Analysis of Glucocorticoids as Potential Adulterants in Cosmetic Products: A Dual Approach for Qualitative and Quantitative Evaluation Based on ELISA and HPLC-MS Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Standard Solutions and Sample Preparation
2.3. Apparatus and Analytical Procedures
2.4. Method Validation
3. Results and Discussion
3.1. LC-MS Method Development
3.2. HPLC-MS Method Validation
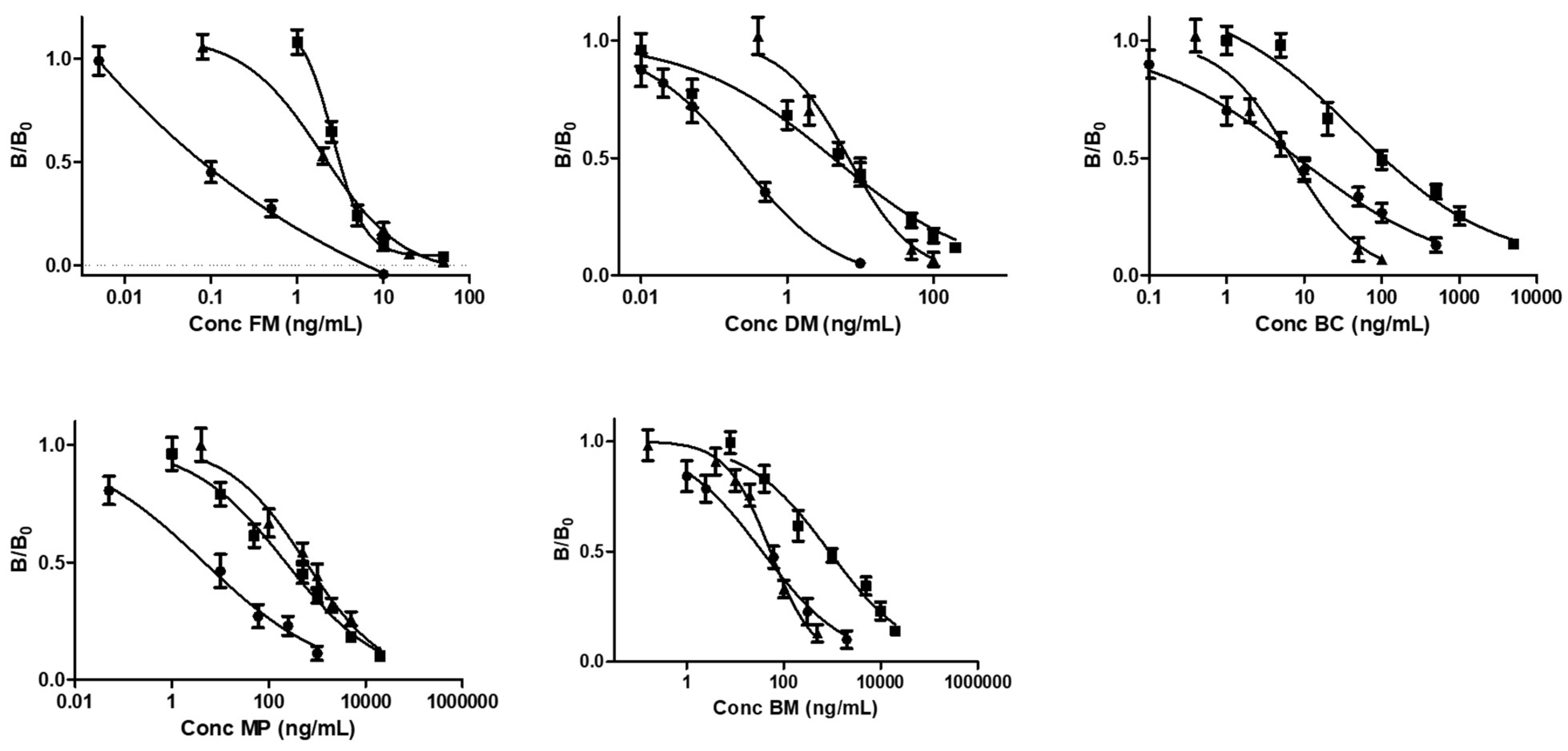
3.3. ELISA Method Validation
3.4. Comparison Between HPLC-MS Method and Immunoassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- del Rosso, J.; Friedlander, S.F. Corticosteroids: Options in the era of steroid-sparing therapy. J. Am. Acad. Dermatol. 2005, 53, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Godthelp, T.; Holm, A.F.; Blom, H.; Klein-Jan, A. Allergic rhinitis and inflammation: The effect of nasal corticosteroid therapy. Allergy 1997, 52, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Rowe, B.H.; Edmonds, M.L.; Spooner, C.H.; Diner, B.; Camargo, C.A. Corticosteroid therapy for acute asthma. Respir. Med. 2004, 98, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Möricke, A.; Zimmermann, M.; Valsecchi, M.G.; Stanulla, M.; Biondi, A.; Mann, G.; Locatelli, F.; Cazzaniga, G.; Niggli, F.; Aricò, M.; et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016, 127, 2101–2112. [Google Scholar] [CrossRef]
- Benedek, T.G. History of the development of corticosteroi therapy. Clin. Exp. Rheumatol. 2011, 29 (Suppl. S68), 5–12. [Google Scholar]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. The Epidemiology of Corticosteroid-Induced Osteoporosis: A Meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef]
- Peppa, M.; Krania, M.; Raptis, S.A. Hypertension and other morbidities with Cushing’s syndrome associated with corticosteroids: A review. Integr. Blood Press. Control 2011, 4, 7–16. [Google Scholar] [CrossRef]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-Induced Hyperglycemia. Endocr. Pract. 2009, 15, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Schoepe, S.; Schäcke, H.; May, E.; Asadullah, K. Glucocorticoid therapy-induced skin atrophy. Exp. Dermatol. 2006, 15, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Youm, J.; Park, K.; Uchida, Y.; Chan, A.; Mauro, T.M.; Holleran, W.M.; Elias, P.M. Local blockade of glucocorticoid activation reverses stress- and glucocorticoid-induced delays in cutaneous wound healing. Wound Repair Regen. 2013, 21, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Westfall, A.O.; Allison, J.; Bijlsma, J.W.; Freeman, A.; George, V.; Kovac, S.H.; Spettell, C.M.; Saag, K.G. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthrit. Care Res. 2006, 55, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mfds.go.kr/eng/index.do (accessed on 1 December 2024).
