Effects of Active Video Games Combined with Conventional Physical Therapy on Perceived Functionality in Older Adults with Knee or Hip Osteoarthritis: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization and Allocation Concealment
2.4. Interventions
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postler, A.; Ramos, A.L.; Goronzy, J.; Günther, K.-P.; Lange, T.; Schmitt, J.; Zink, A.; Hoffmann, F. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: An analysis based on health insurance claims data. Clin. Interv. Aging 2018, 13, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Guede-Rojas, F.; Ibacache-Saavedra, P.; Leal, M.I.; Tuesta, M.; Durán-Marín, C.; Carrasco-Marín, F.; Cigarroa, I.; Alvarez, C.; Izquierdo, M.; Delgado-Floody, P. A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017. Nutrients 2023, 15, 4263. [Google Scholar] [CrossRef]
- Wood, G.; Neilson, J.; Cottrell, E.; Hoole, S.P. Osteoarthritis in people over 16: Diagnosis and management—Updated summary of NICE guidance. BMJ 2023, 380, p24. [Google Scholar] [CrossRef]
- Lundgren-Nilsson, Å.; Dencker, A.; Palstam, A.; Person, G.; Horton, M.C.; Escorpizo, R.; Küçükdeveci, A.A.; Kutlay, S.; Elhan, A.H.; Stucki, G.; et al. Patient-reported outcome measures in osteoarthritis: A systematic search and review of their use and psychometric properties. RMD Open 2018, 4, e000715. [Google Scholar] [CrossRef]
- Rivera-Torres, S.; Fahey, T.D.; Rivera, M.A. Adherence to Exercise Programs in Older Adults: Informative Report. Gerontol. Geriatr. Med. 2019, 5, 2333721418823604. [Google Scholar] [CrossRef]
- Marks, R. Knee Osteoarthritis and Exercise Adherence: A Review. Curr. Aging Sci. 2012, 5, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Damaševičius, R.; Maskeliūnas, R.; Blažauskas, T. Serious Games and Gamification in Healthcare: A Meta-Review. Information 2023, 14, 105. [Google Scholar] [CrossRef]
- Brepohl, P.C.A.; Leite, H. Virtual reality applied to physiotherapy: A review of current knowledge. Virtual Real. 2023, 27, 71–95. [Google Scholar] [CrossRef]
- Wohlgenannt, I.; Simons, A.; Stieglitz, S. Virtual Reality. Bus. Inf. Syst. Eng. 2020, 62, 455–461. [Google Scholar] [CrossRef]
- Li, X.; Luh, D.-B.; Xu, R.-H.; An, Y. Considering the Consequences of Cybersickness in Immersive Virtual Reality Rehabilitation: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 5159. [Google Scholar] [CrossRef]
- Bilika, P.; Karampatsou, N.; Stavrakakis, G.; Paliouras, A.; Theodorakis, Y.; Strimpakos, N.; Kapreli, E. Virtual Reality-Based Exercise Therapy for Patients with Chronic Musculoskeletal Pain: A Scoping Review. Healthcare 2023, 11, 2412. [Google Scholar] [CrossRef]
- Xu, W.; Liang, H.-N.; Baghaei, N.; Wu Berberich, B.; Yue, Y. Health Benefits of Digital Videogames for the Aging Population: A Systematic Review. Games Health J. 2020, 9, 389–404. [Google Scholar] [CrossRef]
