Abstract
Robotic walking assistance devices support the rehabilitation of patients with neurological impairments. However, most commercialized systems rely on treadmill-based walking, which may not reflect real-world environments. This study aimed to evaluate the usability of a newly developed over-ground walking assistance robot (OWAR-MW) based on mecanum wheels compared with a commercial system (Andago) from the perspectives of physical therapists and patients with stroke. Nine physical therapists and nine stroke patients participated. Each participant walked 100 m using both the OWAR-MW and Andago systems. Subsequently, a satisfaction survey was conducted across three categories—safety, operability and functionality, and convenience—using a questionnaire adapted from the standard usability testing guidelines for walking assistive devices. Additionally, in-depth interviews were conducted to explore user experience and improvement needs. In both participant groups, the OWAR-MW showed a tendency for lower satisfaction scores than Andago across all categories. Stroke patients reported significantly lower scores in all three categories (safety: 4.90 vs. 4.04, operability and functionality: 4.83 vs. 4.33, convenience: 4.87 vs. 4.49, p < 0.05), whereas therapists noted a significant difference only in safety (4.02 vs. 3.37, p < 0.05). Key issues identified included a lack of handles, delay in actuator response, low motion detection sensitivity, non-intuitive controls, and discomfort caused by the harness, particularly the thigh straps. OWAR-MW demonstrated usability limitations in its current prototype form. Technical improvements in user interface, control accuracy, and harness design are necessary before clinical application. This study provides valuable feedback for the future development of user-centered rehabilitation robotics.
1. Introduction
Stroke is a neurological condition caused by the obstruction of cerebral blood flow or damage to cerebral blood vessels. As of 2022, more than 630,000 stroke cases have been reported in South Korea [1]. Post-stroke gait disturbances often include asymmetric gait patterns, impaired balance, and reduced weight-bearing on the paretic limb, leading to functional disability and limited ambulation [2,3]. Gait deficits cannot be improved solely with pharmacological treatment; therefore, neurologically focused physical therapy is required [4]. Traditionally, therapists have employed manual assistance or body weight-supported treadmill training to improve gait. However, these methods have limitations, such as a high physical burden on therapists and insufficient precision or repeatability [5].
Robotic gait assistance systems have been actively developed [6]. Robot-assisted walking therapy has shown promise in aiding patients to walk longer distances and improving endurance, balance, and confidence [7]. A recent Cochrane systematic review reported that combining electromechanical body weight-supported gait devices with physical therapy can significantly enhance the independent walking ability in patients with stroke [8]. Currently, the most commonly used robotic system in clinical practice is exoskeleton-assisted treadmill walking. However, treadmill-based walking does not fully replicate over-ground ambulation, particularly concerning speed adaptation, directional changes, or sensory stimuli from ground contact, factors essential for community reintegration following discharge [9,10].
To address this, over-ground walking assistance robots with body weight support harnesses and motorized tracking capabilities have been developed [11]. One representative example is Andago, a commercial device featuring a mobile support frame and dynamic weight support system [12], which enables patients to walk while preventing falls and reducing physical stress. Previous studies have demonstrated its effectiveness in improving inter-joint coordination during walking in children with gait impairments [13], and reduced fear of falling of stroke patients [12]. Over-ground walking devices provide realistic gait training conditions that promote dynamic balance strategies, and the functional adaptability required for daily mobility [13]. These systems reduce the burden on therapists while enabling quantitative and repetitive training [6,11]. Consistent with global trends, South Korea has actively developed robotic gait trainers. The over-ground walking assistive robot based on the mecanum wheel (OWAR-MW) is a Korean-developed prototype that offers the functionality of Andago with added omnidirectional mobility.
The OWAR-MW recognizes user intentions, provides partial body weight support, and tracks movement in multiple directions using mecanum wheels, allowing forward, lateral, diagonal, and rotational movements. However, the OWAR-MW remains in the prototype stage, and prior to commercialization, its safety, operability, and usability must be rigorously evaluated using user-centered testing [14]. In addition, technical refinements should be guided by specific challenges identified through comprehensive user experience analysis [15]. Sabino et al. (2023) emphasized that the success of wearable systems in clinical ergonomics depends not only on accurate sensor feedback but also on non-intrusiveness, comfort, and intuitive operations, which contribute to high usability [16]. Similarly, in walking-assistive robots, patients’ and clinicians’ feedback on ease of use, stability, and comfort is essential for translating prototypes into clinically viable tools. Additionally, evaluating usability through structured assessments involving both patients and therapists can provide key insights for refining device design [17]. Therefore, this study assessed the usability of the OWAR-MW compared to commercial Andago through satisfaction surveys and in-depth interview. Specifically, this study was the first usability test for the OWAR-MW prototype and aimed to derive advanced improvement requirements from the user perspective (stroke patients and physical therapists) for the future commercialization of OWAR-MW through satisfaction surveys and in-depth interviews.