- Gaudiano, M.C.; Lucente, D.; Antoniella, E.; Bertocchi, P.; Muleri, N.; Manna, L.; Bartolomei, M.; Alimonti, S.; Valvo, L.; Rodomonte, A.L. “For export only” medicines come back to Europe: A RP-LC method for the screening of six glucocorticoids in illegal and counterfeit anti-inflammatory and lightening creams. J. Pharm. Biomed. Anal. 2010, 53, 158–164. [Google Scholar] [CrossRef]
- Wei, C.; Ding, S.; You, H.; Zhang, Y.; Wang, Y.; Yang, X.; Yuan, J. An Immunoassay for Dibutyl Phthalate Based on Direct Hapten Linkage to the Polystyrene Surface of Microtiter Plates. PLoS ONE 2011, 6, e29196. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Kwon, I.K.; Lee, K.B. Monitoring of clobetasol propionate and betamethasone dipropionate as undeclared steroids in cosmetic products manufactured in Korea. Forensic Sci. Int. 2011, 210, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Yoo, G.J.; Lee, J.H.; Park, H.-J.; Cho, S.; Shin, D.W.; Kim, Y.; Baek, S.Y. Determination of 43 prohibited glucocorticoids in cosmetic products using a simultaneous LC-MS/MS method. Anal. Methods 2017, 9, 2104–2115. [Google Scholar] [CrossRef]
- Pellegrini, M.; Marchei, E.; Pacifici, R.; Rotolo, M.C.; Pichini, S. Advances in the analysis of non-allowed pharmacologically active substances in cosmetic products. J. Pharm. Biomed. Anal. 2011, 55, 842–847. [Google Scholar] [CrossRef]
- Johansson, M.; Fransson, D.; Rundlöf, T.; Huynh, N.-H.; Arvidsson, T. A general analytical platform and strategy in search for illegal drugs. J. Pharm. Biomed. Anal. 2014, 100, 215–229. [Google Scholar] [CrossRef]
- Jalili, R.; Miraghaei, S.; Mohamadi, B.; Babaei, A.; Bahrami, G. Detection of corticosteroid compounds and phosphodiesterase inhibitors (PDH-5) as counterfeit in herbal products available in Iranian market by HPLC method. J. Rep. Pharm. Sci. 2015, 4, 75–81. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, E.J.; Ham, H.J.; Yang, Y.J.; Kim, N.S.; Kim, H.I.; Baek, S.Y. Application of LC–MS/MS and UHPLC-Q-Orbitrap methods for determining 54 steroids in illegal dietary supplements and other sample types. Rapid Commun. Mass Spectrom. 2022, 36, e9334. [Google Scholar] [CrossRef]
- Chan, C.Y.; Ng, S.W.; Ching, C.K.; Mak, T.W.L. Detection of 28 Corticosteroids in Pharmaceutical and Proprietary Chinese Medicinal Products Using Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. Sci. 2021, 59, 548–554. [Google Scholar] [CrossRef]