- Marques, L.M.; Uchida, P.M.; Barbosa, S.P. The impact of Exergames on emotional experience: A systematic review. Front. Public. Health 2023, 11, 1209520. [Google Scholar] [CrossRef]
- Ogawa, E.F.; You, T.; Leveille, S.G. Potential Benefits of Exergaming for Cognition and Dual-Task Function in Older Adults: A Systematic Review. J. Aging Phys. Act. 2016, 24, 332–336. [Google Scholar] [CrossRef]
- Elshazly, F.A.A. Comparative study on Virtual Reality Training (VRT) over Sensory Motor Training (SMT) in Unilateral Chronic Osteoarthritis—A Randomized Control Trial. Int. J. Med. Res. Health Sci. 2016, 5, 7–16. [Google Scholar]
- Mete, E.; Sari, Z. The efficacy of exergaming in patients with knee osteoarthritis: A randomized controlled clinical trial. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2022, 27, e1952. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yu, L.-F.; Kuo, S.-F.; Wang, X.-M.; Lu, L.-H.; Lin, C.-H. Effects of computer-aided rowing exercise systems on improving muscle strength and function in older adults with mild knee osteoarthritis: A randomized controlled clinical trial. BMC Geriatr. 2022, 22, 809. [Google Scholar] [CrossRef] [PubMed]
- Nambi, G.; Abdelbasset, W.K.; Elsayed, S.H.; Khalil, M.A.; Alrawaili, S.M.; Alsubaie, S.F. Comparative effects of virtual reality training and sensory motor training on bone morphogenic proteins and inflammatory biomarkers in post-traumatic osteoarthritis. Sci. Rep. 2020, 10, 15864. [Google Scholar] [CrossRef]
- Hernandez-Martinez, J.; Ramos-Espinoza, F.; Muñoz-Vásquez, C.; Guzman-Muñoz, E.; Herrera-Valenzuela, T.; Branco, B.H.M.; Castillo-Cerda, M.; Valdés-Badilla, P. Effects of active exergames on physical performance in older people: An overview of systematic reviews and meta-analysis. Front. Public Health 2024, 12, 1250299. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.; Postolache, G.; Teixeira, L.; Arriaga, P.; Lima, M.L.; Postolache, O. Exergames for motor rehabilitation in older adults: An umbrella review. Phys. Ther. Rev. 2019, 24, 84–99. [Google Scholar] [CrossRef]
- Kong, H.; Wang, X.-Q.; Zhang, X.-A. Exercise for Osteoarthritis: A Literature Review of Pathology and Mechanism. Front. Aging Neurosci. 2022, 14, 854026. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Liu, S.; Liu, H.; Zhang, T.; Luo, J. Exergames improve cognitive function in older adults and their possible mechanisms: A systematic review. J. Glob. Health 2023, 13, 04177. [Google Scholar] [CrossRef]