2. Methods
2.1. Participants
The general characteristics of the participants are listed in Table 1. Eighteen participants were recruited for this study: nine physical therapists and nine patients with stroke. The therapists were employed at rehabilitation hospitals and had experience with commercial ground robotic gait training systems. The mean clinical expertise of all therapists was 5.33 years. The inclusion criteria for patients with strokes were as follows: individuals diagnosed with stroke via CT or MRI, those who scored ≥ 24 on the Korean version of the Mini-Mental State Examination (MMSE-K), those with a Functional Ambulation Category (FAC) score of 2 or 3, and those capable of walking independently for at least 2 min with an assistive device. The exclusion criteria included the presence of other neurological disorders, such as brain tumors or traumatic brain injury, visual field deficits that impair walking, orthopedic diseases or surgical histories that could interfere with gait, and skin lesions that would hinder the safe use of the harness system. All participants provided written informed consent before participation. The study was approved by the Institutional Review Board of the Korea National University of Transportation (Approval No.: KNUT-2024-HR-26-23). This study was conducted in accordance with the principles of the Declaration of Helsinki.

Table 1.
General characteristics of the participants (n = 18).
2.2. Procedure
A usability test was conducted by comparing two robotic walking devices: a commercialized over-ground walking assistance robot (COWAR, Andago) and the newly developed OWAR-MW. The procedure of the usability test is illustrated in Figure 1.
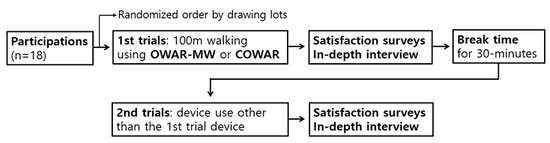
Figure 1.
Flowchart of usability test.
All participants were instructed to walk freely for a total distance of 100 m using both devices. The sequence of device usage was randomly assigned by drawing lots, and a 30 min break was given between the use of the first and second devices. The test was performed in a rectangular corridor measuring 15 × 35 m2. During each trial, the participants wore a harness that provided 10% body weight support. While walking, each robotic system detects the user’s movement intention in real-time and assists in walking by tracking and following the user’s trajectory. No time limit was imposed to complete the 100 m walk. A trained assistant accompanied each participant for safety, offering physical support only in emergencies. Additionally, the participants with stroke used an additional walking aid (a cane) while using the two types of ground-based walking robots if they wished. The walking task was conducted independently using a robotic assistance system. After completing the walking task with each device, the participants completed a usability satisfaction questionnaire covering three domains: safety, operability and functionality, and convenience. Following the survey, one-on-one in-depth interviews were conducted to explore participants’ subjective experiences, perceived limitations, and suggestions for device improvement. The same procedure was applied to both robotic systems.
2.3. Commercialized Over-Ground Walking Assistance Robot (COWAR)
Andago (Hocoma AG, Volketswil, Switzerland) was used as the commercial over-ground walking assistance robot [18]. Andago, a ground-based gait-training system that combines a dynamic body weight support system with a mobile frame and automated tracking, is commonly used in the neurological rehabilitation of patients with gait impairments, such as recovering from stroke or spinal cord injuries (Figure 2A). Andago provides partial body weight support through a motorized harness and allows for natural vertical movement during ambulation, encouraging gait patterns closely resembling over-ground walking. The system is equipped with sensors that detect the user’s movement in real-time, enabling the device to automatically follow the user’s travel direction. This design allows users to walk freely without the restrictions of exoskeletal robotics while receiving fall prevention and body weight support. Unlike treadmill-based systems, Andago allows unrestrained movement across indoor spaces, offering a more realistic walking environment to enhance clinical rehabilitation outcomes.
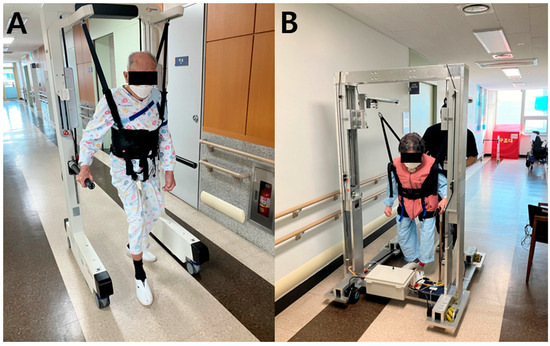
Figure 2.
