Plasma-Assisted Decoration of Gold Nanoparticles on Bioinspired Polydopamine Nanospheres as Effective Catalyst for Organic Pollutant Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
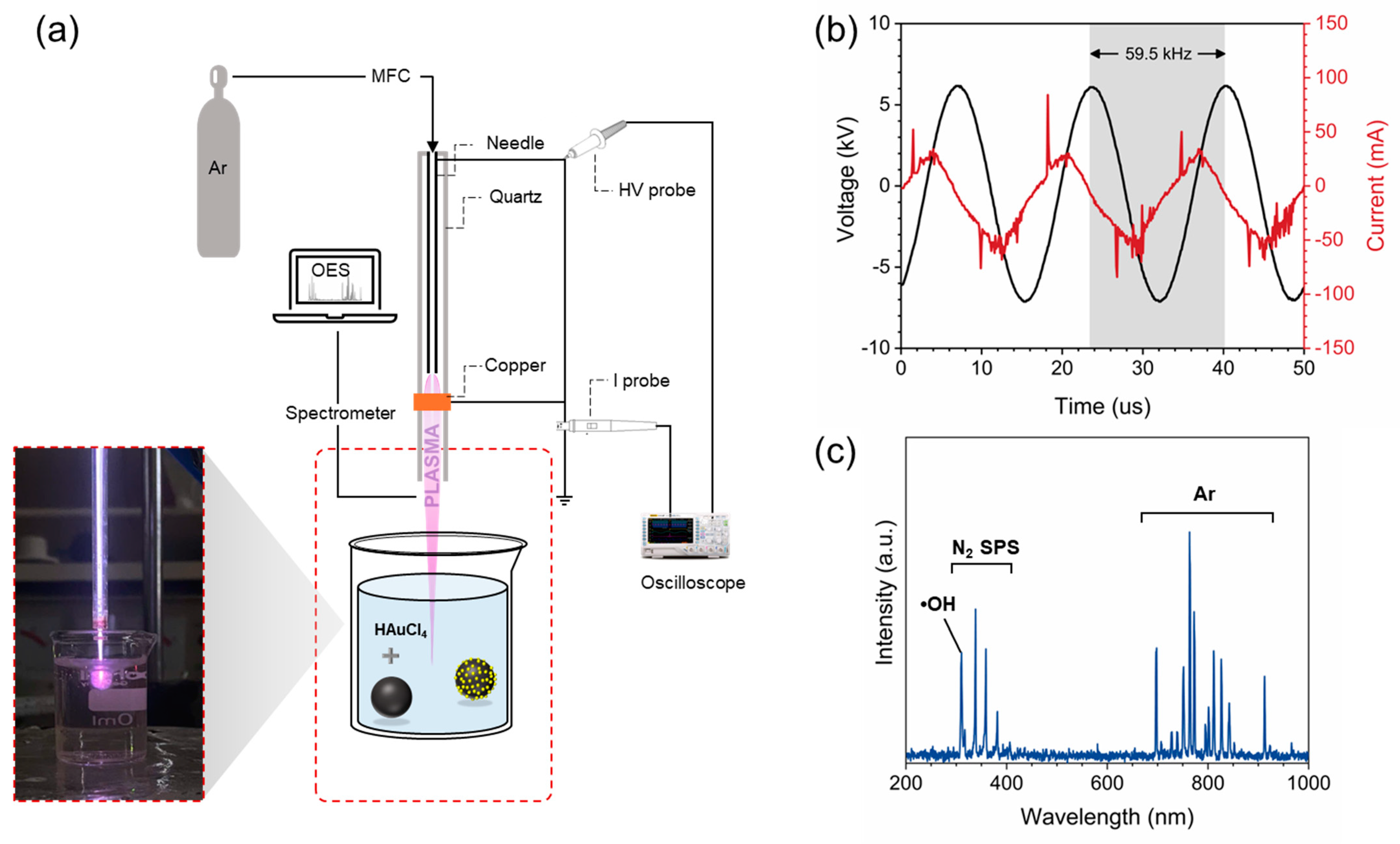
2.2. Plasma Experimental Setup
2.3. Plasma Characterizations
2.4. Synthesis of Polydopamine PDA Nanospheres
2.5. Decoration of PDA with AuNPs by Plasma–Liquid Interaction
2.6. Nanoparticle Characterizations
2.7. Catalytic Reduction
3. Results and Discussion
3.1. Plasma System Characterizations
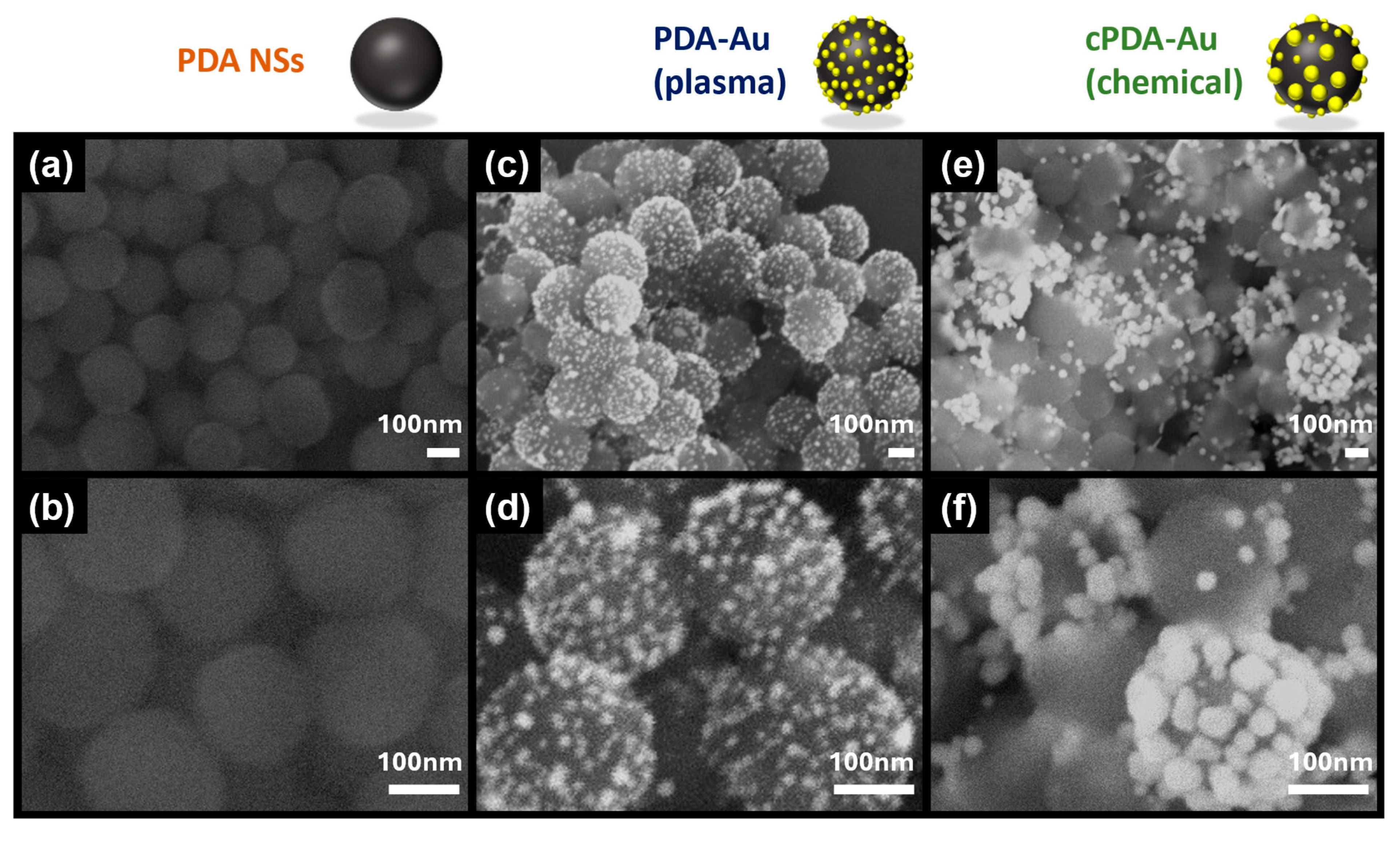
3.2. Morphological and Structural Characterizations
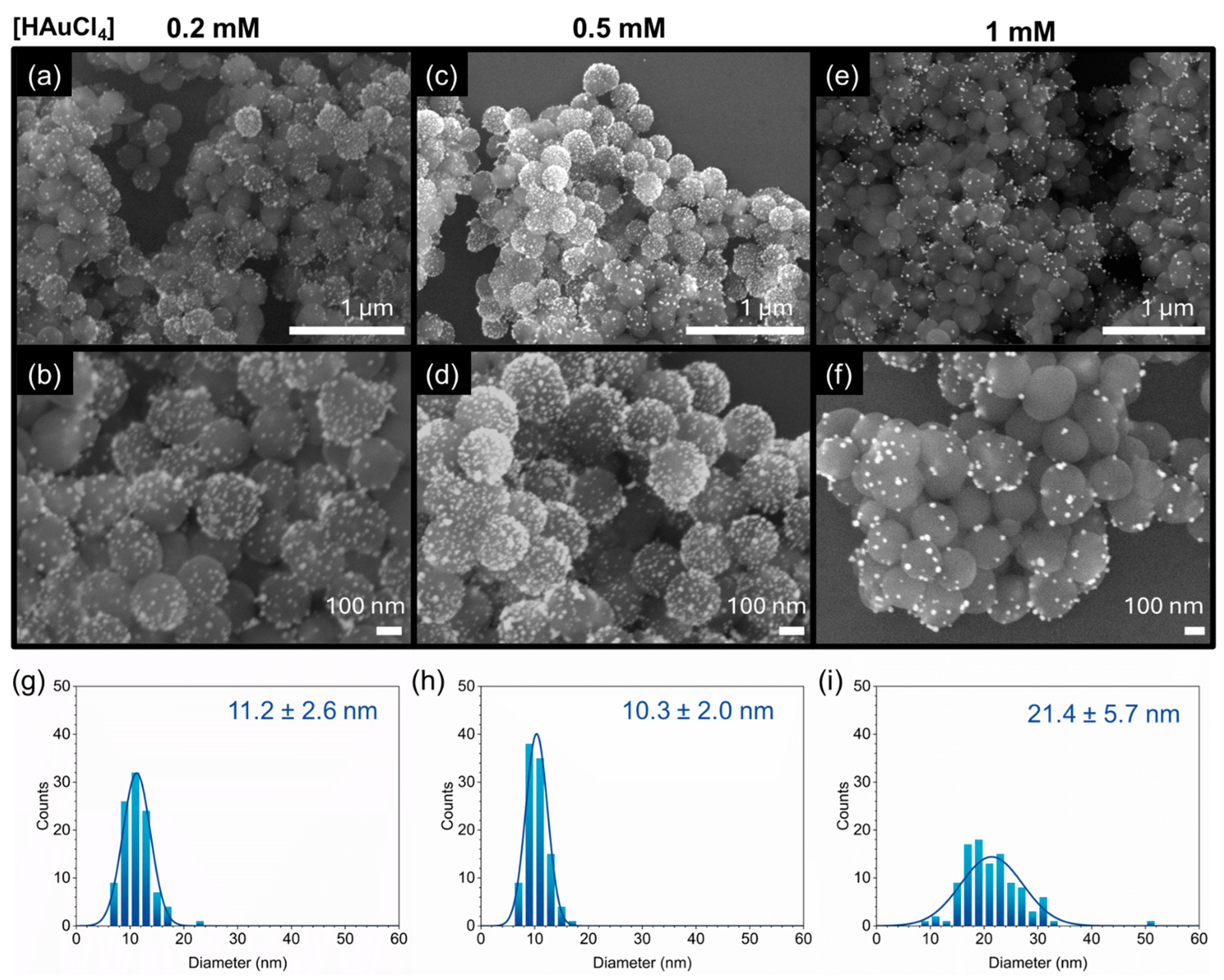
3.3. Impact of HAuCl4 Concentrations on the Morphology of PDA-Au
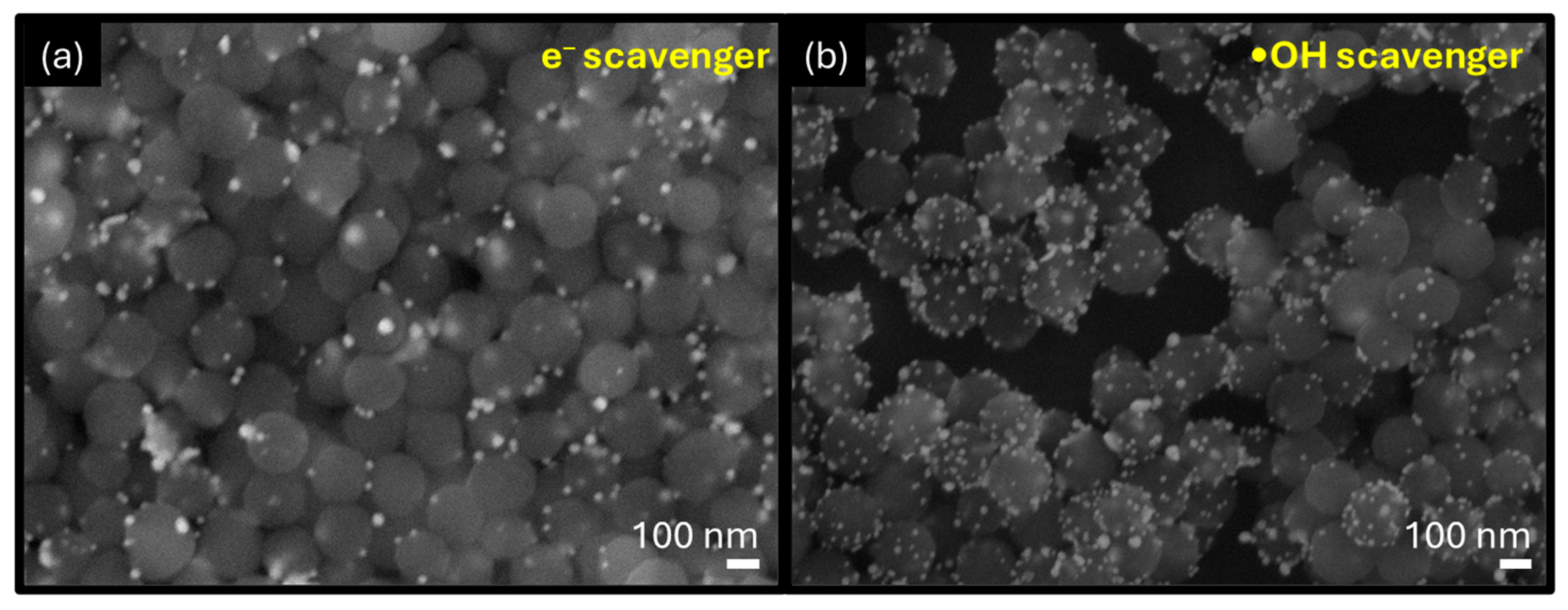
3.4. Insight into Formation of PDA-Au by Plasma-Assisted Method
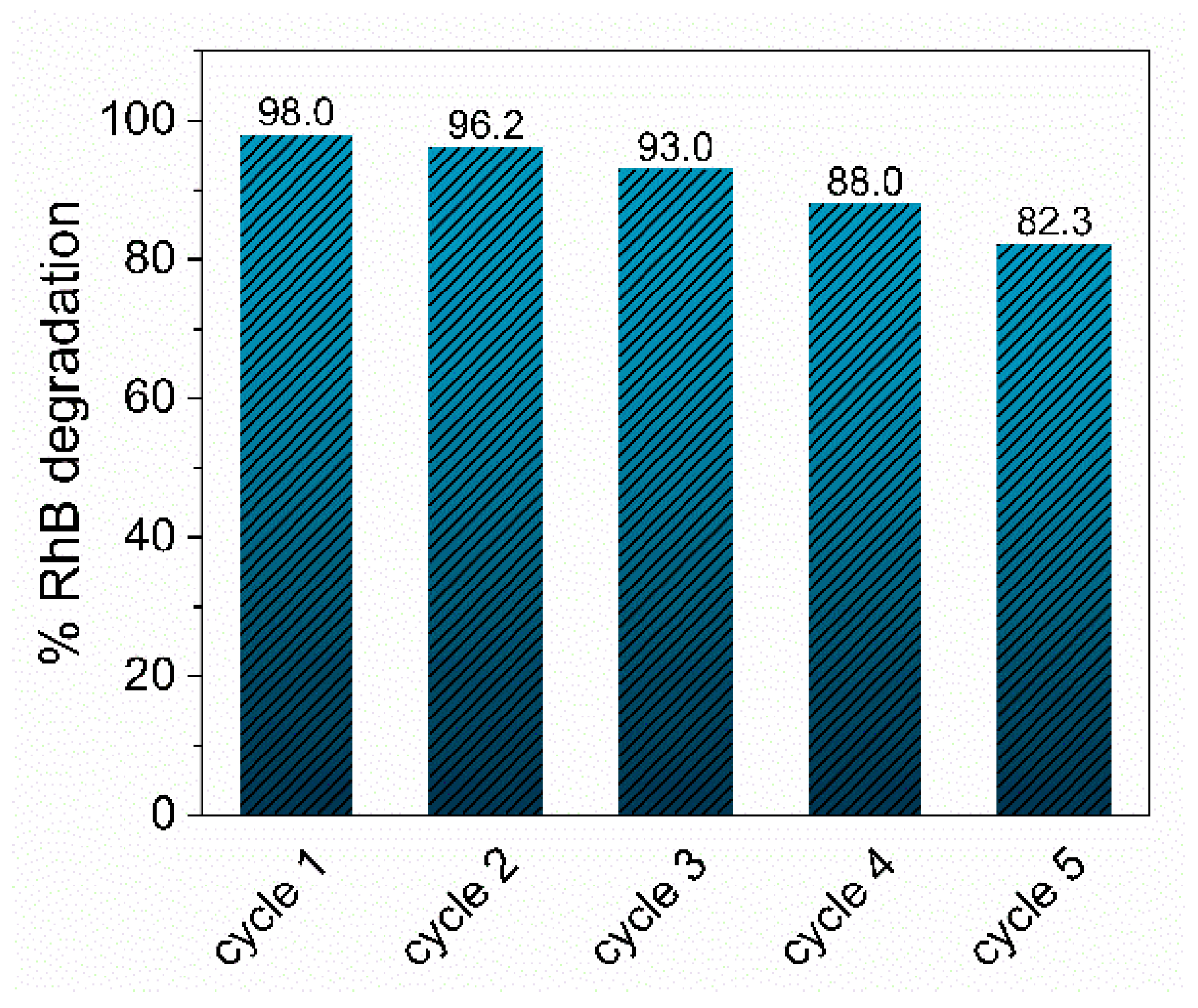
3.5. Catalytic Activity for Organic Dye Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| PDA | Polydopamine |
| PDA NSs | Polydopamine nanospheres |
| PDA-Au | AuNP decorated on PDA nanospheres (synthesized by plasma-assisted method) |
| cPDA-Au | AuNP decorated on PDA nanospheres (synthesized by chemical synthesis method) |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
References
- Rezania, S.; Darajeh, N.; Rupani, P.F.; Mojiri, A.; Kamyab, H.; Taghavijeloudar, M. Recent Advances in the Adsorption of Different Pollutants from Wastewater Using Carbon-Based and Metal-Oxide Nanoparticles. Appl. Sci. 2024, 14, 11492. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and Biological Treatments for Wastewater Decontamination—A Review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods: A General Review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Chen, W.; Mo, J.; Du, X.; Zhang, Z.; Zhang, W. Biomimetic Dynamic Membrane for Aquatic Dye Removal. Water Res. 2019, 151, 243–251. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic Durable Multifunctional Self-Cleaning Nanofibrous Membrane with Outstanding Oil/Water Separation, Photodegradation of Organic Contaminants, and Antibacterial Performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef]