- Fiori, J.; Andrisano, V. LC–MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharm. Biomed. Anal. 2014, 91, 185–192. [Google Scholar] [CrossRef]
- McEwen, I.; Elmsjö, A.; Lehnström, A.; Hakkarainen, B.; Johansson, M. Screening of counterfeit corticosteroid in creams and ointments by NMR spectroscopy. J. Pharm. Biomed. Anal. 2012, 70, 245–250. [Google Scholar] [CrossRef]
- Giaccone, V.; Polizzotto, G.; Macaluso, A.; Cammilleri, G.; Ferrantelli, V. Determination of ten corticosteroids in illegal cosmetic products by a simple, rapid, and high-performance LC-MS/MS method. Int. J. Anal. Chem. 2017, 2017, 3531649. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Sun, S.; Li, N.; Zhang, D.; Chen, M.; Zhang, H. Extraction and determination of hormones in cosmetics by homogeneous ionic liquid microextraction high-performance liquid chromatography. J. Sep. Sci. 2012, 35, 2032–2039. [Google Scholar] [CrossRef]
- Ivković, B.; Crevar, M.; Cvetanović, A.; Ubavkić, K.; Marković, B. Development and validation of RP-HPLC method for quantification of trace levels of topical corticosteroids in ambiphilic cream. Acta Chromatogr. 2023, 35, 46–51. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Wang, M.; Zhao, H.; Zhao, F. Advances on Hormones in Cosmetics: Illegal Addition Status, Sample Preparation, and Detection Technology. Molecules 2023, 28, 1980. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.S.; El-Gahany, W.; Alraeesi, A.; Al-Hajj, L.; Al-Maidalli, A.; Shah, I. Analysis of illicit glucocorticoid levels in camel hair using competitive ELISA—Comparison with LC–MS/MS. Drug Test. Anal. 2020, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Duffort, O.A.; Polo, F.; Lombardero, M.; Díaz-Perales, A.; Sánchez-Monge, R.; García-Casado, G.; Salcedo, G.; Barber, D. Immunoassay to quantify the major peach allergen Pru p 3 in foodstuffs. Differential allergen release and stability under physiological conditions. J. Agric. Food Chem. 2002, 50, 7738–7741. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.; Tothill, I. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef]
- Sharma, G.M.; Rallabhandi, P.; Williams, K.M.; Herrmann, M.; Sadrieh, N. Gluten Quantitation in Cosmetic Products by Enzyme-Linked Immunosorbent Assay. J. AOAC Int. 2016, 99, 586–590. [Google Scholar] [CrossRef]