- Guede-Rojas, F.; Andrades-Torres, B.; Aedo-Díaz, N.; González-Koppen, C.; Muñoz-Fuentes, M.; Enríquez-Enríquez, D.; Carvajal-Parodi, C.; Mendoza, C.; Alvarez, C.; Fuentes-Contreras, J. Effects of exergames on rehabilitation outcomes in patients with osteoarthritis. A systematic review. Disabil. Rehabil. 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Wei, W.; Tang, H.; Luo, Y.; Yan, S.; Ji, Q.; Liu, Z.; Li, H.; Wu, F.; Yang, S.; Yang, X. Efficacy of virtual reality exercise in knee osteoarthritis rehabilitation: A systematic review and meta-analysis. Front. Physiol. 2024, 15, 1424815. [Google Scholar] [CrossRef]
- Wang, Q.; Runhaar, J.; Kloppenburg, M.; Boers, M.; Bijlsma, J.W.J.; Bierma-Zeinstra, S.M.A. the CREDO expert group Evaluation of the Diagnostic Performance of American College of Rheumatology, EULAR, and National Institute for Health and Clinical Excellence Criteria Against Clinically Relevant Knee Osteoarthritis: Data From the CHECK Cohort. Arthritis Care Res. 2024, 76, 511–516. [Google Scholar] [CrossRef]
- Bierma-Zeinstra, S.; Bohnen, A.; Ginai, A.; Prins, A.; Verhaar, J. Validity of American College of Rheumatology criteria for diagnosing hip osteoarthritis in primary care research. J. Rheumatol. 1999, 26, 1129–1133. [Google Scholar] [PubMed]
- Jiménez, D.; Lavados, M.; Rojas, P.; Henríquez, C.; Silva, F.; Guillón, M.; Jiménez, D.; Lavados, M.; Rojas, P.; Henríquez, C.; et al. Performance of an abbreviated mini mental examination to detect dementia in older people. Rev. Médica Chile 2017, 145, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Hawley-Hague, H.; Horne, M.; Skelton, D.A.; Todd, C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open 2016, 6, e011560. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Escobar, A.; Quintana, J.M.; Bilbao, A.; Azkárate, J.; Güenaga, J.I. Validation of the Spanish Version of the WOMAC Questionnaire for Patients with Hip or Knee Osteoarthritis. Clin. Rheumatol. 2002, 21, 466–471. [Google Scholar] [CrossRef]
- Gandek, B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: A systematic review. Arthritis Care Res. 2015, 67, 216–229. [Google Scholar] [CrossRef]
- Piva, S.R.; Gil, A.B.; Moore, C.G.; Fitzgerald, G.K. Responsiveness of the activities of daily living scale of the knee outcome survey and numeric pain rating scale in patients with patellofemoral pain. J. Rehabil. Med. 2009, 41, 129–135. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Wijekularathna, D.K.; Manage, A.B.W.; Scariano, S.M. Power analysis of several normality tests: A Monte Carlo simulation study. Commun. Stat.—Simul. Comput. 2020, 51, 757–773. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; 567p, ISBN 978-0-203-77158-7. [Google Scholar]
- Nascimento, D.d.C.; Rolnick, N.; da Silva Almeida, I.; Cipriano Junior, G.; Durigan, J.L. Frequentist, Bayesian Analysis and Complementary Statistical Tools for Geriatric and Rehabilitation Fields: Are Traditional Null-Hypothesis Significance Testing Methods Sufficient? Clin. Interv. Aging 2024, 19, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Williamson, P.; Batterham, A.M. Issues in the determination of ‘responders’ and ‘non-responders’ in physiological research. Exp. Physiol. 2019, 104, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. The Chi-square test of independence. Biochem. Medica 2013, 23, 143. [Google Scholar] [CrossRef]