- Li, N.; Chen, G.; Zhao, J.; Yan, B.; Cheng, Z.; Meng, L.; Chen, V. Self-Cleaning PDA/ZIF-67@PP Membrane for Dye Wastewater Remediation with Peroxymonosulfate and Visible Light Activation. J. Membr. Sci. 2019, 591, 117341. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.-J.; Wu, G.-P.; Darling, S.B.; Xu, Z.-K. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Chin, H.-K.; Lin, P.-Y.; Chen, J.; Kirankumar, R.; Wen, Z.-H.; Hsieh, S. Polydopamine-Mediated Ag and ZnO as an Active and Recyclable SERS Substrate for Rhodamine B with Significantly Improved Enhancement Factor and Efficient Photocatalytic Degradation. Appl. Sci. 2021, 11, 4914. [Google Scholar] [CrossRef]
- Chae, W.R.; Song, Y.-J.; Lee, N.Y. Polydopamine-Mediated Gold Nanoparticle Coating Strategy and Its Application in Photothermal Polymerase Chain Reaction. Lab Chip 2025, 25, 1429–1438. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Müller, L.; Müller, F.A. Adjustable Synthesis of Polydopamine Nanospheres and Their Nucleation and Growth. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125196. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Li, M. Selecting Water-Alcohol Mixed Solvent for Synthesis of Polydopamine Nano-Spheres Using Solubility Parameter. Sci. Rep. 2014, 4, 6070. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. A Facile Method to Synthesize Mussel-Inspired Polydopamine Nanospheres as an Active Template for in Situ Formation of Biomimetic Hydroxyapatite. Mater. Sci. Eng. C 2019, 94, 729–739. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H. Pt, Pd, and Rh Nanoparticles Supported on Polydopamine Nanospheres as Catalysts for Transfer Hydrogenolysis. ACS Appl. Nano Mater. 