- Tranquet, O.; Lupi, R.; Echasserieau-Laporte, V.; Pietri, M.; Larré, C.; Denery-Papini, S. Characterization of Antibodies and Development of an Indirect Competitive Immunoassay for Detection of Deamidated Gluten. J. Agric. Food Chem. 2015, 63, 5403–5409. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, L.; Yu, M.; Zhao, H. The application of a lateral flow immunographic assay to rapidly test for dexamethasone in commercial facial masks. Anal. Bioanal. Chem. 2019, 411, 5703–5710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yao, T.; Wang, S.; Feng, R.; Chen, L.; Zhu, V.; Hu, G.; Zhang, H.; Yang, G. Upconversion luminescence nanoparticles-based immunochromatographic assay for quantitative detection of triamcinolone acetonide in cosmetics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 214, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Pschenitza, M.; Niessner, R.; Li, Y.; Knopp, D.; Deng, A. Highly sensitive and specific determination of mercury(II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Anal. Bioanal. Chem. 2012, 403, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; Yao, X.; Deng, A.; Li, J. Highly sensitive electroluminescence immunoassay for Hg(II) ions based on the use of CdSe quantum dots, the methylmercury-6-mercaptonicotinic acid-ovalbumin conjugate, and a specific monoclonal antibody. Mikrochim. Acta 2015, 182, 469–477. [Google Scholar] [CrossRef]
- English, D.; Scalici, C.; Hamilton, J.; Destro, C.; Jimenez, L. Evaluation of the TECRA™ visual immunoassay for detecting Staphylococcus aureus in cosmetic/pharmaceutical raw materials and finished products. J. Rapid Methods Autom. Microbiol. 1999, 7, 193–203. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, R.; Li, J.; Wang, X.; Xu, L.; Li, Y.; Li, P. Highly Specific Chemiluminescence Immunoassay for the Determination of Chloramphenicol in Cosmetics. Int. J. Anal. Chem. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.P.; Yan, H.; Liu, J.; Shi, L.L.; Chen, P.; Du, H.W. Development of a High-Throughput Immunoassay for Rapid Detection of Multiple Antibiotic Residues in Cosmetics. Adv. Mater. Res. 2014, 1073–1076, 357–361. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar]
- Dayan-Kenigsberg, J.; Bertocchi, A.; Garber, E.A.E. Rapid detection of ricin in cosmetics and elimination of artifacts associated with wheat lectin. J. Immunol. Methods 2008, 336, 251–254. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.Y.; Zhou, K.; Luo, L.; Xu, Z.L. Development of a competitive indirect ELISA for high-throughput screening of hydrocortisone in cosmetic sample. Food Agric. Immunol. 2019, 30, 594–605. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes 2021, 9, 1982. [Google Scholar] [CrossRef]
- González, O.; Blanco, M.E.; Iriarte, G.; Bartolomé, L.; Maguregui, M.I.; Alonso, R.M. Bioanalytical chromatographic method validation according to current regulations, with a special focus on the non-well defined parameters limit of quantification, robustness and matrix effect. J. Chromatogr. A 2014, 1353, 10–27. [Google Scholar] [CrossRef]