- Fleiss, J.; Levin, B.; Cho Paik, M. Statistical Methods for Rates and Proportions, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2003; ISBN 978-0-471-52629-2. [Google Scholar]
- Karsdal, M.A.; Bihlet, A.; Byrjalsen, I.; Alexandersen, P.; Ladel, C.; Michaels, M.; Andersen, J.R.; Riis, B.J.; Kraus, V.; Bay-Jensen, A.C.; et al. OA phenotypes, rather than disease stage, drive structural progression—Identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthr. Cartil. 2015, 23, 550–558. [Google Scholar] [CrossRef]
- Alkan, B.M.; Fidan, F.; Tosun, A.; Ardıçoğlu, Ö. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod. Rheumatol. 2014, 24, 166–171. [Google Scholar] [CrossRef]
- Freiberger, E.; Sieber, C.C.; Kob, R. Mobility in Older Community-Dwelling Persons: A Narrative Review. Front. Physiol. 2020, 11, 881. [Google Scholar] [CrossRef]
- Özlü, A.; Ünver, G.; Tuna, H.İ.; Menekşeoğlu, A.K. The Effect of a Virtual Reality-Mediated Gamified Rehabilitation Program on Pain, Disability, Function, and Balance in Knee Osteoarthritis: A Prospective Randomized Controlled Study. Game. Health J. 2023, 12, 118–124. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lee, W.-C.; Hsieh, R.-L. Active video games for knee osteoarthritis improve mobility but not WOMAC score: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 63, 458–465. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Guo, Z.; Bao, D.; Zhou, J. Comparison between the effects of exergame intervention and traditional physical training on improving balance and fall prevention in healthy older adults: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2021, 18, 164. [Google Scholar] [CrossRef]
- Debi, R.; Mor, A.; Segal, G.; Segal, O.; Agar, G.; Debbi, E.; Halperin, N.; Haim, A.; Elbaz, A. Correlation between single limb support phase and self-evaluation questionnaires in knee osteoarthritis populations. Disabil. Rehabil. 2011, 33, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Petrella, M.; Gramani-Say, K.; Serrão, P.R.M.S.; Lessi, G.C.; Barela, J.A.; Carvalho, R.P.; Mattiello, S.M. Measuring postural control during mini-squat posture in men with early knee osteoarthritis. Hum. Mov. Sci. 2017, 52, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-Y.; Zhang, Z.-R.; Tang, Z.-M.; Hua, F.-Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef]
- Raimo, S.; Maggi, G.; Ilardi, C.R.; Cavallo, N.D.; Torchia, V.; Pilgrom, M.A.; Cropano, M.; Roldán-Tapia, M.D.; Santangelo, G. The relation between cognitive functioning and activities of daily living in normal aging, mild cognitive impairment, and dementia: A meta-analysis. Neurol. Sci. 2024, 45, 2427–2443. [Google Scholar] [CrossRef]
