Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Olive Defatted Flour Characterization
2.2.1. Humidity and Particle Size
2.2.2. Total Fat Content
2.2.3. Lipid Characterization by Gas Chromatography
2.2.4. Total Dietary Fiber Content
2.2.5. Total Phenolic Content
2.2.6. Phenolic Compound Characterization by UHPLC-ESI-MS
2.2.7. Aqueous Extract of HD
2.3. Film Elaboration
2.4. Characterization of the Obtained Films
2.4.1. Weight and Film Thickness
2.4.2. Film Moisture Content and Water Solubility
2.4.3. Transparency and Film Transmission
2.4.4. Film Total Phenolic Content
2.5. Bioactive Packaging Films to Prevent Lipid Oxidation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of Olive Defatted Flour
3.2. The Characterization of the Phenolic Fraction from the Olive Defatted Flour
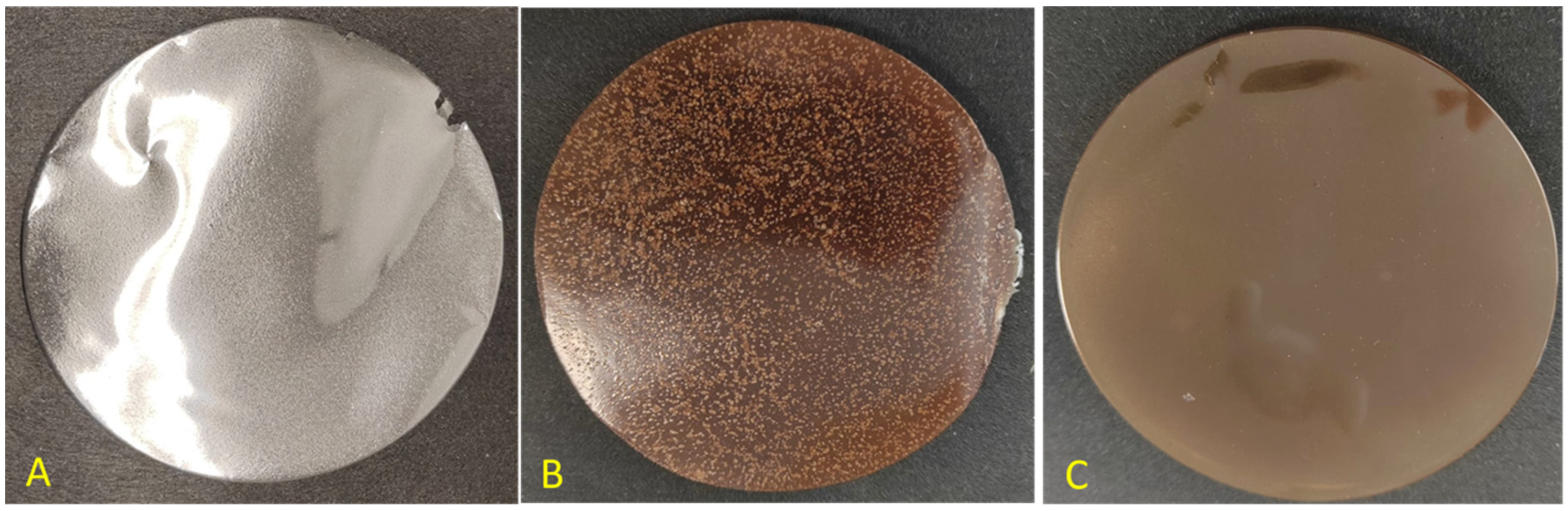
3.3. Film Characterization
3.3.1. Film Weight, Thickness, Moisture Content, and Water Solubility
3.3.2. Light Transmission and Transparency
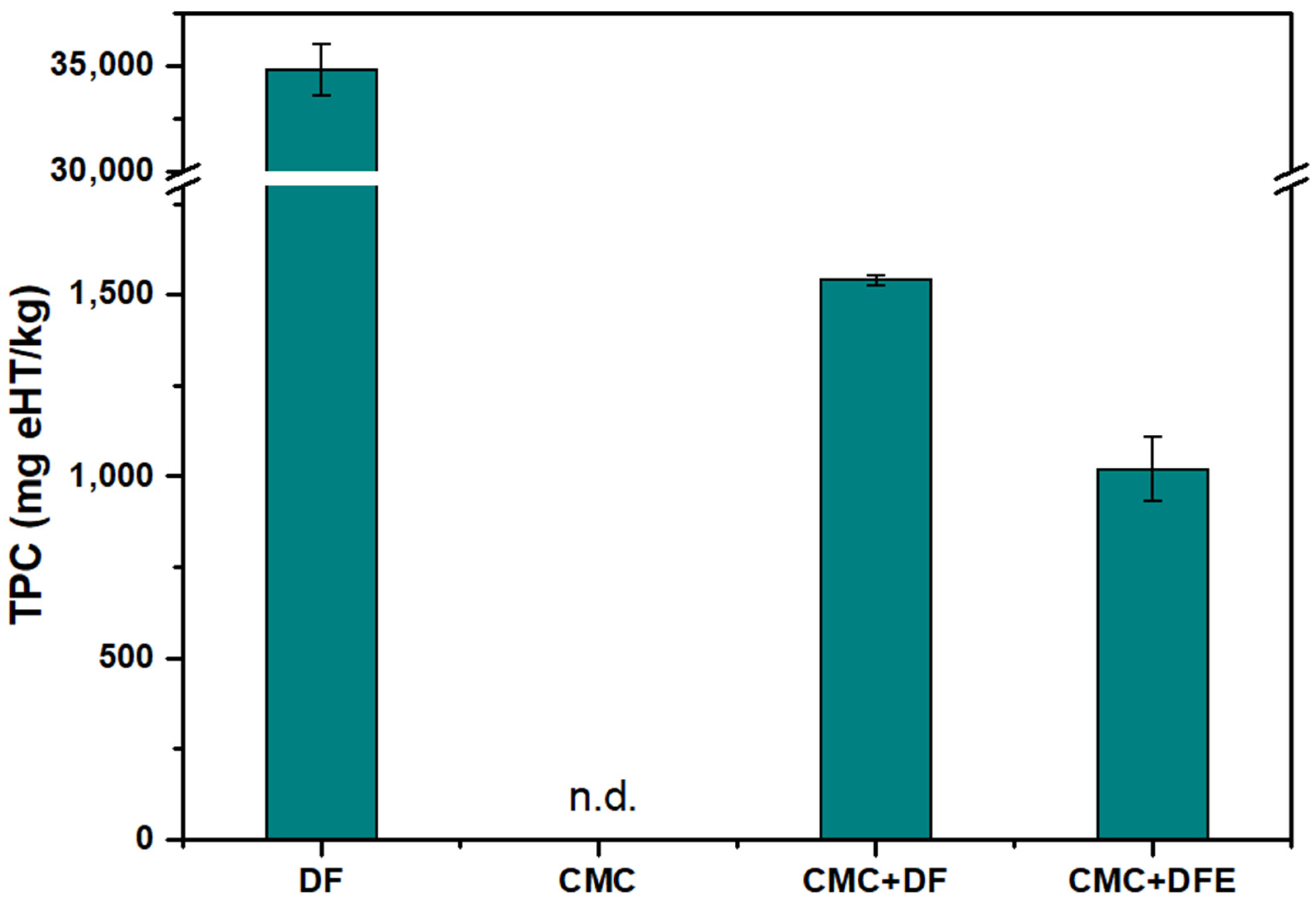
3.3.3. Total Phenolic Content of the Films
3.4. Lipid Oxidation Control Using Bioactive Packaging Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.-P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Chabni, A.; Mathioux, M.I.; López, C. Physicochemical and sensory analysis of Olive oil from roasted olives. Food Humanity 2023, 1, 1388–1397. [Google Scholar] [CrossRef]
- Chabni, A.; Vázquez, L.; Bañares, C.; Torres, C.F. Combination of Dehydration and Expeller as a Novel Methodology for the Production of Olive Oil. Molecules 2023, 28, 6953. [Google Scholar] [CrossRef] [PubMed]
- Bañares, C.; Chabni, A.; de Donlebún, B.P.; Reglero, G.; Torres, C.F. Chemical characterization of pomegranate and alfalfa seed oils obtained by a two-step sequential extraction procedure of expeller and supercritical CO2 technologies. J. Food Compos. Anal. 2023, 115, 105040. [Google Scholar] [CrossRef]