- Raposo, F.; Ibelli-Bianco, C. Performance parameters for analytical method validation: Controversies and discrepancies among numerous guidelines. TrAC Trends Anal. Chem. 2020, 129, 115913. [Google Scholar] [CrossRef]
- Pour, S.R.S.; Calabria, D.; Nascetti, A.; Caputo, D.; de Cesare, G.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Easy-to-Use Chemiluminescent-Based Assay for a Rapid and Low-Cost Evaluation of the Antioxidant Activity of Cosmetic Products. Chemosensors 2024, 12, 25. [Google Scholar] [CrossRef]
- Shao, B.; Cui, X.; Yang, Y.; Zhang, J.; Wu, Y. Validation of a solid-phase extraction and ultra-performance liquid chromatographic tandem mass spectrometric method for the detection of 16 glucocorticoids in pig tissues. J. AOAC Int. 2009, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Hansen, L.G.; Pedersen, M. Optimization of solid phase extraction clean up and validation of quantitative determination of corticosteroids in urine by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2008, 617, 216–224. [Google Scholar] [CrossRef]
- Qin, H.; Yu, S.; Hu, X.; Yang, Y. Surfactant-based ultrasound-assisted dispersive liquid-liquid microextraction determination of corticosteroids followed by HPLC-DAD. Anal. Lett. 2013, 46, 589–604. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of steroid hormones in fish tissues by microwave-assisted extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 237, 1012–1020. [Google Scholar] [CrossRef]
- El-Alfy, M.; Deloche, C.; Azzi, L.; Bernard, B.A.; Bernerd, F.; Coutet, J.; Chaussade, V.; Martel, C.; Leclaire, J.; Labrie, F. Skin responses to topical dehydroepiandrosterone: Implications in antiageing treatment? BJD 2010, 163, 968–976. [Google Scholar] [CrossRef]
| Steroid | Spiked Concentration (ng/mL) | Recovery (±CV%) | LOD (ng/mL) | LOQ (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cream A | Cream B | Serum | Cream A | Cream B | Serum | Cream A | Cream B | Serum | ||
| BM 17-V | 0.3 | 88.1 (±4.3) | 65.3 (±5.5) | 70.2 (±9.2) | 0.02 | 0.067 | 0.19 | 0.07 | 0.13 | 0.36 |
| 0.5 | 95.1 (±7.6) | 71.2(±7.8) | 78.2 (±3.4) | |||||||
| 10 | 83.5 (±5.2) | 62.5 (±7.7) | 76.7 (±5.7) | |||||||
| 20 | 84.9 (±5.3) | 68.6 (±4.9) | 73.5 (±3.7) | |||||||
| BC | 0.3 | 91.5 (±8.2) | 120.8 (±9.6) | 108.7 (±9.1) | 0.07 | 0.15 | 0.28 | 0.2 | 0.49 | 0.51 |
| 0.5 | 89.1 (±4.3) | 109.9 (±7.7) | 103.2 (±6.6) | |||||||
| 10 | 95.3 (±5.6) | 115.7 (±6.7) | 105.1 (±3.6) | |||||||
| 20 | 99.8 (±8.9) | 113.5 (±5.4) | 112.9 (±3.9) | |||||||
