas, and assess the properties of the protective films before and after ageing. The analyses were conducted using a DinoLite AM411-FVW digital microscope (AnMo Electronics Corporation, Taipei, Taiwan), operating at magnifications ranging from 50× to 200× under both visible (VIS) and ultraviolet (UV) light.
2.2.4. Spectrocolorimetry
Spectrocolorimetry was utilised to define colour variations in the painted areas induced by artificial ageing and protective coatins applications. Measurements were performed using the Spectrophotometer 3nh NS810 (Shenzhen ThreeNH Technology Co., Ltd., Shenzhen, China), within the CIELab colour space. Chromatic differences were calculated using the ΔE* (Equation (1)):
The ΔE* value, defined by the three parameters L*, a*, and b* in the CIELab system, is interpreted as follows: ΔE* < 3 indicates imperceptible colour variation to the naked eye, 3 < ΔE* < 5 signifies perceptible but acceptable colour variation, and ΔE* > 5 represents significant colour alteration, suggesting the modification of surface aspect of the artwork [65,66].
This analysis was useful in identifying the areas most susceptible to degradation and in assessing the suitability of the protective films, as conservation treatments should not significantly alter the original colour of the artwork.
2.2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
FTIR was performed to characterise the composition of materials and investigate degradation mechanisms following artificial ageing. Analyses were performed using the attenuated total reflection (ATR) mode, requiring no sample preparation prior to measurement. Spectra were acquired using an FT-IR Nicolet Summit spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with the Everest™ Diamond ATR accessory. Instrumental parameters included 32 scans per sample, a spectral resolution of 8 cm⁻1, and a measurement range from 4000 to 600 cm⁻1, ensuring precise identification of chemical characteristics.
2.2.6. Raman Spectroscopy
Raman spectroscopy was utilised to confirm the identification of materials composing the painted areas. Spectra were recorded in aged and non-aged chromatic areas. The analysis was conducted using a 785 nm Raman Spectrometer RK785-I from Oceanhood (Oceanhood Optoelectronics Technology Co., Ltd., Xiamen, China), a portable Raman device employing a 785 nm laser. The acquisition parameters were as follows: power between 50 and 200 mW, integration time of 3000 ms, Raman shift ranging from 200 to 3000 cm−1, and a spectral resolution of 4 cm−1. The obtained spectra were interpreted using online databases such as RUFF and relevant scientific literature.
2.2.7. SEM-EDS
Scanning electron microscopy (SEM) analysis was performed using SEM Tescan-VEGA 3 (TESCAN ORSAY HOLDING, Brno, Czech Republic) to determine the morphology of the coating before and after the ageing process. SEM images were acquired in COMBO mode: secondary electrons (SE) + back-scattered electron (BSE). Analyses were conducted using an accelerating voltage of 30 kV under high vacuum conditions, which ensures reliable detection of light elements. The SEM analyses were conducted on micro-samples taken from the chromatic areas of the experimental banknote after the application of the protective coatings, in order to observe both surface morphologies; each specimen was examined without prior preparation. The EDS spectra used for elemental mapping were acquired using the INCA2000 probe (Oxford Instruments, Abingdon, UK). However, as they were not deemed essential for the main discussion, they have been included in the Supplementary Materials.
2.2.8. Contact Angle Measurements
Contact angle measurements were conducted using OCA 15EC (DataPhysics Instruments GmbH, Filderstadt, Germany) to evaluate the wettability of the protective films before and after artificial ageing. This analysis aimed to assess potential changes in surface energy and hydrophilic/hydrophobic balance of the coatings, which could influence their interaction with possible pollutants or cleaning agents. Measurements were performed using the sessile drop method, employing deionised water as probe liquid. Each film was tested in triplicate, and the average values were recorded to ensure reproducibility [67,68].
2.2.9. Adhesion Tests (Peel Test)
Adhesion tests were carried out to evaluate the cohesion and film–substrate bonding of the protective coatings. The test was performed using an Instron 3343 dynamometer, (Instron, Norwood, MA, USA) with a fixed angle of 90° and a controlled speed of 5 mm/min. The peel test allowed for a comparative assessment of the mechanical resistance of each formulation, providing insights into the ease of removal and long-term adhesion behaviour. This parameter is crucial in the context of reversibility and minimal intervention in conservation practices.
3. Results
3.1. Multispectral Imaging and and Paint Layer Thickness Measurement
UV and IR imaging of the original banknote artwork by RichardHTT (Table 1) revealed distinct responses from the applied materials, enabling the identification of unpainted areas, integrated security features, and underlying preparatory sketches. Under UV illumination, the hair of the female figure, appearing blue and lilac under visible light, showed differentiated behaviour: the lilac portions emitted a reddish fluorescence, while the blue areas appeared dark, indicating a lack of fluorescence. The red background demonstrated strong UV absorption, as did other colours such as the blue of the hair and the yellow of the necklace beads, all of which lacked notable fluorescence. Infrared reflectography revealed the presence of underlying sketches and preparatory lines, offering insights into the artwork’s execution process. Notably, two specific zones, the soldier’s shoulder and the woman’s hand, exhibited distinct UV fluorescence patterns, revealing unpainted areas or possible use of a white pigment with different optical properties. These anomalies may suggest either incomplete coverage by the POSCA markers or the intentional use of a fluorescent pigment differing from the rest of the palette.

Table 1.
Multispectral imaging investigation on the artist’s banknote. Images were acquired using three acquisition modes: visible light (VIS), ultraviolet fluorescence with a yellow filter (UVF-Y), and infrared reflectography with a long-pass filter at 960 nm (IR960).
The analysis of the artist’s original banknote provided valuable insights into the optical behaviour and material characteristics in a real artistic context (Table 2). In parallel, the experimental banknote was designed to replicate these conditions, allowing for a systematic evaluation of the effects of artificial ageing and protective treatments under controlled laboratory conditions. Before the application of protective coatings, the chromatic fields of the experimental banknote exhibited notable responses under different lighting conditions. Specifically, the pink area displayed an intense and distinctive red fluorescence under UV light, while in infrared reflectography it showed high IR reflectance. The remaining colour fields did not fluoresce under UV and appeared dark in both UVF and UVF-Y images. In infrared reflectography, the black field appeared transparent to IR radiation, clearly revealing the banknote substrate. The white, light blue, yellow, and red areas appeared brighter than the substrate, indicating strong IR reflection.

Table 2.
Multispectral imaging of the experimental banknote (VIS, UVF, UVF-Y, IR60), before and after ageing and coating application.
The artificially aged areas exhibited chromatic changes, particularly in the black and blue fields. The black field showed a reduced covering power, making the substrate visible even under normal light. The blue field displayed a slight decrease in saturation. The other colour fields were more resistant to degradation, although minor variations were detected in infrared reflectography. Notably, the white, light blue, red, and pink fields in aged areas made inscriptions and markings on the banknote more visible, due to decreased IR reflectance within the pigment layer. Following the application of the protective coatings, no significant differences were observed in UV fluorescence responses between treated and untreated areas, either before or after ageing. In infrared reflectography, however, the aged and coated areas showed greater IR absorption compared to the corresponding untreated areas, suggesting that ageing altered the optical properties of the coating materials.
Thickness measurements (Table 3) performed on both the original artwork and the laboratory samples confirmed that the pictorial layer applied by the artist RichardHTT (120 µm ± 62 µm) closely matches the values obtained in the laboratory reproductions using POSCA markers. The coloured paint layers, before the application of any protective treatment, showed an average thickness ranging from approximately 90 μm to 140 μm, depending on pigment colour and application density. The application of chitosan-based protective coatings resulted in a measurable increase in total stratigraphy, with combined thickness values (banknote + POSCA + coating) ranging between 710 μm and 1220 μm, reflecting the variable composition and load of the protective films. After artificial ageing, a moderate reduction in coating thickness was recorded across all samples, ranging from 50 μm to 230 μm. This decrease is likely due to minor physical contraction and moisture loss, commonly associated with hydrophilic polymer systems.

Table 3.
Thickness measurements before and after artificial ageing.
3.2. EDXRF
The analyses performed using portable EDXRF spectroscopy on the original banknote by RichardHTT revealed a characteristic elemental composition about both the banknote substrate and the artist’s chromatic palette (Table 4). A common matrix was detected across all analysed areas, primarily composed of titanium (Ti), silicon (Si), and iron (Fe), corresponding to metal associated with paper fibers and printing inks. In several locations, additional elements such as copper (Cu) and gold (Au) were detected, typical markers of integrated metallic security threads and watermark features embedded in the banknote’s structure. The most distinctive chemical signal differentiating the painted areas from the unaltered substrate was an increase in silicon content. The areas painted with Posca acrylic markers showed significantly elevated silicon levels compared to the banknote surface, where silicon was present only in trace amounts. This difference is consistent with the formulation of many commercial acrylic markers, which include silica-based additives to enhance pigment dispersion, opacity, and film durability. In contrast, aluminium (Al), a major component of the substrate, was significantly reduced or undetected in the painted zones.

Table 4.
EDXRF analysis performed on RichiardHTT banknote.