2022, 5, 11797–11808. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Yan, M.; Liu, H. Synergetic Effect of Polydopamine Particles and In-Situ Fabricated Gold Nanoparticles on Charge-Dependent Catalytic Behaviors. Particuology 2019, 44, 63–70. [Google Scholar] [CrossRef]
- Wang, W.; Dong, X.; Huang, X.; Wu, H.; Zhu, D. Polydopamine-Mediated CdS Crystal Growth Behavior and the Synergistic Photocatalytic–Adsorption Mechanisms in Removing Rhodamine B. Energy Fuels 2023, 37, 18184–18193. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Wamsley, M.; Zhang, D.; Wang, H. Colloidal Polydopamine Beads: A Photothermally Active Support for Noble Metal Nanocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 17560–17569. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H. Phase-Controlled Ruthenium Nanocrystals on Colloidal Polydopamine Supports and Their Catalytic Behaviors in Aerobic Oxidation Reactions. ACS Appl. Mater. Interfaces 2023, 15, 36676–36687. [Google Scholar] [CrossRef]
- Abood, E.A.; Essa, W.K.; Alsuraifi, A.; Yasin, S.A. A Novel Nanogold Composite Fabrication, Its Characterization, and Its Application in the Removal of Methylene Blue Dye from an Aqueous Solution. Appl. Sci. 2024, 14, 5229. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Li, Y.-T.; Li, X.-J.; Zhang, L.; Kouadio Fodjo, E.; Han, S. Controllable in Situ Fabrication of Portable AuNP/Mussel-Inspired Polydopamine Molecularly Imprinted SERS Substrate for Selective Enrichment and Recognition of Phthalate Plasticizers. Chem. Eng. J. 2020, 402, 125179. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Huang, P.; Wu, F.-Y. Colorimetric and Photothermal Immunosensor for Sensitive Detection of Cancer Biomarkers Based on Enzyme-Mediated Growth of Gold Nanostars on Polydopamine. Anal. Chim. Acta 2023, 1279, 341775. [Google Scholar] [CrossRef]