- Chen, F.-T.; Etnier, J.L.; Chan, K.-H.; Chiu, P.-K.; Hung, T.-M.; Chang, Y.-K. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1451–1467. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef]
- Costa, M.T.S.; Vieira, L.P.; Barbosa, E.d.O.; Mendes Oliveira, L.; Maillot, P.; Otero Vaghetti, C.A.; Giovani Carta, M.; Machado, S.; Gatica-Rojas, V.; Monteiro-Junior, R.S. Virtual Reality-Based Exercise with Exergames as Medicine in Different Contexts: A Short Review. Clin. Pract. Epidemiol. Ment. Health CP EMH 2019, 15, 15–20. [Google Scholar] [CrossRef]
- Manser, P.; Herold, F.; de Bruin, E.D. Components of effective exergame-based training to improve cognitive functioning in middle-aged to older adults—A systematic review and meta-analysis. Ageing Res. Rev. 2024, 99, 102385. [Google Scholar] [CrossRef]
- Temprado, J.-J. Can Exergames Be Improved to Better Enhance Behavioral Adaptability in Older Adults? An Ecological Dynamics Perspective. Front. Aging Neurosci. 2021, 13, 670166. [Google Scholar] [CrossRef]
- Tang, M.; Wang, D.; Guerrien, A. A systematic review and meta-analysis on basic psychological need satisfaction, motivation, and well-being in later life: Contributions of self-determination theory. PsyCh J. 2020, 9, 5–33. [Google Scholar] [CrossRef]
- Crane, B.M.; Drazich, B.F.; Taylor, J.L.; Moored, K.D.; Ahmad, O.; Krakauer, J.W.; Carlson, M.C. Older Adults and Three-Dimensional Exergaming: Motivators and Barriers to Participation and Retention. Games Health J. 2023, 12, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yildizeli Topcu, S. Relations among Pain, Pain Beliefs, and Psychological Well-Being in Patients with Chronic Pain. Pain. Manag. Nurs. 2018, 19, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.A.; Kaplan, D.J.; Mojica, E.; Strauss, E.J.; Gonzalez-Lomas, G.; Campbell, K.A.; Alaia, M.J.; Jazrawi, L.M. The Minimal Clinically Important Difference: A Review of Clinical Significance. Am. J. Sports Med. 2023, 51, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.; Hochberg, M.; van der Heijde, D.; et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: The minimal clinically important improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef]
- MacKay, C.; Clements, N.; Wong, R.; Davis, A.M. A systematic review of estimates of the minimal clinically important difference and patient acceptable symptom state of the Western Ontario and McMaster Universities Osteoarthritis Index in patients who underwent total hip and total knee replacement. Osteoarthr. Cartil. 2019, 27, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Ewert, T.; Lehmann, S.; Aeschlimann, A.; Stucki, G. The factor subdimensions of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) help to specify hip and knee osteoarthritis. a prospective evaluation and validation study. J. Rheumatol. 2005, 32, 1324–1330. [Google Scholar]