The EDXRF analysis of the chromatic areas in the laboratory-prepared banknote confirmed elemental compositional consistency with the base substrate but also highlighted specific markers of the applied materials (Table 5). Elemental analysis on several colour applications—black, red, light blue, blue, white, yellow, green, and pink—revealed moderate variations in their elemental amount. Silicon (Si), titanium (Ti), and iron (Fe) were the most recurrent elements, with variable yet systematic presence across the spectrum. Trace elements including zinc (Zn) and copper (Cu) were occasionally detected, reflecting either impurities or additives. Notably, there was an absence of transition metals commonly associated with inorganic pigments, supporting the conclusion that the UniPosca markers used in the experimental setup rely on organic pigments/colourants rather than traditional mineral-based formulations. The general homogeneity in metal content across the various colours, along with the prevalence of light elements and silicon enrichment, aligns with findings from previous studies on modern water-based markers and acrylic paints reported in the bibliography [18,19,69,70].

Table 5.
EDXRF analyses performed on laboratory sample.
3.3. Optical Microscopy
Optical microscopy performed under visible and ultraviolet light allowed for the observation of changes induced by both artificial ageing and the application of protective coatings (Table 6 and Table 7). The black area, identified as an application of ballpoint pen (Biro), showed a glossy black-brown appearance in the non-aged section, completely masking the characteristic weave of the banknote paper. The golden inscriptions from the banknote’s watermark, which emit intense yellow fluorescence, were almost invisible in visible light and only faintly detectable under UV illumination. In the aged area, the same ink appeared lighter and less opaque, allowing the banknote texture to show through. This is further confirmed by the enhanced visibility of the watermark’s golden decorations under both visible and UV light. In the other chromatic areas, a general desaturation of colour was observed after ageing, with the effect being particularly noticeable in the blue and pink fields. Furthermore, aging led to the formation of cracks in the paint layer, especially evident in the blue and white areas, where the banknote substrate became more visible compared to the corresponding non-aged zones.

Table 6.
Optical microscopy of painted areas on laboratory banknote in VIS and UV light, before protective treatments. Magnification: 180×. Aged and non-aged surface morphologies. Scale bar: 0.2 mm, located in the bottom right corner.

Table 7.
Optical microscopy of coated painted areas (red, blue, yellow, green)—VIS/UV, at several magnifications, before and after ageing (scale bar: 0.2 mm, located in the bottom right corner).
Optical microscopy observations performed on the coated samples before and after artificial ageing revealed that the protective films (CS, CS/CNC1.5, CS/CNC3, CS/CNC6) did not introduce any macroscopically detectable changes in the appearance of the painted surfaces under both visible and ultraviolet light (Table 7). For all tested markers (red, blue, white, and green), the coatings maintained uniform coverage and did not interfere with the visual characteristics of the underlying POSCA layers. Only in formulations with higher nanocellulose content, small white aggregates, UV-reflective, were observed under both visible and ultraviolet illumination. These aggregates were localised and did not affect the overall homogeneity of the surface. Post-ageing observations showed that the coatings remained adherent to the painted surfaces and preserved a homogeneous morphology, with no evidence of surface defects or optical discontinuities. No relevant differences in surface texture or structural integrity were observed among the different formulations.
3.4. Spectrocolorimetry
Spectrocolorimetric analysis revealed that unprotected pictorial layers underwent evident chromatic alterations following artificial ageing (Table 8). This is reflected by ΔE values greater than 3 for the majority of tested colours, indicating changes that are perceptible to the naked eye. The most significant shifts were observed in the blue (ΔE = 18.20), black (ΔE = 14.86), red (ΔE = 6.67), and light blue (ΔE = 5.42) areas, demonstrating a high sensitivity of these pigments to environmental stress when left untreated. In the areas treated with polymeric coatings, the chromatic variations observed both immediately after application and following subsequent ageing were minimal. ΔE values remained consistently below the threshold of perceptibility (ΔE < 3) for all tested markers, with the exception of the blue colour, which showed moderate variation (ΔE = 4.27 after treatment and 6.90 after ageing). Nevertheless, these values were markedly lower than those recorded in the aged areas before treatment, confirming the stabilizing effect of the coatings in limiting pigment degradation and preserving overall colour integrity.

Table 8.
Colour differences (ΔE) measured on each treated area, comparing the condition before and after coating application and before and after artificial ageing (0.2 < SD < 0.8).
3.5. FT-IR Spectroscopy
3.5.1. Characterisation Laboratory Sample
Compositional information on the banknote and the applied colourants was obtained through FTIR spectroscopy. Analyses conducted on the unpainted white areas of the banknote confirmed the cellulosic nature of the paper support, with characteristic peaks at 3335 and 3294 cm−1 (O-H stretching), 2920 and 2852 cm−1 (CH2/CH3 stretching), 1643 cm−1 (O-H bending), the region between 1363 and 1000 cm−1 (pyranose ring and glycosidic bond vibrations), and between 1000 and 700 cm−1 (glucosidic ring deformations) [71,72,73]. A weak absorption band at 1724 cm−1, associated with C=O stretching, suggests the presence of hemicellulose or minor lignin residues (Figure 3) [72].
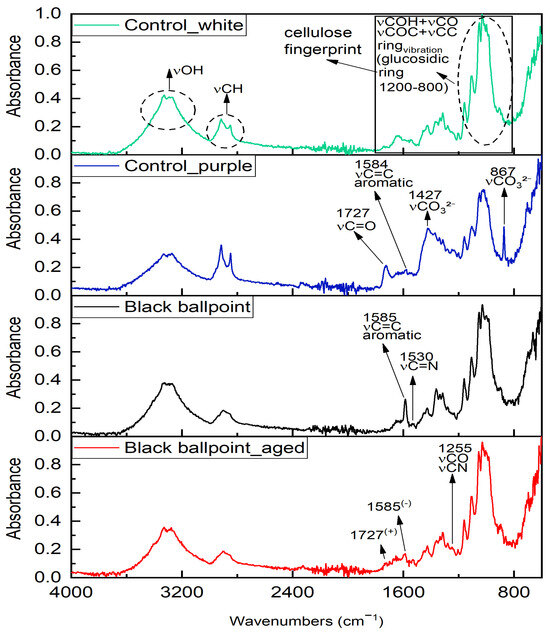
Figure 3.
FTIR spectra collected on black ballpoint pen applied to both non-aged and aged areas. Applications are compared with both white and purple substrates, as they were applied to regions with distinct characteristics (white for the non-aged side and purple for the aged side).
The purple area of the banknote showed a similar profile but with increased intensity at 1727 cm−1 (C=O stretching) and 1427 cm−1 (asymmetric stretching of CO32−), supported by signals at 1792, 867, and 704 cm−1, indicating the possible presence of carbonate compounds [74,75]. The additional peak at 1584 cm−1 suggests the presence of aromatic structures, likely from dye components [76,77].
Spectra collected on the black marker, both aged and unaged, reflected contributions from both the substrate and the marker dye. New bands at 1585, 1530, and 1255 cm−1 were attributed to C=C aromatic stretching, C=N vibrations, and C-N/C-O-C modes, compatible with triarylmethane-based dyes [78,79].
The UniPosca acrylic markers showed spectra distinct from the substrate, confirming the presence of a synthetic acrylic medium. Peaks in the 2969–2858 cm−1 region corresponded to aliphatic stretching, while the strong band at 1737 cm−1 reflected C=O stretching typical of acrylic esters. Additional signals between 1443 and 1200 cm−1 were attributed to C-O and C-C vibrations within the polymer matrix [69,70,80,81,82].
The red marker presented vibrational features coherent with a naphthol-based azo pigment, with peaks between 1670 and 813 cm−1 associated with N=N and C=C stretching, and C-N/C-O modes (Figure S1) [83,84,85].
The yellow marker displayed a distinct profile, consistent with diarylide pigments: the diagnostic bands between 1592 and 860 cm−1 corresponded to N=N bonds, aromatic ring modes, and substituted ring deformations (Figure S2) [85]. Both red and yellow systems appeared spectrally stable after accelerated ageing.
White, pink, and light blue markers exhibited strong features of the acrylic binder (Figures S3–S5). The pink sample presented additional peaks at 1560 and 1497 cm−1 (C=C and/or N=N), consistent with organic chromophores [82].
The light blue area showed alterations after ageing, such as the appearance of new bands at 1088 and 804 cm−1, disappearance of the 973 cm−1 peak, and shifts in the 1100–900 cm⁻1 region, indicating UV-induced degradation or hydrolysis [85,86]. In contrast, white and pink areas were spectrally stable.
The blue marker displayed a spectrum compatible with phthalocyanine pigments, with peaks at 1577 cm−1 (C=C), 1492 cm−1 (C-N in conjugated aromatics), and 1411 cm−1 (isoindole deformation), along with C-N and C-C signals between 1334 and 1025 cm−1 (Figure S6). After ageing, a general intensity decreases and peak shifting below 1130 cm−1 was observed, consistent with partial degradation of both pigment and binder [82,87,88,89,90].