- Liu, Y.; Haghighatbin, M.A.; Shen, W.; Cui, H. Functionalized Polydopamine Nanospheres with Chemiluminescence and Immunoactivity for Label-Free Copeptin Immunosensing. ACS Appl. Nano Mater. 2020, 3, 4681–4689. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Anbu Anjugam Vandarkuzhali, S.; Karthikeyan, G.; Pachamuthu, M.P. Efficient Catalytic Reduction of Xanthene Based Dyes, Methylene Blue, and 4-Nitrophenol Using Gold Nanoparticles Supported on Mesoporous Amine Functionalized SBA-15. Mater. Sci. Eng. B 2024, 302, 117208. [Google Scholar] [CrossRef]
- Stolle, H.L.K.S.; Kluitmann, J.J.; Csáki, A.; Köhler, J.M.; Fritzsche, W. Shape-Dependent Catalytic Activity of Gold and Bimetallic Nanoparticles in the Reduction of Methylene Blue by Sodium Borohydride. Catalysts 2021, 11, 1442. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhuang, J.; Wang, D. Small-Scale Big Science: From Nano- to Atomically Dispersed Catalytic Materials. Small Sci. 2022, 2, 2200036. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple Size-Controlled Synthesis of Au Nanoparticles and Their Size-Dependent Catalytic Activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef]
- Liang, C.; Cheong, J.Y.; Sitaru, G.; Rosenfeldt, S.; Schenk, A.S.; Gekle, S.; Kim, I.-D.; Greiner, A. Size-Dependent Catalytic Behavior of Gold Nanoparticles. Adv. Mater. Interfaces 2022, 9, 2100867. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Mei, S.; Kochovski, Z.; Roa, R.; Gu, S.; Xu, X.; Yu, H.; Dzubiella, J.; Ballauff, M.; Lu, Y. Enhanced Catalytic Activity of Gold@Polydopamine Nanoreactors with Multi-Compartment Structure Under NIR Irradiation. Nano-Micro Lett. 2019, 11, 83. [Google Scholar] [CrossRef]
- Wang, J.-G.; Hua, X.; Li, M.; Long, Y.-T. Mussel-Inspired Polydopamine Functionalized Plasmonic Nanocomposites for Single-Particle Catalysis. ACS Appl. Mater. Interfaces 2017, 9, 3016–3023. [Google Scholar] [CrossRef]