Patient with stroke undergoing a usability test of COWAR (A) and OWAR-MW (B) during over-ground walking. All participants were instructed to walk freely for a total distance of 100 m using both COWAR and OWAR-MW.
2.4. Over-Ground Walking Assistance Robotic Device Based on Mecanum Wheel (OWAR-MW)
The OWAR-MW is a Korean-developed over-ground walking assistance robot designed to support gait rehabilitation in individuals with neurological impairments, such as stroke (Figure 2B). The device features a motorized body weight support system integrated with a mobile base driven by mecanum wheels. The body weight support system uses a motorized harness to provide a quantified level of support, reduce the risk of falls, and promote psychological stability during gait training. The movement intention and direction were detected using cable sensors connected to the upper portion of the harness, allowing the robot to track the user’s motion and provide responsive assistance. The key differentiating feature of the OWAR-MW is its omnidirectional mobility enabled by a mecanum wheel drive system, which allows forward, lateral, diagonal, and in-place rotational movements, enhancing the robot’s ability to assist users in narrow or irregular spaces. The system was designed to closely follow user-initiated motions with improved flexibility and spatial adaptability.
2.5. Usability Test
This study evaluated usability via a satisfaction survey and one-on-one in-depth interviews. The satisfaction survey was conducted after the participants walked freely for 100 m using the COWAR and OWAR-MW devices. To ensure comparability, the same satisfaction questionnaire was administered to both stroke patients and physical therapists for each device. The satisfaction questionnaire consisted of 21 items distributed across three domains: safety, operability and functionality, and convenience. The safety domain included seven items addressing operational safety and fall risk during device use. The operability and functionality domains comprised seven items evaluating ease of control and functional performance. The convenience domain also contained seven items that assessed overall user comfort during use. Each item was rated on a 5-point Likert scale (1 = very dissatisfied, 5 = very satisfied). A satisfaction questionnaire was developed by modifying and supplementing the usability test guidelines for a walking assistive device, provided by the Support Center for Senior-Friendly Industry of the Korea Health Industry [19]. According to these guidelines, average satisfaction scores were interpreted as follows: ≥4.5 indicates adequate, 4.0–4.4 indicates improvement required as needed, 3.0–3.9 indicates improvement recommended, 2.0–2.9 indicates improvement required, and ≤1.9 indicates improvement essential.
In-depth interviews were used to derive specific improvement requirements for the OWAR-MW device compared to the commercial system (COWAR). Each in-depth interview was conducted within 30 min per participant.
2.6. Data Analysis
Statistical analyses were performed using SPSS software (version 27.0; IBM Corp., Armonk, NY, USA). The participants’ general characteristics are presented using descriptive statistics and frequency analysis. The Wilcoxon Signed-Rank Test was used to compare satisfaction between the COWAR and OWAR-MW groups. The significance level was set at p < 0.05.
3. Results
3.1. Satisfaction Results for COWAR and OWAR-MW in Physical Therapists and Patients with Stroke
Table 2 presents the satisfaction levels reported by physical therapists and patients with stroke after walking with the COWAR and OWAR-MW. In all participants (physical therapists and patients with stroke), the OWAR-MW showed a tendency towards lower average satisfaction scores than the COWAR in the three categories (safety, operability and functionality, and convenience). Specifically, in terms of the satisfaction for physical therapists, the OWAR-MW showed significantly lower satisfaction scores in only the safety category (4.02 vs. 3.37, p < 0.05) than the COWAR. However, in the satisfaction for patients with stroke, the OWAR-MW showed significantly lower satisfaction scores in all three categories (safety: 4.90 vs. 4.04, operability and functionality: 4.83 vs. 4.33, convenience: 4.87 vs. 4.49, p < 0.05) than the COWAR.

Table 2.
Comparison of satisfaction scores between COWAR and OWAR-MW among physical therapists and patients with stroke (n = 18).
3.2. Main Findings for In-Depth Interviews
Table 3 summarizes the main findings of the in-depth interviews. The participants identified various limitations of the OWAR-MW. From a safety perspective, both groups reported concerns regarding foot entrapment risks, the absence of handles, and delayed actuator responses. Regarding operability and functionality, the participants highlighted the time lag between motion detection and actuator movement, low detection sensitivity, and non-intuitive operation. For convenience, the harness application was complex and uncomfortable, especially because of the restrictive thigh straps.