- Belbaki, A.; Louaer, W.; Meniai, A.-H. Supercritical CO2 extraction of oil from Crushed Algerian olives. J. Supercrit. Fluids 2017, 130, 165–171. [Google Scholar] [CrossRef]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida CM, P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar]
- Vidal, O.L.; Santos MC, B.; Batista, A.P.; Andrigo, F.F.; Barea, B.; Lecomte, J.; Figueroa-Espinoza, M.C.; Gontard, N.; Villeneuve, P.; Guillard, V. Active packaging films containing antioxidant extracts from green coffee oil by-products to prevent lipid oxidation. J. Food Eng. 2022, 312, 110744. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S. Active flexible films for food packaging: A review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kim, J.T.; Roy, S.; Jayakumar, A. Recent advances in carboxymethyl cellulose-based active and intelligent packaging materials: A comprehensive review. Int. J. Biol. Macromol. 2024, 129194. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Chabni, A.; Bañares, C.; Torres, C.F. Study of the oxidative stability via Oxitest and Rancimat of phenolic-rich olive oils obtained by a sequential process of dehydration and expeller and supercritical CO2 extractions. Front. Nutr. 2024, 11, 1494091. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley GH, S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Torres, C.F.; Tenllado, D.; Senorans, F.J.; Reglero, G. A versatile GC method for the analysis of alkylglycerols and other neutral lipid classes. Chromatographia 2009, 69, 729–734. [Google Scholar] [CrossRef]