- Hemmati, S.; Heravi, M.M.; Karmakar, B.; Veisi, H. In Situ Decoration of Au NPs over Polydopamine Encapsulated GO/Fe3O4 Nanoparticles as a Recyclable Nanocatalyst for the Reduction of Nitroarenes. Sci. Rep. 2021, 11, 12362. [Google Scholar] [CrossRef]
- Zubair, M.; Rafique, M.S.; Khalid, A.; Yaqub, T.; Alomar, S.Y.; Gohar, H. Synthesis of Gold-PVP Nanostructured Composites by Microplasma: A Test to Study Their Inhibiting Tendency of Avian Influenza Virus Activity. Appl. Sci. 2022, 12, 5352. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In Situ Plasma-Assisted Synthesis of Polydopamine-Functionalized Gold Nanoparticles for Biomedical Applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Lee, S.Y.; Do, H.T.; Kim, J.H. Microplasma-Assisted Synthesis of TiO2–Au Hybrid Nanoparticles and Their Photocatalytic Mechanism for Degradation of Methylene Blue Dye under Ultraviolet and Visible Light Irradiation. Appl. Surf. Sci. 2022, 573, 151383. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.C.; Ferreira, L.F. Gold Nanoparticles: A Didactic Step-by-Step of the Synthesis Using the Turkevich Method, Mechanisms, and Characterizations. Analytica 2023, 4, 250–263. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Wang, Y.-Y.; Li, S.-L.; Cao, L.; Jiang, F.-L.; Maskow, T.; Liu, Y. Core–Shell Polydopamine/Cu Nanometer Rods Efficiently Deactivate Microbes by Mimicking Chloride-Activated Peroxidases. ACS Omega 2022, 7, 29984–29994. [Google Scholar] [CrossRef]
- Patel, J.; Němcová, L.; Maguire, P.; Graham, W.G.; Mariotti, D. Synthesis of Surfactant-Free Electrostatically Stabilized Gold Nanoparticles by Plasma-Induced Liquid Chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef]