Table 3.
Main findings of in-depth interviews (n = 18).
4. Discussion
Andago, one of the most widely used over-ground walking-assistance robots in clinical settings, supports natural weight shifting during walking through dynamic body weight support and offers a safe environment for practicing a wide range of real-life walking scenarios, such as turning, stopping, and obstacle avoidance [11]. In addition, its frame is smaller than that of standard door frames, allowing for better spatial accessibility, and users can walk with greater naturalness owing to hands-free operations [20]. These mechanical advantages contribute to improved gait ability in patients with stroke [21] and a reduced fear of falling in older adults [22]. Accordingly, this study conducted a usability evaluation (satisfaction survey and in-depth interview) to compare the newly developed OWAR-MW with the commercial COWAR system (Andago), focusing on three categories: safety, operability and functionality, and convenience, as perceived by physical therapists and patients with stroke.
The satisfaction survey indicated that the OWAR-MW showed a tendency towards lower average satisfaction scores than COWAR in all three categories among all participants. In particular, the average scores in the safety category were significantly lower for OWAR-MW than for COWAR for physical therapists (4.02 vs. 3.37, −0.65, 16.21%) and patients with stroke (4.90 vs. 4.04, −0.87, 17.80%). As Andago has undergone structural optimization through commercialization and has demonstrated clinical and mechanical safety in previous studies, users are likely to feel more confident when using it [4,12,20]. In contrast, the OWAR-MW appeared to induce a sense of insecurity due to incomplete structural features (absence of handles) and technical immaturity (delays in motion tracking and control). Unlike Andago, OWAR-MW does not have handles and requires users to rely solely on the harnesses for support, which may cause feelings of instability. To ensure smooth and stable mobility in various terrains, walking-assistive robots require advanced control algorithms that precisely detect and respond to user movements [23]. However, in the OWAR-MW, delays and low precision between the sensor system and the actuator resulted in discrepancies between the user-intended direction and the robot’s movement during straight walking, turning, and in-place rotation. These structural (e.g., handle addition) and technical (e.g., improved sensor-based control algorithms) factors that negatively affect the perceived safety should be prioritized in further developments for commercialization.
In terms of operability and functionality and convenience, patients with stroke rated the OWAR-MW significantly lower than the COWAR (operability and functionality: 4.83 vs. 4.33, −0.49, 10.20%; convenience: 4.87 vs. 4.49, −0.38, 7.82%). In contrast, physical therapists did not show substantial differences in satisfaction with these categories, suggesting that the OWAR-MW falls short of the clinical expectations of end-users with mobility impairments in terms of ease of operation and functional completeness. Lower satisfaction among patients with stroke seems to be attributable to a lack of intuitiveness, non-user-centered control systems, and difficulty in putting on and adjusting devices. In-depth interviews also revealed recurring concerns from patients regarding the difficulty in changing direction or delays in stopping when intended. Therapists generally rated operability and functionality more favorably, possibly due to their greater familiarity and adaptability. In particular, it is important to note the difference in perspective between the two groups of participants. While physical therapists did use the devices during testing, their feedback likely reflects a clinical evaluative standpoint—considering how the device would perform in rehabilitation practice—rather than purely personal comfort or ease. In contrast, the stroke patients’ feedback stems from direct, personal use as end-users with mobility impairments. This difference may better explain why therapists reported smaller drops in satisfaction for operability and convenience; they could adapt to the device’s interface or judge it acceptable for clinical use, whereas patients experienced more immediate challenges (e.g., difficulty with controls or discomfort) that lowered their ratings. This study was the first usability evaluation conducted for the commercialization of OWAR-MW, assessing user satisfaction from the perspectives of stroke patients and physical therapists. Accordingly, despite the differing viewpoints of the two groups, a single questionnaire was applied to provide a common evaluation framework; however, this approach may have oversimplified the unique concerns of each group. In future studies, tailored questionnaires or separate rating scales for different stakeholders could better capture the nuances of each group’s experience. Additionally, according to previous research, to maximize the contribution of assistive technology to enhance the independence and quality of life of individuals with disabilities, it is essential to actively incorporate user feedback during the development phase through a user-centered design approach [16,17,24]. Thus, the future development of the OWAR-MW must emphasize user-centered design by improving the precision and responsiveness of motion tracking, simplifying donning and adjustment procedures and enhancing the intuitiveness of the user interface.