The green marker showed similar features, including peaks at 1593 and 1500 cm−1 and in the 1330–1020 cm−1 region (Figure S7). Slight spectral differences from the blue marker (e.g., a new peak at 1675 cm−1, associated with conjugated C=O stretching) indicate peripheral substitutions (e.g., chlorine) or the presence of an additional pigmentary component [82,91]. Ageing produced minor changes, with new peaks below 1200 cm⁻1 suggesting rearrangements in the binder matrix [88,89,90].
3.5.2. Analyses After Coating Application and Removal
To investigate the influence of the applied protective coatings and their subsequent removal, FTIR spectra were acquired on selected chromatic areas (red, yellow, blue, green), both before and after treatment, and after accelerated ageing.
In all cases, the application of chitosan and nanocellulose-based coatings led to spectral changes primarily in the hydroxyl stretching region (~3300 cm−1) and in the 1200–850 cm⁻1 fingerprint region. These variations are consistent with the presence of polysaccharide coatings at the surface, rather than with chemical interactions or modifications of the acrylic polymer itself. Indeed, both chitosan and nanocellulose are known to form hydrogen bonds with existing functional groups, but do not react covalently with acrylic matrices under mild conditions [92,93,94,95].
In the red and yellow marker samples, the appearance of broader O-H stretching at ~3300 cm−1 and new bands in the 1150–850 cm−1 region can be attributed to characteristic vibrations of chitosan (C-O-C, glycosidic bond, C-O of primary and secondary OH), confirming their presence on the surface after treatment (Figure 4). Similar profiles have been described in polysaccharide-coated acrylic films and model systems [92,94].
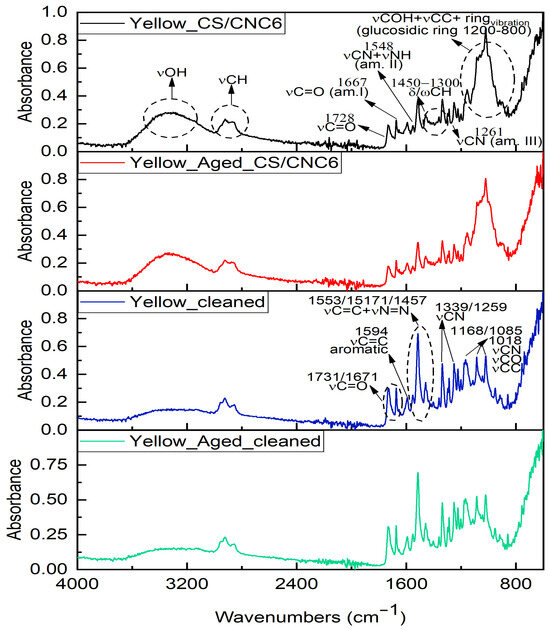
Figure 4.
FTIR spectra collected on red Posca after CS coating application (black line), after ageing process (red line), and after cleaning test on both non-aged (blue line) and aged (green line) coating.
The analyses carried out on the yellow-coloured area after the application of the coating revealed highly similar changes, including an increase in the intensity and broadening of the band at approximately ~3300 cm−1, as well as the appearance of new bands in the low-frequency region (Figure S8). These variations can be attributed partly to chitosan—due to the increased presence of hydroxyl groups and the vibrational modes associated with C-O-C linkages, the glycosidic bond, and C-O stretching—and partly to the nanocellulose used to reinforce the coating. The latter contributes further hydroxyl groups and exhibits characteristic low-frequency vibrations related to C-O bonds (from primary and secondary alcohols), pyranose ring deformations, and glycosidic bond vibrations [92,94].
In the case of the blue marker, after treatment with chitosan and nanocellulose, a decrease in the intensity of the original C=O stretching peak at 1730 cm−1 was observed, along with the appearance of amide I and II bands at 1650 and 1580 cm−1, respectively, typical of chitosan (Figure S9) [92]. Multiple additional bands between 1400 and 900 cm−1 were attributed to the overlapping spectral features of the coating materials. It should be emphasised that these spectral changes are not evidence of chemical interaction with the acrylic binder but rather reflect the spectral contribution of the coating over the pigmented surface, consistent with previous findings in layered systems [93,94].
The green marker area showed similar trends: an increased O-H signal and amide-related peaks (1650, 1580 cm−1), as well as new absorptions in the fingerprint region, indicating the presence of the coating without altering the chemical integrity of the underlying acrylic paint (Figure S10). Minor spectral differences between aged and non-aged samples were mainly attributable to superficial oxidative changes or moisture-induced reorganisation, rather than to degradation of the base layer.
After enzymatic removal using α-amylase, FTIR spectra showed a complete recovery of the original acrylic spectral features, with the disappearance of coating-specific bands. This confirms the effective reversibility of the treatments and the absence of chemical residues, a critical requirement in conservation protocols [71,72,73,90,91,92,93].
3.6. Raman Spectroscopy
The Raman analysis performed on Posca markers, and the black ballpoint colour reveals peaks associated to both organic and inorganic fractions (Table 9).

Table 9.
Vibrational bands detected in the coloured areas of the experimental banknote and their corresponding vibrational mode assignments.
The black ballpoint colour Raman spectrum shows intense peaks at 424, 522, 622, and 726 cm−1, related to aromatic ring deformations and C-H bending vibrations, typical of triphenylmethane dyes (Figure 5). The peak at 1172 cm−1 corresponds to in-plane C-H vibrations in substituted aromatic systems, while peaks between 1532 and 1620 cm−1 indicate conjugated C=C stretching, characteristic of aromatic molecules like Crystal Violet. Secondary peaks between 800 and 1450 cm−1 suggest the presence of a solvent, likely benzyl alcohol or phenoxyethanol [96,97,98].
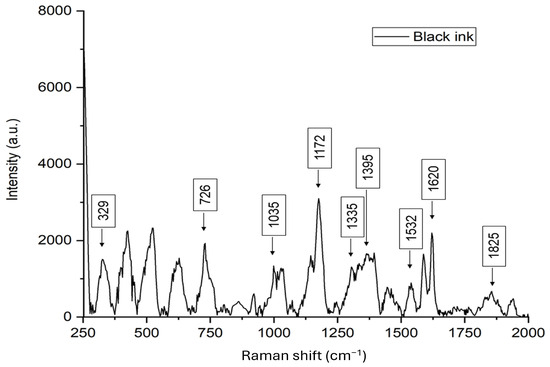
Figure 5.
Raman spectrum collected on black ballpoint ink.
The red Posca marker spectrum, dominated by peaks at 738, 1165, 1249, 1290, 1367, 1425, 1456, and 1616 cm−1, points to an azo pigment with conjugated aromatic systems (Figure S11). Low-frequency peaks at 452 and 613 cm−1 correspond to rutile-phase titanium dioxide, while secondary peaks in the 329–483 range and 522 cm⁻1 suggest silica vibration. The region between 1370 and 1450 cm−1 may also reflect contributions from the acrylic polymer, characterised by C-C and CH vibrational modes [82,99,100,101,102,103,104,105,106,107].
The pink Posca marker shares similarities with the red marker but shows distinct features (Figure S12). Intense peaks at 322, 452, and 615 cm−1 are related to aromatic ring vibrations and rutile-phase titanium dioxide. Additional peaks at 1272, 1325, and 1365 cm−1 suggest an organic pigment, potentially from the azo family [82,99,100,107]. The white marker spectrum is dominated by peaks at 450 and 613 cm⁻1, indicating titanium dioxide in its rutile phase, with additional peaks from Si-O, and the acrylic matrix (Figure S13) [82,99].
The yellow marker spectrum shows peaks between 1200 and 1350 cm−1, with strong peaks at 1265 and 1335 cm−1, related to in-plane C-H vibrations and substituted aromatic ring deformations (Figure S14). The 1597 cm−1 peak suggests C=C stretching in a conjugated aromatic system, consistent with the diarylide pigment class [82,99,108].
The light blue marker spectrum reveals characteristic features of the acrylic matrix and inorganic components, with distinct peaks at 1456, 1532, and 1609 cm−1, typical of metal phthalocyanines (Figure S15). The region between 682 and 854 cm−1 includes skeletal vibrations of the isoindole ring, and the 1309 cm⁻1 peak indicates C-N=N vibrations. The 1100–1250 cm−1 region reflects interactions between the pigment and the matrix. The blue and green markers show similar spectra to the light blue, confirming the presence of metal phthalocyanines, acrylic matrix, and inorganic components (Figures S16 and S17) [82,89,91,99].
3.7. Contact Angle and Peel Tests
Contact angle measurements performed before and after coating application revealed clear distinctions in surface wettability across the different formulations (Figure 6, Figure S18). The uncoated (pre-treated surface) chromatic fields, consistent with the hydrophobic nature of acrylic-based POSCA markers, displayed high contact angles averaging around 98°, both before and after artificial ageing, with no significant variations detected. In contrast, the chitosan-only (CS) coating exhibited substantially lower contact angles, averaging approximately 62°, indicative of a more hydrophilic surface. Following artificial ageing, this value remained largely unchanged (around 60°), suggesting stable surface behaviour but relatively low water repellency. The inclusion of nanocellulose in the coating formulations significantly influenced wettability. The CS/CNC1.5 coating showed a moderate increase in contact angle to about 74° before ageing, with only a slight reduction post-ageing (~71°). The CS/CNC3 formulation further increased the contact angle to approximately 80°, which decreased marginally after ageing (~78°), maintaining its water-repellent characteristics. The CS/CNC6 formulation, which included the highest nanocellulose content, demonstrated the highest contact angles among the coated samples (~87° before ageing), and showed only a minimal drop to 83° after ageing, preserving its enhanced hydrophobic performance. Overall, contact angle measurements confirmed that all protective coatings retained their surface characteristics after artificial ageing, with no evidence of surface degradation or significant changes in wettability across the different chromatic fields.