- Sun, D.; McLaughlan, J.; Zhang, L.; Falzon, B.G.; Mariotti, D.; Maguire, P.; Sun, D. Atmospheric Pressure Plasma-Synthesized Gold Nanoparticle/Carbon Nanotube Hybrids for Photothermal Conversion. Langmuir 2019, 35, 4577–4588. [Google Scholar] [CrossRef]
- De Vos, C.; Baneton, J.; Witzke, M.; Dille, J.; Godet, S.; Gordon, M.J.; Sankaran, R.M.; Reniers, F. A Comparative Study of the Reduction of Silver and Gold Salts in Water by a Cathodic Microplasma Electrode. J. Phys. D Appl. Phys. 2017, 50, 105206. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, M.D.; Nguyen, T.H.; Gao, M.; Kaushik, N.; Vasilyevich, O.V.; Petrovna, A.A.; Andreevich, O.N.; Kaushik, N.K.; Nguyen, T.T.; et al. Microwave-Assisted Synthesis of Copper Ferrite Nanocatalysts for Synergistic Plasma-Fenton Degradation of Rhodamine B. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135681. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, Q.; Zhu, D.; Ding, Z.; Niu, J.; Liu, D.; Zhao, Y.; Xia, Y.; Zhao, Z.; Wang, X. The Reaction Pathways of H2O2(Aq) in the He Plasma Jet with a Liquid System. Plasma Chem. Plasma Process. 2020, 40, 1001–1018. [Google Scholar] [CrossRef]
- Skiba, M.; Zaporozhets, J.; Vorobyova, V. Gold Nanoparticles with Natural Polymer Synthesized by Plasma–Liquid Interactions: Size-Control, Characterization and Colorimetric Detection of Melamine Based on the Size Effect of Gold Nanoparticles. Nano-Struct. Nano-Objects 2024, 37, 101113. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Khan, M.A.; Pareek, V.; Dilip, R.V.; Panwar, J. Utilizing Metal Tolerance Potential of Soil Fungus for Efficient Synthesis of Gold Nanoparticles with Superior Catalytic Activity for Degradation of Rhodamine B. J. Environ. Manag. 2016, 183, 22–32. [Google Scholar] [CrossRef]