- Faschingbauer, M.; Kasparek, M.; Schadler, P.; Trubrich, A.; Urlaub, S.; Boettner, F. Predictive values of WOMAC, KOOS, and SF-12 score for knee arthroplasty: Data from the OAI. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3333–3339. [Google Scholar] [CrossRef]
- Walker, L.C.; Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. The WOMAC score can be reliably used to classify patient satisfaction after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3333–3341. [Google Scholar] [CrossRef]
- Hawker, G.A.; Wright, J.G.; Coyte, P.C.; Williams, J.I.; Harvey, B.; Glazier, R.; Badley, E.M. Differences between Men and Women in the Rate of Use of Hip and Knee Arthroplasty. N. Engl. J. Med. 2000, 342, 1016–1022. [Google Scholar] [CrossRef]
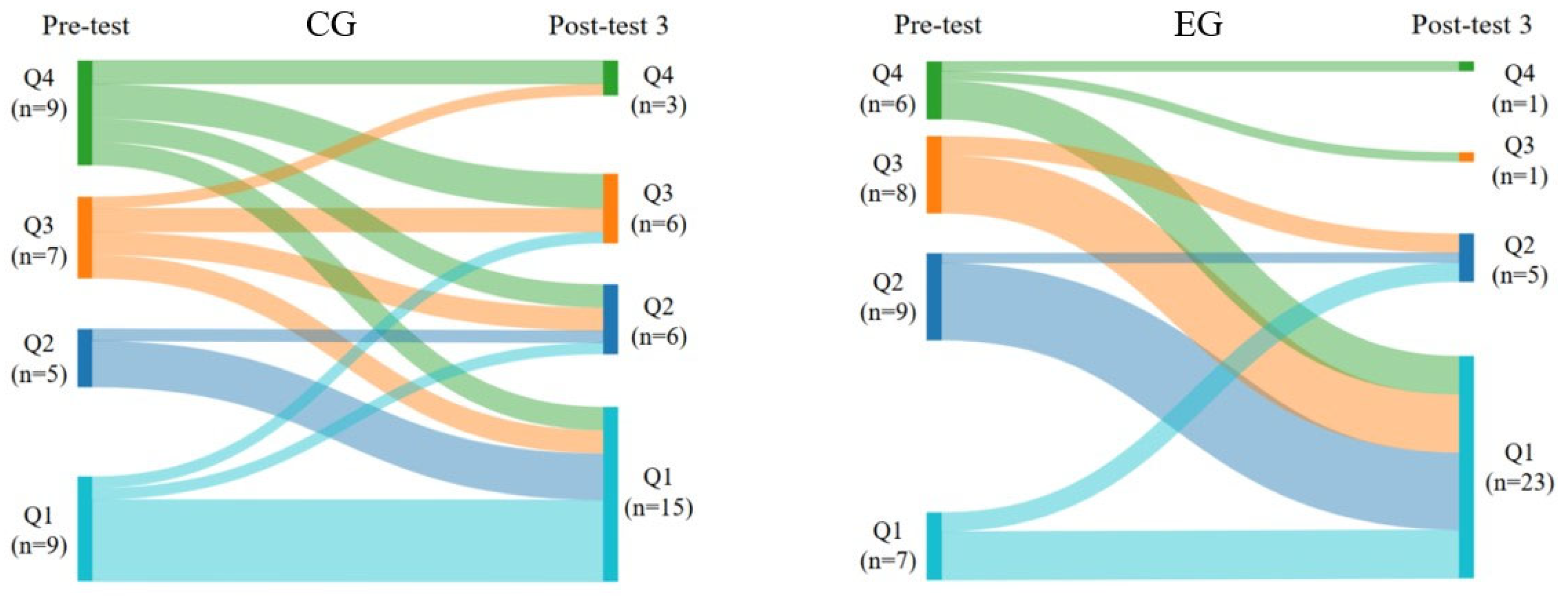
- Otto, E.; Culakova, E.; Meng, S.; Zhang, Z.; Xu, H.; Mohile, S.; Flannery, M.A. Overview of Sankey flow diagrams: Focusing on symptom trajectories in older adults with advanced cancer. J. Geriatr. Oncol. 2022, 13, 742–746. [Google Scholar] [CrossRef]
- Allen, K.D.; Jordan, J.M.; Doherty, M.; Renner, J.B.; Kraus, V.B. Performance of global assessments of hip, knee, and back symptom change. Clin. Rheumatol. 2011, 30, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Abbott, J.H. Global Ratings of Change Do Not Accurately Reflect Functional Change Over Time in Clinical Practice. J. Orthop. Sports Phys. Ther. 2015, 45, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lawford, B.J.; Bennell, K.L.; Campbell, P.K.; Kasza, J.; Hinman, R.S. Association Between Therapeutic Alliance and Outcomes Following Telephone-Delivered Exercise by a Physical Therapist for People with Knee Osteoarthritis: Secondary Analyses from a Randomized Controlled Trial. JMIR Rehabil. Assist. Technol. 2021, 8, e23386. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, H.; Smart, K.M.; Moloney, N.A.; Blake, C.; Doody, C.M. Pain sensitization associated with nonresponse after physiotherapy in people with knee osteoarthritis. PAIN 2018, 159, 1877. [Google Scholar] [CrossRef]


| CG (n = 30) | EG (n = 30) | p-Value | |
|---|---|---|---|
| Age (years), M ± SD | 69.0 ± 5.5 | 68.7 ± 5.4 | 0.852 |
| Height (cm), M ± SD | 1.5 ± 0.0 | 1.5 ± 0.0 | 0.621 |
| Weight (kg), M ± SD | 72.2 ± 11.0 | 70.7 ± 12.5 | 0.612 |
| BMI (kg/m2), M ± SD | 30.1 ± 4.3 | 29.8 ± 4.4 | 0.761 |
| Sex (female/male), no. | 25/5 | 25/5 | 1.000 |
| Control Group (n = 30) | Experimental Group (n = 30) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test 1 | Post-Test 2 | Post-Test 3 | Follow-Up | Pre-Test | Post-Test 1 | Post-Test 2 | Post-Test 3 | Follow-Up | |
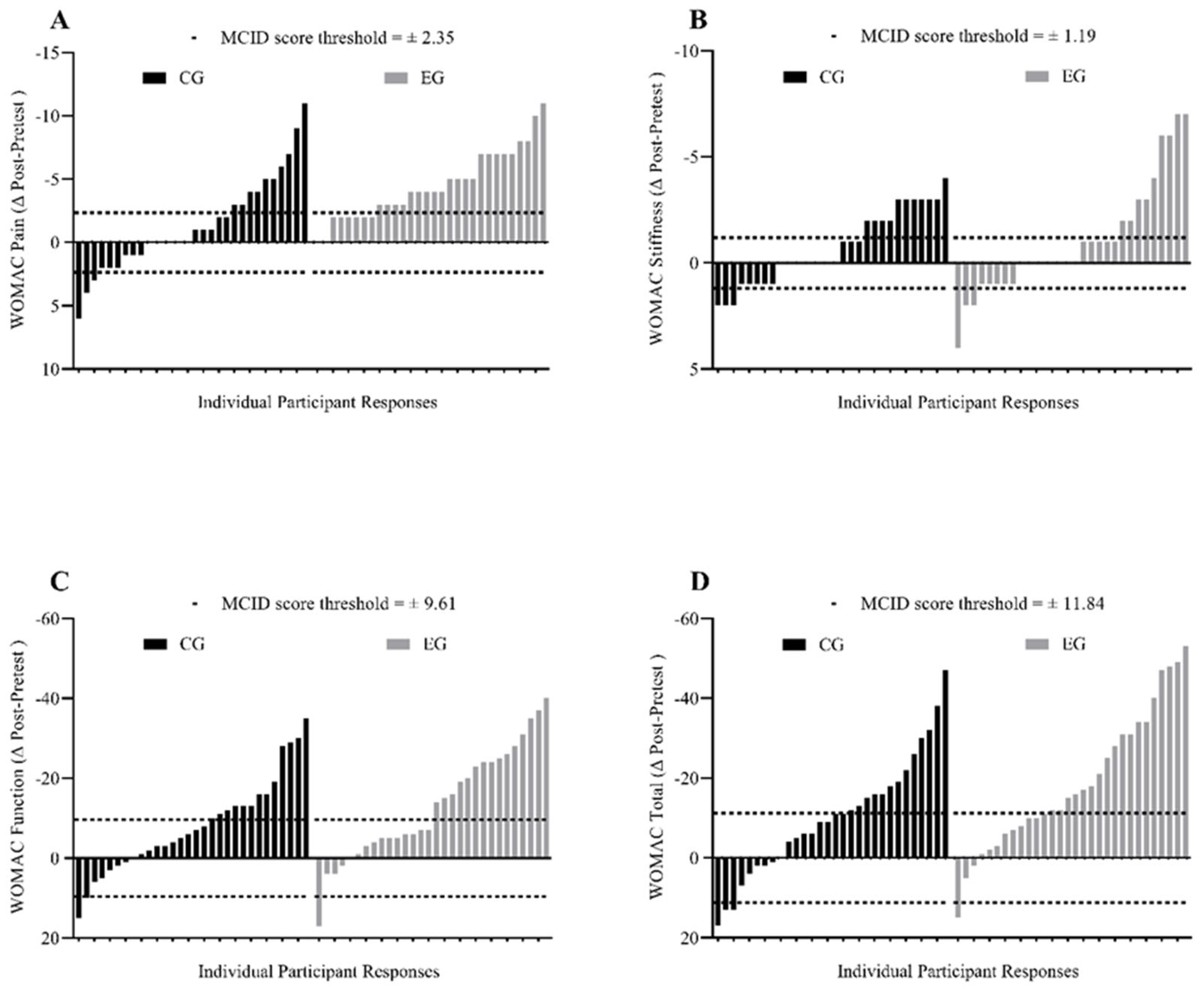