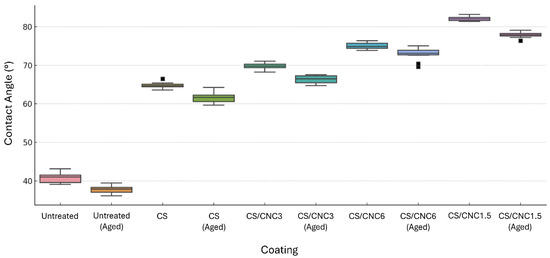
Figure 6.
Contact angle values for untreated (pre-coating) and coated chromatic areas. Measurements were recorded prior to and following artificial ageing. The dispersion of errors is shown by the swarm plots superimposed on the boxplots. Squares indicate statistical outliers. Static contact angle images are included in Supplementary Materials.
The chitosan-only coating (CS) exhibited the lowest adhesion values, with an average peel strength of 1.2 N/cm before ageing and a reduced value of 0.9 N/cm after artificial ageing (Figure 7).
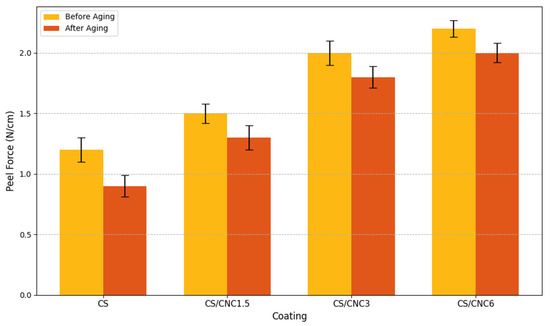
Figure 7.
Peel force (N/cm) for different coatings (CS, CS/CNC1.5, CS/CNC3, CS/CNC6) before and after ageing.
In this formulation, slight delamination was visually observed at the film edges post-ageing, indicating decreased mechanical stability under stress. Incorporation of nanocellulose into the chitosan matrix resulted in a measurable improvement in adhesion. The CS/CNC1.5 formulation demonstrated moderate adhesion values, with 1.5 N/cm before ageing and 1.3 N/cm after ageing, reflecting a good balance between stability and removability. The CS/CNC3 and CS/CNC6 formulations, which contained higher concentrations of nanocellulose, exhibited the highest adhesion forces, with average values reaching 2.0 and 2.2 N/cm before ageing, respectively. Following artificial ageing, these values showed only minor reductions (1.8 and 2.0 N/cm), confirming the strong cohesion and durability of the protective layer even under accelerated degradation conditions. The results indicate that nanocellulose significantly enhances the mechanical performance of chitosan-based coatings, particularly in terms of adhesion to acrylic-painted substrates.
3.8. SEM/EDS
The morphological analysis performed via scanning electron microscopy (SEM) allowed for the detailed observation of surface features across all POSCA-coloured areas, both before and after artificial ageing (Figure 8). The non-aged surfaces displayed homogeneous layers, with limited roughness and well-adhered pigment films. In contrast, the aged areas showed more pronounced micro-roughness, slight textural discontinuities, and, in some cases, particularly blue and light blue, incipient cracking or shrinkage phenomena. The accompanying EDS mapping (Figures S19–S33) supported these observations, revealing the elemental distribution across each sample. Titanium was consistently detected across all colour areas, consistent with its known use as a white pigment base (TiO2) in acrylic formulations. In contrast, silicon (Si) and aluminium (Al) appeared more prominently in coloured layers than in the background substrate, confirming their attribution to the pigment composition of the Posca inks. This aligns with the elemental contrast previously noted between the unpainted banknote—characterised by higher aluminium and lower silicon content—and the painted areas. In some aged samples (e.g., green, red), the presence of additional elements such as copper (Cu), chlorine (Cl), or sulphur (S) was detected, possibly associated with specific pigment compositions or degradation products.
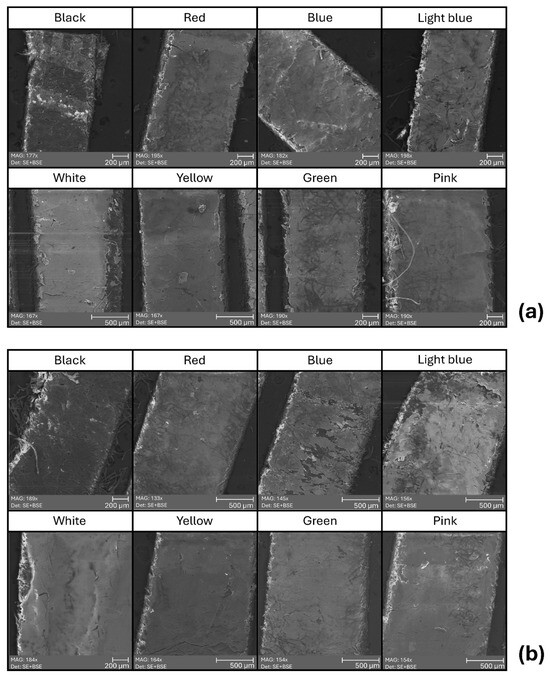
Figure 8.
Top-view SEM images of chromatic areas treated with biopolymer coatings, acquired before (a) and after (b) artificial ageing. Imaging conditions: magnification 100–200×; accelerating voltage (HV): 30 kV; working distance (WD): 13–15 mm; view field: 1–2 mm; detector: SE + BSE.
Notably, the green ink showed localised peaks of Cl and Cu after ageing, potentially indicating a copper phthalocyanine-based formulation. Post-ageing images did not reveal dramatic surface disruptions, suggesting that while photochemical degradation may occur at the molecular level (as seen in FTIR results), the physical integrity of the film remains generally stable.
4. Discussion
The analysis carried out on the original banknote by the artist RichardHTT, alongside the study of experimentally painted samples, enabled a comprehensive investigation into the morphological and compositional characteristics of UniPosca acrylic markers. The samples prepared on zero-euro banknotes were consistent with the original artwork in both substrate and average paint thickness (0.120 mm ± 0.062), validating their suitability for accelerated ageing and conservation testing.
EDXRF analysis revealed silicon and aluminium in all colour fields. However, FTIR and Raman spectroscopy confirmed that the main colouring agents were organic in nature, with inorganic components such as silicon functioning as extenders or dispersants. This finding aligns with established practices in commercial coatings, where silica is often added to improve pigment dispersion and opacity [108,109]. The observed discrepancy in aluminium concentration, low in painted areas and high in unpainted regions, suggests a masking or substitution effect, corroborating similar findings in the field of banknote diagnostics [110].
Vibrational spectroscopy enabled detailed classification of the pigments. FTIR spectra from untreated areas exhibited characteristic bands of the acrylic binder—C=O stretching (1730–1725 cm−1) and C-O-C vibrations (1145–1075 cm−1)—consistent with copolymer systems based on methacrylates and acrylates. Variations in these bands among colour fields indicate differences in binder composition, suggesting formulation variability among marker hues. Post-ageing spectra revealed a significant attenuation and downshift of the C=O band (to ~1715–1710 cm−1), indicative of photooxidative cleavage and carboxylic acid formation, consistent with literature reports on polymer degradation [71,72,73,85,86,90].
The most severe degradation was noted in blue fields, which showed diminished FTIR signal intensity and band resolution, suggesting both binder breakdown and pigment destabilisation, potentially due to aggregation or changes in chromophore dispersion. Raman spectroscopy complemented this analysis, revealing strong, pigment-specific vibrational features: phthalocyanines in blue and green (680, 740, and 1320 cm−1), naphthol-azo in red (1350–1450 cm−1), and diarylide markers in yellow (1380 and 1600 cm−1) [70,90,109,111,112,113,114].
Following ageing, the blue pigments exhibited decreased Raman intensity and minor shifts—indicative of molecular rearrangement or pigment–binder dissociation. Although phthalocyanines are inherently photostable, these effects are attributed to dispersion state and particle size—critical parameters in photostability models involving electronic excitation and non-radiative decay on reflective substrates [108,113].
Combined FTIR, Raman and multispectral imaging consistently highlighted the chromatic instability of the blue and black fields after UV ageing. Visual and analytical evidence—such as surface cracking, colour shifts (ΔE > 5), and binder recession—were linked both to photodegradation processes and to the interaction with the complex banknote surface, which includes metallic inks and optically variable features that influence pigment adhesion and behaviour [88,90,110].
The application of biopolymer-based protective coatings demonstrated a substantial stabilizing effect. Chitosan and nanocellulose-containing films reduced colorimetric variation (ΔE < 3), confirming their ability to mitigate both chemical and mechanical degradation under UV stress, as supported by the recent literature [90,91,92,93]. Morphological analysis showed that pure chitosan produced homogeneous, transparent films, while higher CNC content (e.g., in CS/CNC6) induced microscale surface irregularities and UV-activated aggregate formation. SEM imaging confirmed CNC agglomeration, though no detachment or cracking occurred, even after artificial ageing.