- Benítez, V.; Cantera, S.; Aguilera, Y.; Mollá, E.; Esteban, R.M.; Díaz, M.F.; Martín-Cabrejas, M.A. Impact of germination on starch, dietary fiber and physicochemical properties in non-conventional legumes. Food Res. Int. 2013, 50, 64–69. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Aturki, Z.; Fanali, S.; D’Orazio, G.; Rocco, A.; Rosati, C. Analysis of phenolic compounds in extra virgin olive oil by using reversed-phase capillary electrochromatography. Electrophoresis 2008, 29, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D. Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Paié-Ribeiro, J.; Baptista, F.; Teixeira, J.; Guedes, C.; Gomes, M.J.; Teixeira, A.; Barros, A.N.; Pinheiro, V.; Outor-Monteiro, D. From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients. Appl. Sci. 2024, 14, 5586. [Google Scholar] [CrossRef]
- Zhao, H.; Avena-Bustillos, R.J.; Wang, S.C. Extraction, purification and in vitro antioxidant activity evaluation of phenolic compounds in California olive pomace. Foods 2022, 11, 174. [Google Scholar] [CrossRef]
- Sharma, A.; Ray, A.; Singhal, R.S. A biorefinery approach towards valorization of spent coffee ground: Extraction of the oil by supercritical carbon dioxide and utilizing the defatted spent in formulating functional cookies. Future Foods 2021, 4, 100090. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods—A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhong, Y.; Liu, S.; Liu, L.; Mu, X.; Chen, C.; Yang, S.; Li, G.; Zhang, D. Insoluble/soluble fraction ratio determines effects of dietary fiber on gut microbiota and serum metabolites in healthy mice. Food Funct. 2024, 15, 338–354. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Guo, Y.; Yan, X.; Liu, W.; Huang, S. Preparation and Characterization of Soluble Dietary Fiber Edible Packaging Films Reinforced by Nanocellulose from Navel Orange Peel Pomace. Polymers 2024, 16, 315. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi TR, I.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard CM, G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 85, 21–32. [Google Scholar] [CrossRef]