| W-pain | 9.2 ± 3.3 | 8.3 ± 2.8 s | 7.8 ± 3.6 s | 7.8 ± 3.3 s | 8.1 ± 3.0 s | 9.1 ± 2.6 | 6.2 ± 2.7 *,l | 6.0 ± 3.2 *,l | 4.5 ± 2.6 *,l,† | 6.1 ± 3.2 *,l |
| W-stiffness | 3.4 ± 1.0 | 3.2 ± 1.4 n | 3.1 ± 1.4 s | 2.7 ± 1.5 m | 3.1 ± 1.3 s | 3.3 ± 1.8 | 2.7 ± 0.9 s | 2.7 ± 1.5 s | 2.2 ± 1.6 *,m | 2.5 ± 1.3 m |
| W-function | 31.7 ± 12.3 | 29.5 ± 11.5 n | 27.4 ± 11.8 s | 23.6 ± 8.5 *,m | 27.2 ± 9.3 s | 30.1 ± 11.7 | 23.6 ± 9.7 *,m | 23.1 ± 10.9 *,m | 16.8 ± 11.1 *,l | 20.8 ± 12.0 *,l |
| W-total | 44.4 ± 15.9 | 41.0 ± 15.1 s | 38.4 ± 15.9 s | 34.2 ± 11.6 *,m | 38.5 ± 12.9 s | 42.5 ± 14.4 | 32.6 ± 12.0 *,m | 31.9 ± 14.1 *,m | 23.6 ± 14.4 *,l | 29.5 ± 15.2 *,l |
| Control Group (n = 30) | Experimental Group (n = 30) | |||||
|---|---|---|---|---|---|---|
| Rs | NRs | ARs | Rs | NRs | ARs | |
| W-pain, no. (%) | 10 (33.3) | 17 (56.7) | 3 (10.0) | 22 (73.3) † | 8 (26.7) ‡ | 0 (0.0) |
| W-stiffness, no. (%) | 13 (43.3) | 15 (50.0) | 2 (6.7) | 15 (50.0) | 14 (46.7) | 1 (3.3) |
| W-function, no. (%) | 11 (36.7) | 16 (53.3) | 3 (10.0) | 9 (30.0) | 18 (60.0) | 3 (10.0) |
| W-total, no. (%) | 13 (43.3) | 14 (46.7) | 3 (10.0) | 18 (60.0) | 12 (40.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guede-Rojas, F.; Mendoza, C.; Fuentes-Contreras, J.; Alvarez, C.; Agurto Tarbes, B.; Muñoz-Gutiérrez, J.K.; Soto-Martínez, A.; Carvajal-Parodi, C. Effects of Active Video Games Combined with Conventional Physical Therapy on Perceived Functionality in Older Adults with Knee or Hip Osteoarthritis: A Randomized Controlled Trial. Appl. Sci. 2025, 15, 93. https://doi.org/10.3390/app15010093
Guede-Rojas F, Mendoza C, Fuentes-Contreras J, Alvarez C, Agurto Tarbes B, Muñoz-Gutiérrez JK, Soto-Martínez A, Carvajal-Parodi C. Effects of Active Video Games Combined with Conventional Physical Therapy on Perceived Functionality in Older Adults with Knee or Hip Osteoarthritis: A Randomized Controlled Trial. Applied Sciences. 2025; 15(1):93. https://doi.org/10.3390/app15010093
Chicago/Turabian StyleGuede-Rojas, Francisco, Cristhian Mendoza, Jorge Fuentes-Contreras, Cristian Alvarez, Bárbara Agurto Tarbes, Javiera Karina Muñoz-Gutiérrez, Adolfo Soto-Martínez, and Claudio Carvajal-Parodi. 2025. "Effects of Active Video Games Combined with Conventional Physical Therapy on Perceived Functionality in Older Adults with Knee or Hip Osteoarthritis: A Randomized Controlled Trial" Applied Sciences 15, no. 1: 93. https://doi.org/10.3390/app15010093
APA StyleGuede-Rojas, F., Mendoza, C., Fuentes-Contreras, J., Alvarez, C., Agurto Tarbes, B., Muñoz-Gutiérrez, J. K., Soto-Martínez, A., & Carvajal-Parodi, C. (2025). Effects of Active Video Games Combined with Conventional Physical Therapy on Perceived Functionality in Older Adults with Knee or Hip Osteoarthritis: A Randomized Controlled Trial. Applied Sciences, 15(1), 93. https://doi.org/10.3390/app15010093