Mechanical resistance improved notably with CNC incorporation, as shown by increased peel strength (1.2 N/cm for CS, 2.2 N/cm for CS/CNC6) and higher contact angles (up to 87°), enhancing the barrier properties of the coating, particularly advantageous in high-humidity or pollution-prone display environments [94].
Critically, no chemical interaction was observed between the coatings and the acrylic binder. FTIR spectra showed that the biopolymers contributed distinct vibrational signatures (1150–850 cm−1), yet did not alter the spectral markers of the acrylic system. Raman analysis further confirmed the absence of shifts or structural changes in pigment or binder peaks. This indicates a surface-level adhesion mediated by physical interactions (e.g., hydrogen bonding, Van der Waals forces), without covalent bonding or cross-linking—an interpretation consistent with recent analytical studies of polysaccharide-based coatings [91,93].
The lack of chemical interaction is a key criterion in the validation of protective systems for contemporary artworks. It ensures treatment reversibility, material compatibility, and long-term stability. Reversibility tests using α-amylase demonstrated the complete removal of the coating, with post-cleaning FTIR and Raman spectra indistinguishable from untreated controls. These findings confirm the suitability of CS/CNC systems in conservation protocols that prioritise ethical standards of minimal intervention and full reversibility.
5. Conclusions
This study offers a dual perspective on the conservation challenges and solutions associated with contemporary artworks created using UniPosca acrylic markers. On one hand, it provides a detailed characterisation of Posca materials applied on unconventional supports such as zero-euro banknotes, revealing both their aesthetic qualities and their vulnerabilities. Spectroscopic and morphological analyses highlighted the organic nature of the pigments and the presence of technological fillers such as silica and alumina, which contribute to pigment dispersion but do not prevent degradation. After artificial ageing, several hues, particularly blue and black, showed pronounced chromatic and structural instability, associated with binder breakdown and, in some cases, pigment alteration.
On the other hand, the research validates the use of protective coatings based on chitosan and nanocellulose as a preventive conservation strategy for these sensitive materials. Coated samples demonstrated reduced chromatic variation (ΔE < 3), improved mechanical resistance, and minor hydrophilicity, with contact angles up to 87°. Optical and SEM imaging confirmed that although higher CNC content may lead to slight surface heterogeneity, the coatings remained well adhered and stable after ageing. Furthermore, both IR and Raman analyses confirmed the complete reversibility of treatments removed with α-amylase, fulfilling key criteria for conservation-grade materials.
By addressing both the characterisation of the original artistic materials and the development of protective systems tailored to their properties, this study proposes a comprehensive methodology for preserving contemporary artworks executed with industrial media. Future work may explore the long-term behaviour of these systems under real display or storage conditions, as well as the adaptability of coatings to other non-traditional artistic media.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15105355/s1. Figure S1. FTIR spectra collected on red Posca applied to both non-aged and aged areas. Applications are compared with both white and purple substrates, as they were applied to regions with distinct characteristics (white for the non-aged side and purple for the aged side). Figure S2. FTIR spectra collected on yellow Posca applied to both non-aged and aged areas. Applications are compared with purple substrate, as they were applied to regions with the same control characteristics. Figure S3. FTIR spectra collected on white Posca applied to both non-aged and aged areas. Applications are compared with purple substrate, as they were applied to regions with the same control characteristics. Figure S4. FTIR spectra collected on pink Posca applied to both non-aged and aged areas. Applications are compared with purple substrate, as they were applied to regions with the same control characteristics. Figure S5. FTIR spectra collected on light blue Posca applied to both non-aged and aged areas. Applications are compared with both white and purple substrates, as they were applied to regions with distinct characteristics (white for the non-aged side and purple for the aged side). Figure S6. FTIR spectra collected on blue Posca applied to both non-aged and aged areas. Applications are compared with both white and purple substrates, as they were applied to regions with distinct characteristics (white for the non-aged side and purple for the aged side). Figure S7. FTIR spectra collected on green Posca applied to both non-aged and aged areas. Applications are compared with purple substrates, as they were applied to regions with the same control characteristics. Figure S8. FTIR spectra collected on yellow Posca after CS/CNC6 coating application (black line), after ageing process (red line), and after cleaning test on both non-aged (blue line) and aged (green line) coating. Figure S9. FTIR spectra collected on blue Posca after CS/CNC3 coating application (black line), after ageing process (red line), and after cleaning test on both non-aged (blue line) and aged (green line) coating. Figure S10. FTIR spectra collected on green Posca after CS/CNC1.5 coating application (black line), after ageing process (red line), and after cleaning test on both non-aged (blue line) and aged (green line) coating. Figure S11. Raman spectrum collected on black ballpoint ink. Figure S12. Raman spectrum collected on red Posca. Figure S13. Raman spectrum collected on pink Posca. Figure S14. Raman spectrum collected on white Posca. Figure S15. Raman spectrum collected on yellow Posca. Figure S16. Raman spectrum collected on light blue Posca. Figure S17. Raman spectrum collected on blue Posca. Figure S18. Raman spectrum collected on green Posca. Figure S19. Static water contact angle measured on the experimental banknote before and after protective treatments. For each measurement, both non-aged and aged areas are shown. Figure S20. Black ballpoint ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E), Ca (F) distribution on sample. Figure S21. Black ballpoint ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E), Ca (F) distribution on sample. Figure S22. White Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Al (E), Si (F) distribution on sample. Figure S23. White Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Ca (E) distribution on sample. Figure S24. Blue Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E) distribution on sample. Figure S25. Blue Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E), Ca (F) distribution on sample. Figure S26. Light blue Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E) distribution on sample. Figure S27. Light blue Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E), S (F) distribution on sample. Figure S28. Yellow Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E) distribution on sample. Figure S29. Yellow Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E) distribution on sample. Figure S30. Pink Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Ca (E) distribution on sample. Figure S31. Pink Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Ca (E) distribution on sample. Figure S32. Green Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Cl (E) distribution on sample. Figure S32. Green Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), Cl (E), Cu (F) distribution on sample. Figure S33. Red Posca ink section images before ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E) distribution on sample. Figure S34. Red Posca ink section images after ageing, acquired on secondary electron (A); EDS composition (B); and Ti (C), Na (D), C (E), Al (F) distribution on sample.
Author Contributions
Conceptualisation, A.M.; methodology, A.M., I.A.C., F.I.B., and F.V.; formal analysis, I.A.C., F.I.B., and G.F.; investigation, I.A.C., F.I.B., C.Z., and M.D.; resources, F.V.; data curation, J.S., C.Z., and A.M.; writing—original draft preparation, A.M, I.A.C., F.I.B., and C.Z.; writing—review and editing, F.V. and G.F.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to sincerely thank the artist RichardHTT for kindly providing his original artwork, which was fundamental to the development of this study. The research was conducted within the framework of the PhD Programme in Cultural Heritage, Education, and Territory at Tor Vergata University of Rome.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CNC | Cellulose Nanocrystals |
| CS | Chitosan |
| FTIR | Fourier Transform Infrared Spectroscopy |
| IR | Infrared |
| SEM-EDS | Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy |
| UVF | Ultraviolet Fluorescence |
| UVF-Y | Ultraviolet Fluorescence with Yellow Filter |
| ΔE | Colour Difference |
| POSCA | UniPosca Acrylic Marker |
References
- Haiven, M. Art and Money: Three Aesthetic Strategies in an Age of Financialisation. Financ. Soc. 2015, 1, 38. [Google Scholar] [CrossRef]
- Klamer, A.; Petrova, L. Financing the Arts: The Consequences of Interaction Among Artists, Financial Support, and Creativity Motivation. In Anthologie Kulturpolitik; Tröndle, M., Steigerwald, C., Eds.; transcript Verlag: Bielefeld, Germany, 2019; pp. 305–318. [Google Scholar] [CrossRef]
- Taylor, S.; Littleton, K. Art Work or Money: Conflicts in the Construction of a Creative Identity. Sociol. Rev. 2008, 56, 275–292. [Google Scholar] [CrossRef]
- Banks, M. The colours of money: Artmoney as community currency. Int. J. Community Currency Res. 2011, 15, 77–81. [Google Scholar] [CrossRef]
- POSCA USA. High-Quality All Surface Paint Markers. Available online: https://poscausa.com (accessed on 7 April 2025).