| BCDP | 0.3 | 112.1 (±7.1) | 101.4 (±7.5) | 77.8 (±8.2) | 0.02 | 0.04 | 0.48 | 0.08 | 0.13 | 1.16 |
| 0.5 | 101.1 (±8.4) | 99.6 (±3.9) | 80.6 (±7.5) | |||||||
| 10 | 105.1 (±8.6) | 93.8 (±4.1) | 75.0 (±8.3) | |||||||
| 20 | 111.8 (±4.4) | 98.6 (±8.4) | 85.3 (±9.1) | |||||||
| MP | 0.3 | 76.0 (±3.4) | 77.9 (±2.9) | 79.5 (±7.3) | 0.05 | 0.04 | 0.10 | 0.15 | 0.13 | 0.32 |
| 0.5 | 70.3 (±8.2) | 85.8 (±6.4) | 73.5 (±8.3) | |||||||
| 10 | 79.2 (±10.1) | 81.9 (±4.6) | 71.3 (±8.4) | |||||||
| 20 | 72.4 (±3.7) | 83.4 (±8.8) | 75.3 (±5.6) | |||||||
| BD | 0.3 | 77.4 (±9.2) | 78.0 (±7.2) | 106.0 (±4.6) | 0.12 | 0.16 | 0.13 | 0.42 | 0.53 | 0.45 |
| 0.5 | 72.4 (±6.2) | 75.3 (±7.7) | 115.2 (±7.1) | |||||||
| 10 | 75.0 (±5.5) | 82.8 (±9.2) | 110.3 (±9.1) | |||||||
| 20 | 79.0 (±9.8) | 79.2 (±7.6) | 111.2 (±8.8) | |||||||
| FN | 0.3 | 72.4 (±9.8) | 102.0 (±7.8) | 96.0 (±4.8) | 0.04 | 0.08 | 0.13 | 0.13 | 0.25 | 0.43 |
| 0.5 | 75.4 (±8.9) | 100.6 (±8.8) | 106.5 (±4.4) | |||||||
| 10 | 82.5 (±9.6) | 106.7 (±9.1) | 103.3 (±8.1) | |||||||
| 20 | 79.0 (±5.8) | 105.4 (±8.3) | 99.2 (±8.4) | |||||||
| FM | 0.3 | 93.5 (±3.9) | 74.2 (±8.8) | 99.9 (±5.5) | 0.05 | 0.07 | 0.21 | 0.12 | 0.22 | 0.72 |
| 0.5 | 89.4 (±4.3) | 74.8 (±9.9) | 102.0 (±7.5) | |||||||
| 10 | 101.1 (±4.9) | 76.7 (±2.8) | 109.9 (±8.4) | |||||||
| 20 | 96.6 (±5.9) | 72.1 (±3.7) | 103.54(±8.2) | |||||||
| DM | 0.3 | 85.7 (±6.6) | 94.1 (±8.8) | 96.1 (±8.8) | 0.02 | 0.05 | 0.12 | 0.18 | 0.36 | 0.41 |
| 0.5 | 91.4 (±6.9) | 82.9 (±8.4) | 91.7 (±7.2) | |||||||
| 10 | 79.1 (±7.1) | 93.8 (±9.4) | 93.7 (±6.2) | |||||||
| 20 | 89.7 (±8.3) | 89.0 (±4.8) | 87.6 (±4.4) | |||||||
| Steroid | Spiked Concentration (ng/mL) | Matrix Effect % | ||
|---|---|---|---|---|
| Cream A | Cream B | Serum | ||
| BM 17-V | 0.3 | 0.47 | −0.48 | −0.47 |
| 0.5 | 0.26 | −0.25 | 0.21 | |
| 10.0 | 0.04 | −0.32 | 0.26 | |
| 20.0 | 0.01 | −0.01 | −0.29 | |
| BC | 0.3 | 0.46 | 0.81 | 0.71 |
| 0.5 | 0.21 | 0.06 | −0.02 | |
| 10.0 | 0.12 | −0.03 | 0.08 | |
| 20.0 | −0.49 | 0.82 | −0.12 | |
| BCDP | 0.3 | 0.91 | −0.66 | −0.97 |
| 0.5 | 0.11 | −0.44 | −0.14 | |
| 10.0 | 0.11 | −0.28 | −0.12 | |
| 20.0 | 0.06 | 0.21 | −0.18 | |
| MP | 0.3 | 0.87 | −0.33 | −0.33 |
| 0.5 | 0.19 | −0.07 | −0.41 | |
| 10.0 | 0.18 | −0.06 | −0.36 | |
| 20.0 | 0.01 | −0.48 | −0.38 | |
| BD | 0.3 | 0.15 | 0.07 | 1.01 |
| 0.5 | 0.09 | 0.12 | −0.14 | |
| 10.0 | 0.09 | −0.38 | −0.51 | |
| 20.0 | −0.11 | −0.36 | 0.71 | |
| FN | 0.3 | 0.33 | −0.40 | −0.40 |
| 0.5 | 0.05 | −0.53 | −0.52 | |
| 10.0 | 0.15 | −0.48 | −0–47 | |
| 20.0 | −0.04 | −0.23 | −0.45 | |
| FM | 0.3 | 0.72 | −0.27 | −0.53 |
| 0.5 | 0.16 | −0.07 | 0.08 | |
| 10.0 | 0.07 | −0.12 | −0.07 | |