Another notable finding is that the quantitative (satisfaction survey) and qualitative (in-depth interviews) results of this study showed consistent interpretations. Survey data (Table 2) indicated that among stroke patients, the largest drops in satisfaction scores with OWAR-MW—compared to COWAR—were in items related to directional changes, in-place rotation, and stopping control, suggesting that the safety and responsiveness of the device in dynamic conditions require particular attention. Similarly, interview findings (Table 3) revealed recurring concerns about delayed actuator response and low motion detection sensitivity, which explain the observed dissatisfaction in control and movement synchronization. The lack of intuitive operation and harness-related discomfort—especially due to restrictive thigh straps—were also repeatedly highlighted in interviews, aligning with the lower convenience scores among patients. These integrated findings emphasize the need to refine OWAR-MW’s sensing precision, simplify the control interface, and enhance physical comfort to ensure its viability in clinical practice.
This study has some limitations. First, the sample size was small, restricting the generalizability of the findings. Second, this usability test was based on a single-session trial and, therefore, did not capture long-term adaptation, experience, or training effects. Potential changes in user satisfaction, fatigue, and adverse effects due to repeated clinical use warrant further studies. Third, the evaluation relied on subjective surveys and interviews; therefore, individual perceptions and experiences might have influenced the results. More objective assessments should include quantitative indicators, such as control accuracy, response time, walking distance, and duration. Finally, while COWAR is a commercially available device with established stability and clinical application experience, OWAR-MW remains at the prototype stage, introducing inherent differences in the evaluation conditions. These differences might have affected satisfaction outcomes, necessitating a more refined comparative framework in future development stages.
5. Conclusions
This study conducted a usability comparison between OWAR-MW, an over-ground walking assistance robot developed in Korea, and the commercially available Andago (COWAR), to evaluate the former’s potential for clinical application. Based on satisfaction surveys and in-depth interviews with physical therapists and patients with stroke, the OWAR-MW was rated lower than the COWAR in safety, operability and functionality, and convenience, with a particularly marked decline in satisfaction among patients with strokes. This result may stem from multiple factors, including the lack of structural features (e.g., absence of handles), discomfort associated with the harness system, the low precision of the control algorithm, and insufficient user-centered interface design. These findings suggest that technical improvements tailored to user needs are essential for the future commercialization of OWAR-MWs. In particular, improving the accuracy of real-time motion tracking, developing a control interface based on user intuition, and enhancing the comfort and anatomical fit of the harness system are critical in increasing the clinical applicability and user acceptance of the device. In addition, future studies should conduct more comprehensive usability evaluations that include long-term usage effects, performance in various real-world environments, and quantitative analyses of gait and mobility outcomes. This study provided foundational user-based evidence for the clinical advancement of OWAR-MWs. Further studies should examine long-term usability and clinical outcomes in larger and more diverse populations.
Author Contributions
Conceptualization, K.C.; methodology, K.C.; investigation, E.C.; writing—original draft preparation, D.H.; writing—review and editing, K.C.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Changwon Medical-Bio Advanced Device Manufacturing Industry Promotion Project 2023.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Korea National University of Transportation (Approval No.: KNUT-2024-HR-26-23).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Health Insurance Review & Assessment Service (HIRA). Current Status of Treatment for Cerebrovascular Diseases; Health Insurance Review & Assessment Service (HIRA): Wonju, Republic of Korea, 2023.