- Zmeu, C.N.; Bosch-Roig, P. Risk Analysis of Biodeterioration in Contemporary Art Collections: The Poly-Material Challenge. J. Cult. Herit. 2022, 58, 33–48. [Google Scholar] [CrossRef]
- Llamas-Pacheco, R. Some Theory for the Conservation of Contemporary Art. Stud. Conserv. 2020, 65, 487–498. [Google Scholar] [CrossRef]
- Van de Vall, R.; Van Saaze, V. Conservation of Contemporary Art: Bridging the Gap Between Theory and Practice; Springer Nature: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Arbizzani, R.; Casellato, U.; Fiorin, E.; Nodari, L.; Russo, U.; Vigato, P.A. Decay Markers for the Preventative Conservation and Maintenance of Paintings. J. Cult. Herit. 2004, 5, 167–182. [Google Scholar] [CrossRef]
- Macro, N.; Ioele, M.; Cattaneo, B.; De Cesare, G.; Di Lorenzo, F.; Storari, M.; Lazzari, M. Detection of Bronze Paint Degradation Products in a Contemporary Artwork by Combined Non-Invasive and Micro-Destructive Approach. Microchem. J. 2020, 159, 105482. [Google Scholar] [CrossRef]
- Lazzari, M.; Reggio, D. What Fate for Plastics in Artworks? An Overview of Their Identification and Degradative Behaviour. Polymers 2021, 13, 883. [Google Scholar] [CrossRef]
- Zaratti, C.; Brunetti, S.; Fondi, V.; Alisi, C.; Prestileo, F.; de Caro, T.; Montorsi, S.; Macchia, A. Luca Vitone: Monitoring of Four Living Canvases. Heritage 2024, 7, 1438–1452. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Macchia, A.; Troisi, J.; Zaratti, C.; Hansen, A.; Ilardi, S.; Russo, G.; Lahoz, E.; Pacifico, S. Lavender Essential Oil for a Contactless Application for Contemporary Art Conservation: A Case Study. npj Herit. Sci. 2025, 13, 8. [Google Scholar] [CrossRef]
- Brandi, C. Teoria del Restauro; Einaudi: Turin, Italy, 2000; ISBN 978-8806155650. [Google Scholar]
- Verbeeck, M. Brandi and the restoration of contemporary art. One side and the other the Teoria. Conversaciones 2019, 7, 211–226. [Google Scholar]
- Jones, B.J.; Cammidge, J.W.; Evans, C.; Scott, G.; Sherriffs, P.B.; Breen, F.; Andersen, P.M.B.; Popov, K.T.; O’Hara, J. Degradation of Polymer Banknotes through Handling, and Effect on Fingermark Visualisation. Sci. Justice 2022, 62, 644–656. [Google Scholar] [CrossRef]
- Kyrychok, T.; Shevchuk, A.; Nesterenko, V.; Kyrychok, P. Banknote Paper Deterioration Factors: Circulation Simulator Method. BioResources 2013, 9, 710–724. [Google Scholar] [CrossRef]
- Yousef, S.; Hamdy, M.; Tatariants, M.; Tuckute, S.; El-Abden, S.Z.; Kliucininkas, L.; Baltusnikas, A. Sustainable Industrial Technology for Recovery of Cellulose from Banknote Production Waste and Reprocessing into Cellulose Nanocrystals. Resour. Conserv. Recycl. 2019, 149, 510–520. [Google Scholar] [CrossRef]
- Chalhoub, G.; Hamdan, M. Banknote Substrate Durability: A Live Circulation Comparative Study; Banque du Liban: Beirut, Lebanon, 2015; p. 4. Available online: https://www.academia.edu/74702937 (accessed on 31 March 2025).
- Ion, R.-M.; Nuta, A.; Sorescu, A.-A.; Iancu, L. Photochemical Degradation Processes of Painting Materials from Cultural Heritage. In Photochemistry and Photophysics: Fundamentals to Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Barni, D.; Raimondo, L.; Galli, A.; Yivlialin, R.; Caglio, S.; Martini, M.; Sassella, A. Chemical Separation of Acrylic Color Components Enabling the Identification of the Pigment Spectroscopic Response. Eur. Phys. J. Plus 2021, 136, 254. [Google Scholar] [CrossRef]
- Christensen, P.A.; Dilks, A.; Egerton, T.A. Infrared Spectroscopic Evaluation of the Photodegradation of Paint. Part II. The Effect of UV Intensity and Wavelength on the Degradation of Acrylic Films Pigmented with Titanium Dioxide. J. Mater. Sci. 2000, 35, 5353–5358. [Google Scholar] [CrossRef]
- Mejía-González, A.; Zetina, S.; Espinosa-Pesqueira, M.E.; Esturau-Escofet, N. Characterisation of Commercial Artists’ Acrylic Paints and the Influence of UV Light on Aging. Int. J. Polym. Anal. Charact. 2017, 22, 473–482. [Google Scholar] [CrossRef]
- Iscen, A.; Forero-Martinez, N.C.; Valsson, O.; Kremer, K. Molecular Simulation Strategies for Understanding the Degradation Mechanisms of Acrylic Polymers. Macromolecules 2023, 56, 3272–3285. [Google Scholar] [CrossRef]
- Iscen, A.; Forero-Martinez, N.C.; Valsson, O.; Kremer, K. Acrylic Paints: An Atomistic View of Polymer Structure and Effects of Environmental Pollutants. J. Phys. Chem. B 2021, 125, 10854–10865. [Google Scholar] [CrossRef]
- Melo, M.J.; Ferreira, J.L.; Parola, A.J.; de Melo, J.S.S. Photochemistry for Cultural Heritage. In Applied Photochemistry; Bergamini, G., Silvi, S., Eds.; Lecture Notes in Chemistry; Springer: Cham, Switzerland, 2016; Volume 92. [Google Scholar] [CrossRef]
- Macchia, A.; Schuberthan, L.M.; Ferro, D.; Colasanti, I.A.; Montorsi, S.; Biribicchi, C.; Barbaccia, F.I.; La Russa, M.F. Analytical Investigations of XIX–XX Century Paints: The Study of Two Vehicles from the Museum for Communications of Frankfurt. Molecules 2023, 28, 2197. [Google Scholar] [CrossRef]
- Germinario, G.; Logiodice, A.L.; Mezzadri, P.; Di Fusco, G.; Ciabattoni, R.; Melica, D.; Calia, A. Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch. Sustainability 2024, 16, 5069. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage—An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Nodari, L.; Tresin, L.; Benedetti, A.; Tufano, M.K.; Tomasin, P. Conservation of Contemporary Art: Alteration Phenomena in a XXI Century Artwork. From Contactless in Situ Investigations to Laboratory Accelerated Ageing Tests. J. Cult. Herit. 2018, 35, 288–296. [Google Scholar] [CrossRef]
- Clare, T.L.; Swartz, N.A. Characterisation of High Performance Protective Coatings for Use on Culturally Significant Works. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 641–671. [Google Scholar] [CrossRef]
- Masi, G.; Josse, C.; Esvan, J.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Monticelli, C.; Zanotto, F.; Balbo, A.; et al. Evaluation of the Protectiveness of an Organosilane Coating on Patinated Cu-Si-Mn Bronze for Contemporary Art. Prog. Org. Coat. 2019, 127, 286–299. [Google Scholar] [CrossRef]
- Alonso-Villar, E.M.; Rivas, T.; Pozo-Antonio, J.S.; Pellis, G.; Scalarone, D. Efficacy of Colour Protectors in Urban Art Paintings under Different Conditions: From a Real Mural to the Laboratory. Heritage 2023, 6, 3475–3498. [Google Scholar] [CrossRef]
- Macchia, A.; Ruffolo, S.A.; Rivaroli, L.; Malagodi, M.; Licchelli, M.; Rovella, N.; Randazzo, L.; La Russa, M.F. Comparative Study of Protective Coatings for the Conservation of Urban Art. J. Cult. Herit. 2020, 41, 232–237. [Google Scholar] [CrossRef]
- Cianci, C.; Andriulo, F.; Giorgi, R. Formulation of a New Sustainable Hybrid Coating for the Conservation of Street-Art: Characterization and Application. Prog. Org. Coat. 2024, 200, 109026. [Google Scholar] [CrossRef]
- Macchia, A.; Capriotti, S.; Rivaroli, L.; Ruffolo, S.A.; La Russa, M.F. Protection of Urban Art Painting: A Laboratory Study. Polymers 2021, 14, 162. [Google Scholar] [CrossRef]
- Andrés-Herguedas, L.; Pozo-Antonio, J.S.; Alonso-Villar, E.M. Protection of Contemporary Mural Artwork: Evaluation of a Fixing Primer and Colour Protectors. Prog. Org. Coat. 2025, 204, 109252. [Google Scholar] [CrossRef]
- Swarupa, S.; Thareja, P. Techniques, Applications and Prospects of Polysaccharide and Protein Based Biopolymer Coatings: A Review. Int. J. Biol. Macromol. 2024, 266, 131104. [Google Scholar] [CrossRef]