| 20.0 | −0.08 | −0.15 | −0.06 | |
| DM | 0.3 | 0.64 | −0.05 | −0.13 |
| 0.5 | 0.10 | −0.15 | −0.02 | |
| 10.0 | 0.26 | −0.15 | −0.03 | |
| 20.0 | 0.05 | −0.11 | −0.05 | |
| Steroid | PBS/THF | CREAM | SERUM | |||
|---|---|---|---|---|---|---|
| LOD (ng/mL) | Upper Limit (mg/mL) | LOD (ng/mL) | Upper Limit (mg/mL) | LOD (ng/mL) | Upper Limit (mg/mL) | |
| FM | 0.02 | 0.002 | 0.59 | 0.015 | 1.610 | 0.009 |
| DM | 0.04 | 0.005 | 1.8 | 0.067 | 0.03 | 0.12 |
| BC | 0.80 | 0.88 | 1.8 | 0.068 | 12.1 | 6.20 |
| BM 17-V | 4.7 | 3.27 | 13.8 | 0.53 | 67 | 65.46 |
| MP | 0.22 | 3.13 | 51.2 | 36.2 | 5 | 30.76 |
| Steroid | Spiked Concentration (ng/mL) | Recovery (%) | ||
|---|---|---|---|---|
| Cream | Serum | Cream | Serum | |
| FM | 0.7 | 2 | 75 | 71 |
| 7 | 4 | 82 | 79 | |
| 12 | 7 | 90 | 89 | |
| DM | 2 | 1 | 72 | 70 |
| 30 | 60 | 85 | 87 | |
| 50 | 100 | 92 | 90 | |
| BC | 2 | 15 | 73 | 79 |
| 30 | 3000 | 79 | 80 | |
| 50 | 5000 | 88 | 85 | |
| BM 17-V | 20 | 80 | 72 | 71 |
| 250 | 700 | 75 | 78 | |
| 400 | 50,000 | 83 | 81 | |
| MP | 60 | 10 | 71 | 73 |
| 20,000 | 15,000 | 79 | 78 | |
| 30,000 | 25,000 | 81 | 85 | |
| Steroid | LOQ (ng mL−1) | Linearity Range | Sample Preparation | Technique | Ref. |
|---|---|---|---|---|---|
| Mometasone furoate | 12.5 | 0.0125–2 (mg mL−1) | The extraction of 1 g of ambiphilic cream by 10 mL of acetonitrile was performed in an ultrasonic bath for 10 min at room temperature. The extract was filtered through a 0.45 mm cellulose acetate filter and then injected into the HPLC system. | HPLC-DAD | [29] |
| Hydrocortisone acetate | 15.625 | 0.01563–5 (mg mL−1) | |||
| Fluocinonide | 9 | 0.009–2.88 (mg mL−1) | |||
| Fluocinolone acetonide | 31.25 | 0.03125–7.5 (mg mL−1) | |||
| Betamethasone | 21 | 0.021–6.72 (mg mL−1) | |||
| Betamethasone dipropionate | 10.5 | 0.0105–3.36 (mg mL−1) | |||
| Triamcinolone acetonide | 21 | 0.0105–6.72 (mg mL−1) | |||
| 17α-Estradiol | 0.79 | 0.625–125 (ng mL−1) | A total of 20 mL of cosmetic sample were transferred to a 50 mL polyterafluoroethylene (PTFE) centrifuge tube. A total of 0.6 g of NaCl was added and 1 mol/L NaOH was added to adjust the pH value to 10. After vortex mixing for 30 s, 70 μL of [C6MIM][BF4] were added to the homogenized sample. The solution was homogenized for 10 s. A total of 1 mL of water solution containing 0.3588 g of NH4PF6 was added. A cloudy solution was formed as a result of the formation of fine droplets of [C6MIM][PF6], which homogeneously dispersed in the solution. After vortexing for 2 min, the resulting sample solution was centrifuged at 15,000 rpm for 8 min at −4 °C and the [C6MIM][PF6] was deposited at the bottom of the centrifuge tube. The resulting IL phase was filtered with a 0.22 μm PTFE filter membrane before HPLC analysis. | RP-HPLC-UV | [30] |