- Nguyen, T.T.-N.; Vo, T.-T.; Nguyen, B.N.-H.; Nguyen, D.-T.; Dang, V.-S.; Dang, C.-H.; Nguyen, T.-D. Silver and Gold Nanoparticles Biosynthesized by Aqueous Extract of Burdock Root, Arctium Lappa as Antimicrobial Agent and Catalyst for Degradation of Pollutants. Environ. Sci Pollut. Res. 2018, 25, 34247–34261. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, W.-Y.; Hu, C.-C.; Chiu, T.-C. Tannic Acid-Decorated Bimetallic Copper–Gold Nanoparticles with High Catalytic Activity for the Degradation of 4-Nitrophenol and Rhodamine B. ACS Omega 2024, 9, 24970–24977. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, X.-Y.; Feng, J.-J.; Mei, L.-P.; Wang, A.-J. Simple Fabrication of Bimetallic Platinum-Rhodium Alloyed Nano-Multipods: A Highly Effective and Recyclable Catalyst for Reduction of 4-Nitrophenol and Rhodamine B. J. Colloid Interface Sci. 2021, 582, 701–710. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, C.-G.; Guo, Q.; Wei, X. Preparation of Stable AgNPs@PAN/GO-SH Nanocompsite by Electrospinning for Effective Degradation of 4-Nitrophenol, Methylene Blue and Rhodamine B. Mater. Lett. 2020, 265, 127409. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Baadhe, R.R. Study of Parameters Affecting the Degradation of Rhodamine-B and Methyl Orange Dyes by Annona Muricata Leaf Extract Synthesized Nanoparticles as Well as Their Recyclability. J. Mol. Struct. 2021, 1236, 130287. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Green Synthesis of Silver Nanoparticles Using Nervalia Zeylanica Leaf Extract and Evaluation of Their Antioxidant, Catalytic, and Antimicrobial Potentials. Part. Sci. Technol. 2019, 37, 809–819. [Google Scholar] [CrossRef]




| Catalyst | RhB Concentration | Treatment Volume | Degradation Time | Degradation Efficiency | Pseudo-First-Order Rate Constant | Ref. |
|---|---|---|---|---|---|---|
| AuNPs | 2.5 × 10−5 M | 3 mL | 7 min | - | 0.762 min−1 * | [46] |
| Br-AuNPs | 0.1 mM | 3 mL | 12 min | - | 0.364 min−1 * | [47] |
| BR-AgNPs | 0.1 mM | 3 mL | 10 min | - | 0.424 min−1 * | [47] |
| TA-CuAu NPs | 0.1 mM | 4 mL | 10 min | - | 0.2628 min−1 | [48] |