- Marx, Í.M.; Casal, S.; Rodrigues, N.; Cruz, R.; Veloso, A.C.; Pereira, J.A.; Peres, A.M. Does water addition during the industrial milling phase affect the chemical-sensory quality of olive oils? The case of cv. Arbequina oils. Food Chem. 2022, 395, 133570. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Collevecchio, C.; Spogli, R.; Epifano, F.; Genovese, S. Novel procedures for olive leaves extracts processing: Selective isolation of oleuropein and elenolic acid. Food Chem. 2024, 447, 139038. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Diekemper, D.; Pölloth, B.; Schwarzer, S. From agricultural waste to a powerful antioxidant: Hydroxytyrosol as a sustainable model substance for understanding antioxidant capacity. J. Chem. Educ. 2021, 98, 2610–2617. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Flôres, S.H. Active food packaging prepared with chitosan and olive pomace. Food Hydrocoll. 2018, 74, 139–150. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Costa, T.M.; de Oliveira Rios, A.; Flôres, S.H. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crops Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Zhang, X.; Zhou, Z.; Wang, C.; Pan, Y.; Hu, B.; Liu, C.; Pan, C.; Shen, C. Transparent ultrahigh-molecular-weight polyethylene/MXene films with efficient UV-absorption for thermal management. Nat. Commun. 2024, 15, 3076. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Banares, C.; Chabni, A.; Reglero, G.; Torres, C.F. Oxidative stability of microalgae oil and its acylglycerol mixture obtained by enzymatic glycerolysis and the antioxidant effect of supercritical rosemary extract. LWT 2022, 171, 114150. [Google Scholar] [CrossRef]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 long-chain polyunsaturated fatty acid content and oxidation state of fish oil supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef] [PubMed]