| 17α-Ethinylestradiol | 0.63 | 0.625–125 (ng mL−1) | |||
| Estrone | 0.59 | 0.625–125 (ng mL−1) | |||
| 17α-Hydroxyprogesterone | 0.23 | 0.25–50 (ng mL−1) | |||
| Medroxyprogesterone | 0.30 | 0.25–50 (ng mL−1) | |||
| Megestrol-17-acetate | 0.10 | 0.125–100 (ng mL−1) | |||
| Norethisterone acetate | 0.17 | 0.25–125 (ng mL−1) | |||
| Progesterone | 0.10 | 0.125–125 (ng mL−1) | |||
| 43 Glucocorticoids | Comprised between 1.0–90.0 | 100.0–2000.0 (ng mL−1) | One gram of each sample was taken and dissolved in 100% methanol in a 20 mL volumetric flask, and 1 mL of 0.1% formic acid in methanol solution and 0.25 mL of ISTD (200 μg mL−1) were added. After sonication for 30 min and centrifugation (at 2000 rpm) for 10 min, 4 mL of supernatant liquid were diluted with 0.1% formic acid in 80% methanol (1:5). The stock solution was filtered through a 0.22 μm PVDA filter (Millipore, Milford, CT, USA) prior to UPLC analysis. | UPLC-MS/MS | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariati Pour, S.R.; Emamiamin, A.; Zangheri, M.; Calabria, D.; Guardigli, M.; Porru, E.; Fiori, J.; Mirasoli, M. Analysis of Glucocorticoids as Potential Adulterants in Cosmetic Products: A Dual Approach for Qualitative and Quantitative Evaluation Based on ELISA and HPLC-MS Methods. Appl. Sci. 2025, 15, 414. https://doi.org/10.3390/app15010414
Shariati Pour SR, Emamiamin A, Zangheri M, Calabria D, Guardigli M, Porru E, Fiori J, Mirasoli M. Analysis of Glucocorticoids as Potential Adulterants in Cosmetic Products: A Dual Approach for Qualitative and Quantitative Evaluation Based on ELISA and HPLC-MS Methods. Applied Sciences. 2025; 15(1):414. https://doi.org/10.3390/app15010414
Chicago/Turabian StyleShariati Pour, Seyedeh Rojin, Afsaneh Emamiamin, Martina Zangheri, Donato Calabria, Massimo Guardigli, Emanuele Porru, Jessica Fiori, and Mara Mirasoli. 2025. "Analysis of Glucocorticoids as Potential Adulterants in Cosmetic Products: A Dual Approach for Qualitative and Quantitative Evaluation Based on ELISA and HPLC-MS Methods" Applied Sciences 15, no. 1: 414. https://doi.org/10.3390/app15010414
APA StyleShariati Pour, S. R., Emamiamin, A., Zangheri, M., Calabria, D., Guardigli, M., Porru, E., Fiori, J., & Mirasoli, M. (2025). Analysis of Glucocorticoids as Potential Adulterants in Cosmetic Products: A Dual Approach for Qualitative and Quantitative Evaluation Based on ELISA and HPLC-MS Methods. Applied Sciences, 15(1), 414. https://doi.org/10.3390/app15010414