- Janesch, J.; Czabany, I.; Hansmann, C.; Mautner, A.; Rosenau, T.; Gindl-Altmutter, W. Transparent Layer-By-Layer Coatings Based on Biopolymers and CeO2 to Protect Wood from UV Light. Prog. Org. Coat. 2020, 138, 105409. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Hayat, S.; Siddique, M.H.; Aslam, B.; Ashraf, A.; Saqalein, M.; Khurshid, M.; Sarfraz, M.F.; Afzal, M.; Muzammil, S. Chitosan Based Coatings and Films: A Perspective on Antimicrobial, Antioxidant, and Intelligent Food Packaging. Prog. Org. Coat. 2024, 188, 108235. [Google Scholar] [CrossRef]
- Camargos, C.H.M.; Poggi, G.; Chelazzi, D.; Baglioni, P.; Rezende, C.A. Protective Coatings Based on Cellulose Nanofibrils, Cellulose Nanocrystals, and Lignin Nanoparticles for the Conservation of Cellulosic Artifacts. ACS Appl. Nano Mater. 2022, 5, 13245–13259. [Google Scholar] [CrossRef]
- Macchia, A.; Marinelli, L.; Barbaccia, F.I.; de Caro, T.; Hansen, A.; Schuberthan, L.M.; Izzo, F.C.; Pintus, V.; Chiari, K.T.; Francesco, M. Mattel’s ©Barbie: Preventing Plasticizers Leakage in PVC Artworks and Design Objects through Film-Forming Solutions. Polymers 2024, 16, 1888. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; Salzano de Luna, M.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-Based Coatings for Corrosion Protection of Copper-Based Alloys: A Promising More Sustainable Approach for Cultural Heritage Applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Ermolyuk, A.; Avdanina, D.; Khayrova, A.; Lopatin, S.; Shumikhin, K.; Kolganova, T.; Simonenko, N.; Lunkov, A.; Varlamov, V.; Shitov, M.; et al. Search for New Materials Based on Chitosan for the Protection of Cultural Heritage. Herit. Sci. 2024, 12, 330. [Google Scholar] [CrossRef]
- Zhgun, A.; Avdanina, D.; Shagdarova, B.; Nuraeva, G.; Shumikhin, K.; Zhuikova, Y.; Il’ina, A.; Troyan, E.; Shitov, M.; Varlamov, V. The Application of Chitosan for Protection of Cultural Heritage Objects of the 15–16th Centuries in the State Tretyakov Gallery. Materials 2022, 15, 7773. [Google Scholar] [CrossRef]
- Silva, N.C.; Madureira, A.R.; Pintado, M.; Moreira, P.R. Chitosan Coatings Reinforced with Cellulose Crystals and Oregano Essential Oil as Antimicrobial Protection against the Microbiological Contamination of Stone Sculptures. Cellulose 2024, 31, 9825–9845. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef]
- N.Hattawi, S.; Ahmed, A.G.; Fadhil, F.M.; Kuot, S.R.; Alsubaie, M.S.; L.Alazmi, M.; Fetouh, H.A. New Approach for Processing Chitosan as Low Cost Protective Hybrid Coating for C-Steel in Acid Media. Heliyon 2024, 10, e33743. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in Coating Technologies: Unveiling the Potential of Chitosan for the Preservation of Fruits and Vegetables. Int. J. Biol. Macromol. 2024, 254, 127677. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and Applications of Chitosan and Cellulose Composite Materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.Y.; Mubarak, N.M.; Tanjung, F.A. Structure–Property Relationship of Cellulose Nanowhiskers Reinforced Chitosan Biocomposite Films. J. Environ. Chem. Eng. 2017, 5, 6132–6136. [Google Scholar] [CrossRef]
- H.P.S., A.K.; Saurabh, C.K.; A.S., A.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A Review on Chitosan–Cellulose Blends and Nanocellulose Reinforced Chitosan Biocomposites: Properties and Their Applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Dehnad, D.; Emam-Djomeh, Z.; Mirzaei, H.; Jafari, S.-M.; Dadashi, S. Optimization of Physical and Mechanical Properties for Chitosan–Nanocellulose Biocomposites. Carbohydr. Polym. 2014, 105, 222–228. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin Modified Cellulose Nanofiber as a Reinforcing and Co-Antimicrobial Agents in Polylactic Acid /Chitosan Composite Film for Food Packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Membrane Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef]
- Sakai, Y.; Hayano, K.; Yoshioka, H.; Fujieda, T.; Saito, K.; Yoshioka, H. Chitosan-Coating of Cellulosic Materials Using an Aqueous Chitosan-CO2 Solution. Polym. J. 2002, 34, 144–148. [Google Scholar] [CrossRef][Green Version]
- Kang, X.; Kuga, S.; Wang, C.; Zhao, Y.; Wu, M.; Huang, Y. Green Preparation of Cellulose Nanocrystal and Its Application. ACS Sustain. Chem. Eng. 2018, 6, 2954–2960. [Google Scholar] [CrossRef]
- UNI EN ISO 16474-2:2014; Paints and Varnishes-Methods of Exposure to Laboratory Light Sources-Part 2: Xenon-Arc Lamps. International Organization for Standardization: Geneva, Switzerland, 2014.
- Pan, S.; Wu, S.; Kim, J. Preparation of Glucosamine by Hydrolysis of Chitosan with Commercial α-Amylase and Glucoamylase. J. Zhejiang Univ. Sci. B 2011, 12, 931–934. [Google Scholar] [CrossRef]
- Qian, J.; Shi, B.; Mo, L.; Shu, D.; Guo, H. Preparation of Chitooligosaccharides by α-Amylase from Chitosan with Oxidative Pretreatment. J. Chem. Technol. Biotechnol. 2021, 96, 3408–3413. [Google Scholar] [CrossRef]
- Rokhati, N.; Widjajanti, P.; Pramudono, B.; Susanto, H. Performance Comparison of α- and β-Amylases on Chitosan Hydrolysis. ISRN Chem. Eng. 2013, 2013, 186159. [Google Scholar] [CrossRef]
- Tortora, M.; Gherardi, F.; Ferrari, E.; Colston, B. Biocleaning of Starch Glues from Textiles by Means of α-Amylase-Based Treatments. Appl. Microbiol. Biotechnol. 2020, 104, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Nurhaeni, N.; Ridhay, A.; Laenggeng, A.H. Depolymerisation of Chitosan from Snail (Pilla ampullaceae) Field Shell Using α-Amylase. J. Phys. Conf. Ser. 2019, 1242, 012005. [Google Scholar] [CrossRef]
- Pinto, A.P.F.; Rodrigues, J.D. Impacts of Consolidation Procedures on Colour and Absorption Kinetics of Carbonate Stones. Stud. Conserv. 2014, 59, 79–90. [Google Scholar] [CrossRef]
- Valentini, F.; Pallecchi, P.; Relucenti, M.; Donfrancesco, O.; Sottili, G.; Pettiti, I.; Mussi, V.; De Angelis, S.; Scatigno, C.; Festa, G. SiO2 Nanoparticles as New Repairing Treatments toward the Pietraforte Sandstone in Florence Renaissance Buildings. Crystals 2022, 12, 1182. [Google Scholar] [CrossRef]
- Rbihi, S.; Aboulouard, A.; Laallam, L.; Jouaiti, A. Contact Angle Measurements of Cellulose Based Thin Film Composites: Wettability, Surface Free Energy and Surface Hardness. Surf. Interfaces 2020, 21, 100708. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Atari, M.; Parhizkar, M. Polymeric Nanocomposite Structures Based on Functionalised Graphene with Tunable Properties for Nervous Tissue Replacement. ACS Biomater. Sci. Eng. 2021, 7, 4591–4601. [Google Scholar] [CrossRef]
- Atanassova, V.; Ghervase, L.; Cortea, I.M. Laser Removal of Marker Tags from a Contemporary Graffiti Painting. J. Phys. Conf. Ser. 2021, 1859, 012001. [Google Scholar] [CrossRef]
- Marazioti, V.; Douvas, A.M.; Katsaros, F.; Koralli, P.; Chochos, C.; Gregoriou, V.G.; Boyatzis, S.; Facorellis, Y. Chemical Characterisation of Artists’ Spray-Paints: A Diagnostic Tool for Urban Art Conservation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122375. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Tohamy, H.-A.S. Preparation and Infrared Study of Cellulose Based Amphiphilic Materials. Available online: https://www.researchgate.net/publication/326294827_Preparation_and_infrared_study_of_cellulose_based_amphiphilic_materials (accessed on 7 April 2025).