- Bastante, C.C.; Cardoso, L.C.; Ponce MT, F.; Serrano, C.M.; De La Ossa-Fernández EJ, M. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]

| Olive Defatted Flour | |
|---|---|
| Humidity (%) | 2.17 ± 0.33 |
| Granulometry (%) | |
| Diameter (µm) | |
| >500 | 32.31 ± 1.23 |
| 250–500 | 27.81 ± 0.50 |
| 100–250 | 24.70 ± 1.12 |
| 45–100 | 13.93 ± 0.63 |
| <45 | 1.37 ± 0.22 |
| TFC (%) | 2.43 ± 0.21 |
| Lipid composition (%) | |
| Fatty acid | 0.71 ± 0.19 |
| DAG | 1.74 ± 0.14 |
| Sterol esters | 1.58 ± 0.28 |
| TAG | 42.72 ± 9.69 |
| TPC (ppm) | 34,856 ± 1220 |
| Total dietary fiber (%) | |
| IDF | 28.40 ± 1.75 |
| SDF | 2.28 ± 0.34 |
| IDF:SDF | 12.62 ± 1.11 |
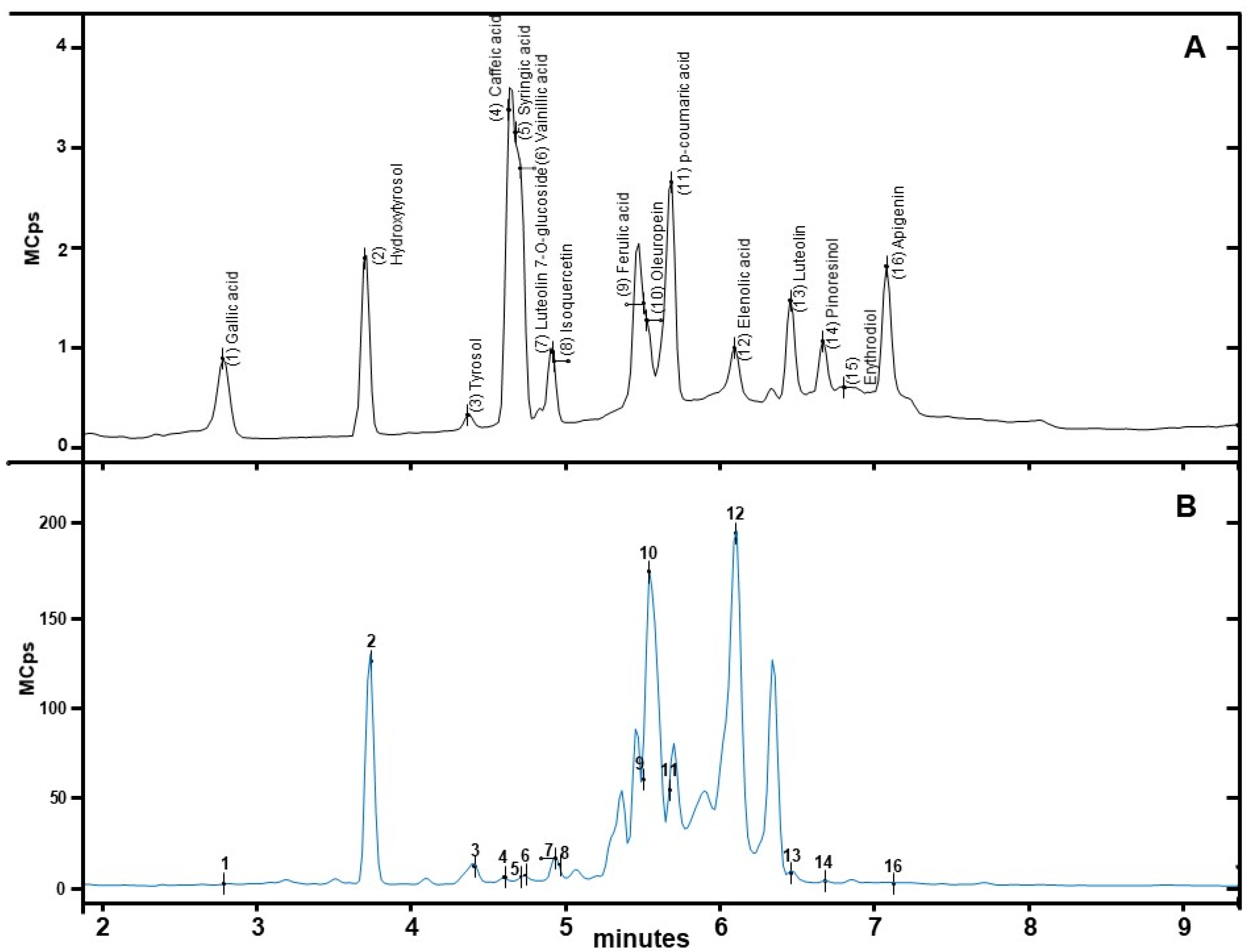
| Peak No. | Class/Phenolic Compounds | Rt | [M − H]− (m/z) | mg/kg DF |
|---|---|---|---|---|
| 1 | Gallic acid | 2.78 ± 0.00 | 169 | 1.69 ± 0.33 |
| 2 | Hydroxytyrosol (HT) | 3.69 ± 0.02 | 153 | 1524.74 ± 178.16 |
| 3 | Tyrosol (TYR) | 4.92 ± 0.57 | 137 | 156.86 ± 41.95 |
| 4 | Caffeic acid | 4.63 ± 0.00 | 179 | 0.76 ± 0.12 |
| 5 | Syringic acid | 4.66 ± 0.01 | 197 | 0.25 ± 0.03 |
| 6 | Vanillic acid | 4.72 ± 0.00 | 167 | 1.40 ± 0.17 |
| 7 | Luteolin-7-O-glucoside | 4.92 ± 0.00 | 447 | 210.56 ± 10.10 |
| 8 | Isoquercitine | 4.93 ± 0.00 | 463 | 2.27 ± 0.39 |
| 9 | Ferulic acid | 5.50 ± 0.00 | 193 | 1.02 ± 0.00 |
| 10 | Oleuropein | 5.53 ± 0.01 | 539 | 4324.74 ± 408.87 |
| 11 | p-Coumaric acid | 5.66 ± 0.02 | 163 | 0.05 ± 0.00 |
| 12 | Elenolic acid (EA) | 6.08 ± 0.02 | 241 | 3602.75 ± 1555.62 |
| 13 | Luteolin | 6.47 ± 0.00 | 285 | 13.31 ± 0.68 |
| 14 | Pinoresinol | 6.66 ± 0.00 | 357 | 9.22 ± 0.63 |