- Kelly-Hayes, M.; Beiser, A.; Kase, C.S.; Scaramucci, A.; D’Agostino, R.B.; Wolf, P.A. The influence of gender and age on disability following ischemic stroke: The Framingham study. J. Stroke Cerebrovasc. Dis. 2003, 12, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM & R 2014, 6, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.; Schweinfurther, R.; Dewor, A.; Huster, T.; Paredes, L.P.; Zutter, D.; Möller, J.C. The Andago for overground gait training in patients with gait disorders after stroke—Results from a usability study. Physiother. Res. Rep. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Koceska, N.; Koceski, S. Robot devices for gait rehabilitation. Int. J. Comput. Appl. 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.S.; Neo, E.J.; Tam, M.M.; Tan, P.L. Post-critical care COVID-19 patient benefits from a robotic patient-guided suspension system for pulmonary rehabilitation. Ann. Acad. Med. Singap. 2020, 49, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef] [PubMed]
- Hidler, J.; Brennan, D.; Black, I.; Nichols, D.; Brady, K.; Nef, T. ZeroG: Overground gait and balance training system. J. Rehabil. Res. Dev. 2011, 48, 287–298. [Google Scholar] [CrossRef]
- Schmidt, H.; Hesse, S.; Berhardt, R.; Kruger, J. HapticWalker—A novel haptic foot device. ACM Trans. Appl. Percept. 2005, 2, 166–180. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Ciobanu, I.; Badea, D.I.; Iliescu, A.; Pizzamiglio, S.; Schauer, T.; Seel, T.; Seiciu, P.L.; Turner, D.L.; Berteanu, M. Advanced technology for gait rehabilitation: An overview. Adv. Mech. Eng. 2018, 10, 1687814018783627. [Google Scholar] [CrossRef]
- Fay, A.; Synott, E.; McDaid, E.; Barrett, E. A comparison of the immediate effects of the Andago over ground body weight support trainer versus over ground walking on selected gait parameters in a post-acute rehabilitation population. Physiother. Theory Pract. 2024, 40, 767–777. [Google Scholar] [CrossRef] [PubMed]
- van Hedel, H.J.; Rosselli, I.; Baumgartner-Ricklin, S. Clinical utility of the over-ground bodyweight-supporting walking system Andago in children and youths with gait impairments. J. Neuroeng. Rehabil. 2021, 18, 29. [Google Scholar] [CrossRef]
- Li, X.Y.; An, Q.; Choi, K.R. A study on behavior analysis and scenario-based furniture design through user observation—Focus on seniors walker design. Korean Soc. Sci. Art 2023, 41, 403–413. [Google Scholar] [CrossRef]
- Kim, J. Efforts and challenges for medical robots in South Korea. J. Inst. Control Robot. Syst. 2024, 30, 423–427. [Google Scholar] [CrossRef]
- Sabino, I.; do Carmo Fernandes, M.; Cepeda, C.; Quaresma, C.; Gamboa, H.; Nunes, I.L.; Gabriel, A.T. Ergo4workers: Usability testing of the second prototype of an app for the ergonomic assessment of healthcare professionals. In Occupational and Environmental Safety and Health V, Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2023; pp. 99–108. [Google Scholar] [CrossRef]
- Moustris, G.; Kardaris, N.; Tsiami, A.; Chalvatzaki, G.; Koutras, P.; Dometios, A.; Oikonomou, P.; Tzafestas, C.; Maragos, P.; Efthimiou, E.; et al. The i-walk lightweight assistive rollator: First evaluation study. Front. Robot. AI 2024, 100, 103570. [Google Scholar] [CrossRef]
- Hocoma. Andago–Robotic Gait Training. Available online: https://www.hocoma.com/solutions/andago/ (accessed on 1 April 2025).
- The Support Center for Senior Friendly Industry Under the Korea Health Industry Development Institute. Available online: https://www.khidi.or.kr/board?menuId=MENU00310&siteId=SITE00003 (accessed on 1 April 2025).
- van Hedel, H.J.; Bulloni, A.; Gut, A. Prefrontal cortex and supplementary motor area activation during robot-assisted weight-supported over-ground walking in young neurological patients: A pilot fNIRS study. Front. Rehabil. Sci. 2021, 2, 788087. [Google Scholar] [CrossRef]
- San Tay, S.; Visperas, C.A.; Abideen, A.B.Z.; Tan, M.M.J.; Zaw, E.M.; Lai, H.; Neo, E.J.R. Effectiveness of adjunct robotic therapy with a patient-guided suspension system for stroke rehabilitation using a 7-days-a-week model of care: A comparison with conventional rehabilitation. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100144. [Google Scholar] [CrossRef]
- Fay, A.; Synott, E.; McDaid, E.; Barrett, E. The influence of the ANDAGO® on gait parameters among older adults in the post-acute rehabilitation setting: A pilot study. Age Ageing 2022, 51, afac218.091. [Google Scholar] [CrossRef]
- Cowan, R.E.; Fregly, B.J.; Boninger, M.L.; Chan, L.; Rodgers, M.M.; Reinkensmeyer, D.J. Recent trends in assistive technology for mobility. J. Neuroeng. Rehabil. 2012, 9, 20. [Google Scholar] [CrossRef]
- Demiral, D.G. Emerging assistive technologies and challenges encountered. In Current Studies in Technology, Innovation and Entrepreneurship; ISRES Publisher: Konya, Turkey, 2023; Volume 1, pp. 1–21. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).