- Geminiani, L.; Campione, F.P.; Corti, C.; Motella, S.; Rampazzi, L.; Recchia, S.; Luraschi, M. Unveiling the Complexity of Japanese Metallic Threads. Heritage 2021, 4, 4017–4039. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Hwang, H.; Jo, H.-S.; Choi, S.; Kim, H.-J.; Kim, S.-E.; Park, K. Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects. Int. J. Mol. Sci. 2021, 22, 4614. [Google Scholar] [CrossRef]
- dos Santos, V.H.J.M.; Pontin, D.; Ponzi, G.G.D.; e Stepanha, A.S.D.G.; Martel, R.B.; Schütz, M.K.; Einloft, S.M.O.; Dalla Vecchia, F. Application of Fourier Transform Infrared Spectroscopy (FTIR) Coupled with Multivariate Regression for Calcium Carbonate (CaCO3) Quantification in Cement. Constr. Build. Mater. 2021, 313, 125413. [Google Scholar] [CrossRef]
- Jha, S.; Mehta, S.; Chen, Y.; Ma, L.; Renner, P.; Parkinson, D.Y.; Liang, H. Correction to “Design and Synthesis of Lignin-Based Flexible Supercapacitors”. ACS Sustain. Chem. Eng. 2020, 8, 9597–9598. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Hadi, I.S.; Yas, R.M. Gamma Radiation Effect on Characterizations of Gold Nanoparticles Synthesized Using Green Method. Iraqi J. Sci. 2022, 63, 4305–4313. [Google Scholar] [CrossRef]
- Jini, D.; Aravind, M.; Alsaiari, N.S.; Alzahrani, F.M.; Sillanpää, M. Investigation on Structural, Optical, Morphological, Electrical, Mechanical and Biological Activities of Pure and Crystal Violet Dye-Doped Epsomite Single Crystals. Journal of materials science. Mater. Electron. 2023, 34, 61. [Google Scholar] [CrossRef]
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. 2018, 130, 7523–7529. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. Evaluation of the Impacts of Four Weathering Methods on Two Acrylic Paints: Showcasing Distinctions and Particularities. Coatings 2019, 9, 121. [Google Scholar] [CrossRef]
- Ortiz-Herrero, L.; Cardaba, I.; Setien, S.; Bartolomé, L.; Alonso, M.L.; Maguregui, M.I. OPLS Multivariate Regression of FTIR-ATR Spectra of Acrylic Paints for Age Estimation in Contemporary Artworks. Talanta 2019, 205, 120114. [Google Scholar] [CrossRef]
- Lakshmi, D.; Rajendran, S.; Sathiyabama, J.; Joseph Rathis, R.; Santhana Prabha, S. Application of Infra-Red Spectroscopy in Corrosion Inhibition Studies. J. Corros. Sci. Eng. 2016, 3, 181–203. [Google Scholar]
- Lomax, S.Q.; Lomax, J.F.; Graham, T.K.; Moore, T.J.T.; Knapp, C.G. Historical Azo Pigments: Synthesis and Characterization. J. Cult. Herit. 2019, 35, 218–224. [Google Scholar] [CrossRef]
- Allen, N.S.; Regan, C.J.; McIntyre, R.; Johnson, B.; Dunk, W.A.E. The Photostabilisation of Water-Borne Acrylic Coating Systems. Polym. Degrad. Stab. 1996, 52, 67–72. [Google Scholar] [CrossRef]
- Pintus, V.; Schreiner, M. Characterization and Identification of Acrylic Binding Media: Influence of UV Light on the Ageing Process. Anal. Bioanal. Chem. 2010, 399, 2961–2976. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.-M.; Ion, M.L.; Niculescu, V.I.R.; Serban, S. Spectral Analysis of Original and Restaurated Ancient Paper from Romanian Gospel. Rom. J. Phys. 2008, 53, 781–791. [Google Scholar]
- Guarnieri, N.; Di Benedetto, A.; Comelli, D.; Mirani, F.; Dellasega, D.; Pagnin, L.; Goidanich, S.; Toniolo, L. Rapid Chromatic Alteration of Street Art: Mechanisms of Deterioration of the Painting Materials of the 20 Years of Freedom and Democracy Mural. Dye. Pigment. 2025, 239, 112733. [Google Scholar] [CrossRef]
- Balakhnina, I.A.; Chikishev, A.Y.; Brandt, N.N. Raman Spectroscopy of Thermo- and Laser-Induced Transformations of Gouache Paint Layer of Copper Phthalocyanine Blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 318, 124430. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Pintus, V.; Schreiner, M. Photostability and Influence of Phthalocyanine Pigments on the Photodegradation of Acrylic Paints under Accelerated Solar Radiation. Polym. Degrad. Stab. 2017, 146, 13–23. [Google Scholar] [CrossRef]
- Bauer, E.M.; De Caro, T.; Tagliatesta, P.; Carbone, M. Unraveling the Real Pigment Composition of Tattoo Inks: The Case of Bi-Components Phthalocyanine Based Greens. Dye. Pigment. 2019, 167, 225–235. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, H.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhang, H.; Fan, D. The Physicochemical Properties of Chitosan Prepared by Microwave Heating. Food Sci. Nutr. 2020, 8, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Queiroz, M.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Abhulimen, K.E.; Adeleke, A.I.; Njoku, C.V.; Amoo, K.O. Production and Characterisation of Derived Composite Biosorbents from Animal Bone. Afr. J. Pure Appl. Chem. 2019, 13, 12–26. [Google Scholar] [CrossRef]
- Kaya, M.; Seyyar, O.; Baran, T.; Turkes, T. Bat Guano as New and Attractive Chitin and Chitosan Source. Front. Zool. 2014, 11, 59. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Surface-Enhanced Raman Scattering (SERS)-Active Substrates from Silver Plated-Porous Silicon for Detection of Crystal Violet. Appl. Surf. Sci. 2015, 331, 241–247. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, T.; Tan, X.; Wu, W.; Tang, Y.; Zhang, H. A Facile Surface-Enhanced Raman Scattering (SERS) Detection of Rhodamine 6G and Crystal Violet Using Au Nanoparticle Substrates. Appl. Surf. Sci. 2015, 347, 569–573. [Google Scholar] [CrossRef]
- Badawi, H.M. Vibrational Spectra and Assignments of 2-Phenylethanol and 2-Phenoxyethanol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Fremout, W.; Saverwyns, S. Identification of Synthetic Organic Pigments: The Role of a Comprehensive Digital Raman Spectral Library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Germinario, G.; van der Werf, I.D.; Sabbatini, L. Chemical Characterisation of Spray Paints by a Multi-Analytical (Py/GC–MS, FTIR, μ-Raman). Approach. Microchem. J. 2016, 124, 929–939. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Moens, L.; Edwards, H.G.M.; Dams, R. Raman Spectroscopic Database of Azo Pigments and Application to Modern Art Studies. J. Raman Spectrosc. 2000, 31, 509–517. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Mokhtar, S.M.; Soon, C.F.; Nafarizal, N.; Suriani, A.B.; Mohamed, A.; Mamat, M.H.; Malek, M.F.; Shimomura, M.; Murakami, K. Raman Investigation of Rutile-Phased TiO2 Nanorods/Nanoflowers with Various Reaction Times Using One Step Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2016, 27, 7920–7926. [Google Scholar] [CrossRef]
- Sacco, A.; Mandrile, L.; Tay, L.-L.; Itoh, N.; Raj, A.; Moure, A.; Del Campo, A.; Fernandez, J.F.; Paton, K.R.; Wood, S.; et al. Quantification of Titanium Dioxide (TiO2) Anatase and Rutile Polymorphs in Binary Mixtures by Raman Spectroscopy: An Interlaboratory Comparison. Metrologia 2023, 60, 055011. [Google Scholar] [CrossRef]
- Chen, G. Rapid Synthesis of Rutile TiO2 Powders Using Microwave Heating. J. Alloys Compd. 2015, 651, 503–508. [Google Scholar] [CrossRef]
- Borowicz, P.; Taube, A.; Rzodkiewicz, W.; Latek, M.; Gierałtowska, S. Raman Spectra of High-κ Dielectric Layers Investigated with Micro-Raman Spectroscopy Comparison with Silicon Dioxide. Sci. World J. 2013, 2013, e208081. [Google Scholar] [CrossRef] [PubMed]
- Fridrichová, J.; Bačík, P.; Illášová, Ľ.; Kozáková, P.; Škoda, R.; Pulišová, Z.; Fiala, A. Raman and Optical Spectroscopic Investigation of Gem-Quality Smoky Quartz Crystals. Vib. Spectrosc. 2016, 85, 71–78. [Google Scholar] [CrossRef]
- Bouchard, M.; Rivenc, R.; Menke, C.; Learner, T. Micro-FTIR and Micro-RAMAN Study of Paints Used by Sam Francis. Carrie Menke. Available online: https://ucmerced.d8.theopenscholar.com/cmenke/publications/micro-ftir-and-micro-raman-study-paints-used-sam-francis (accessed on 31 March 2025).
- Tian, Y.; Ya, G.; Qiang, L.; Maoling, T.; Xiaodong, S. Removal and recovery of triphenylmethane dyes from wastewater with temperature-sensitive magnetic ionic liquid aqueous two-phase system. J. Clean. Prod. 2021, 328, 129648. [Google Scholar] [CrossRef]
- Freeman, H.S.; Peters, A.T. Colorants for Non-Textile Applications; Elsevier: Amsterdam, The Netherlands, 2000; ISBN 978-0-444-82888-0. Available online: https://www.sciencedirect.com/book/9780444828880/colorants-for-non-textile-applications (accessed on 7 April 2025).
- Zamalloa Jara, M.A.; Luízar Obregón, C.; Araujo Del Castillo, C. Exploratory Analysis for the Identification of False Banknotes Using Portable X-Ray Fluorescence Spectrometer. Appl. Radiat. Isot. 2018, 135, 212–218. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I. Investigating the Fragmentation Pathways of β-Naphthol Pigments by Liquid Chromatography—Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8789. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, T.; Zhou, C. Modification study involving a Naphthol as red pigment. Dye. Pigment. 2000, 44, 75–80. [Google Scholar] [CrossRef]
- Christie, R.; Abel, A. Phthalocyanine Pigments: General Principles. Phys. Sci. Rev. 2021, 6, 671–677. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Diab, H.A.; Adams, J. Preparation and Characterization of UV Curable-Encapsulated Phthalocyanine Blue Pigment. Prog. Org. Coat. 2015, 84, 70–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).