| PtRh ANMPs | 0.3 mM | 2.36 mL | 12 min | 97.22% | 0.354 min−1 | [49] |
| Pt black | 0.3 mM | 2.36 mL | 40 min | 47.29% | 0.056 min−1 | [49] |
| AgNPs@PAN/Go-SH | 10 mg L−1 | 50 mL | 14 min | - | 0.266 min−1 | [50] |
| Ag-AgCl NPs | 50 mg/100 mL | 5 mL | 5 min | 96% | 0.748 min−1 | [51] |
| AgNPs | 0.05 mM | 3.5 mL | 10 min | - | 0.600 min−1 | [52] |
| PDA-Au | 10 ppm | 3.5 mL | 7 min | >98% | 1.405 min−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.M.; Kaushik, N.; Nguyen, L.T.; Nguyen, G.T.; Nguyen, T.H.; Pham, H.S.; Choi, E.H.; Kaushik, N.K.; Nguyen, L.N. Plasma-Assisted Decoration of Gold Nanoparticles on Bioinspired Polydopamine Nanospheres as Effective Catalyst for Organic Pollutant Removal. Appl. Sci. 2025, 15, 5280. https://doi.org/10.3390/app15105280
Nguyen TM, Kaushik N, Nguyen LT, Nguyen GT, Nguyen TH, Pham HS, Choi EH, Kaushik NK, Nguyen LN. Plasma-Assisted Decoration of Gold Nanoparticles on Bioinspired Polydopamine Nanospheres as Effective Catalyst for Organic Pollutant Removal. Applied Sciences. 2025; 15(10):5280. https://doi.org/10.3390/app15105280
Chicago/Turabian StyleNguyen, Thu Minh, Neha Kaushik, Loan Thu Nguyen, Giang Thi Nguyen, Tung Hoang Nguyen, Hieu Sy Pham, Eun Ha Choi, Nagendra Kumar Kaushik, and Linh Nhat Nguyen. 2025. "Plasma-Assisted Decoration of Gold Nanoparticles on Bioinspired Polydopamine Nanospheres as Effective Catalyst for Organic Pollutant Removal" Applied Sciences 15, no. 10: 5280. https://doi.org/10.3390/app15105280
APA StyleNguyen, T. M., Kaushik, N., Nguyen, L. T., Nguyen, G. T., Nguyen, T. H., Pham, H. S., Choi, E. H., Kaushik, N. K., & Nguyen, L. N. (2025). Plasma-Assisted Decoration of Gold Nanoparticles on Bioinspired Polydopamine Nanospheres as Effective Catalyst for Organic Pollutant Removal. Applied Sciences, 15(10), 5280. https://doi.org/10.3390/app15105280