| 15 | Erythrodiol | 6.81 ± 0.02 | 441 | n.q. |
| 16 | Apigenin | 7.08 ± 0.00 | 269 | 0.45 ± 0.02 |
| Σ Polyphenols | 8891.11 ± 763.72 | |||
| Films | Weight (g) | Thickness (µm) | Moisture (%) | WS (%) |
|---|---|---|---|---|
| CMC | 0.68 ± 0.05 b | 62 ± 7.49 c | 6.8 ± 0.25 c | 100 a |
| CMC + DF | 0.82 ± 0.02 a | 108 ± 16 a | 12.5 ± 1.51 a | 52.6 ± 2.62 b |
| CMC + DFE | 0.72 ± 0.03 b | 80 ± 8.10 b | 9.7 ± 0.73 b | 100 a |
| Films | Transmittance (%) | Transparency (%) | |||||
|---|---|---|---|---|---|---|---|
| 200 nm | 300 nm | 400 nm | 500 nm | 600 nm | 800 nm | 600 nm | |
| CMC | 0.07 ± 0.01 a | 67.3 ± 0.04 a | 82.8 ± 0.00 a | 85.5 ± 0.01 a | 86.7 ± 0.00 a | 88.2 ± 0.01 a | 1.03 ± 0.03 c |
| CMC + DF | 0.02 ± 0.01 b | 0.02 ± 0.00 b | 0.00 ± 0.00 c | 0.71 ± 0.00 c | 3.72 ± 0.00 c | 14.2 ± 0.00 c | 11.5 ± 1.70 a |
| CMC + DFE | 0.03 ± 0.01 b | 0.02 ± 0.00 b | 1.23 ± 0.01 b | 19.6 ± 0.01 b | 38.9 ± 0.01 b | 64.7 ± 0.00 b | 5.0 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabni, A.; Bañares, C.; Sanchez-Rey, I.; Torres, C.F. Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products. Appl. Sci. 2025, 15, 312. https://doi.org/10.3390/app15010312
Chabni A, Bañares C, Sanchez-Rey I, Torres CF. Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products. Applied Sciences. 2025; 15(1):312. https://doi.org/10.3390/app15010312
Chicago/Turabian StyleChabni, Assamae, Celia Bañares, Irene Sanchez-Rey, and Carlos F. Torres. 2025. "Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products" Applied Sciences 15, no. 1: 312. https://doi.org/10.3390/app15010312
APA StyleChabni, A., Bañares, C., Sanchez-Rey, I., & Torres, C. F. (2025). Active Biodegradable Packaging Films Based on the Revalorization of Food-Grade Olive Oil Mill By-Products. Applied Sciences, 15(1), 312. https://doi.org/10.3390/app15010312







