Aqueous Theta-Phase Aluminum Oxide Nanofluid for Energy Applications: Experimental Study on Thermal Conductivity
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Nanofluids
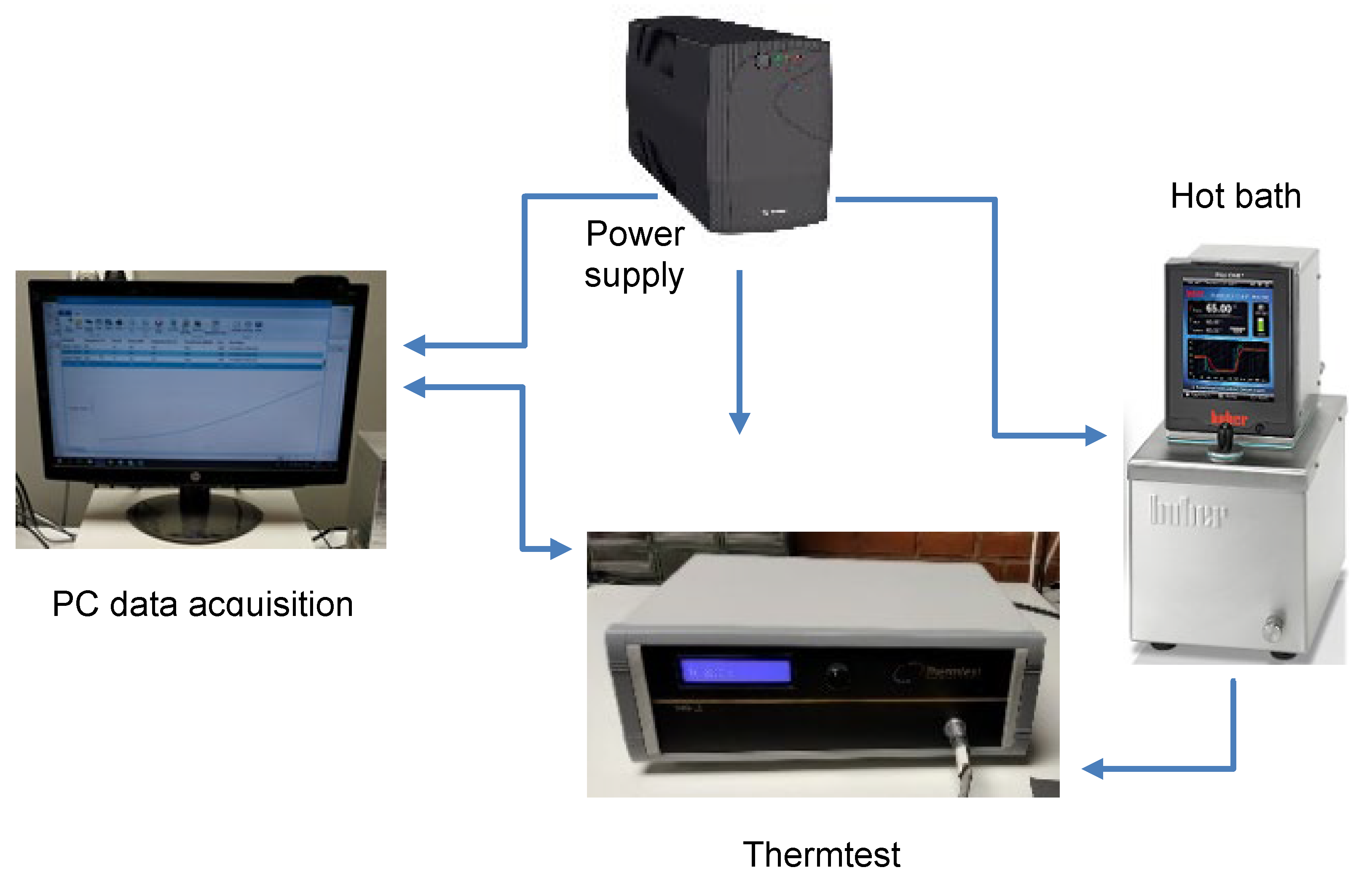
2.2. Equipment and Measurement Procedure
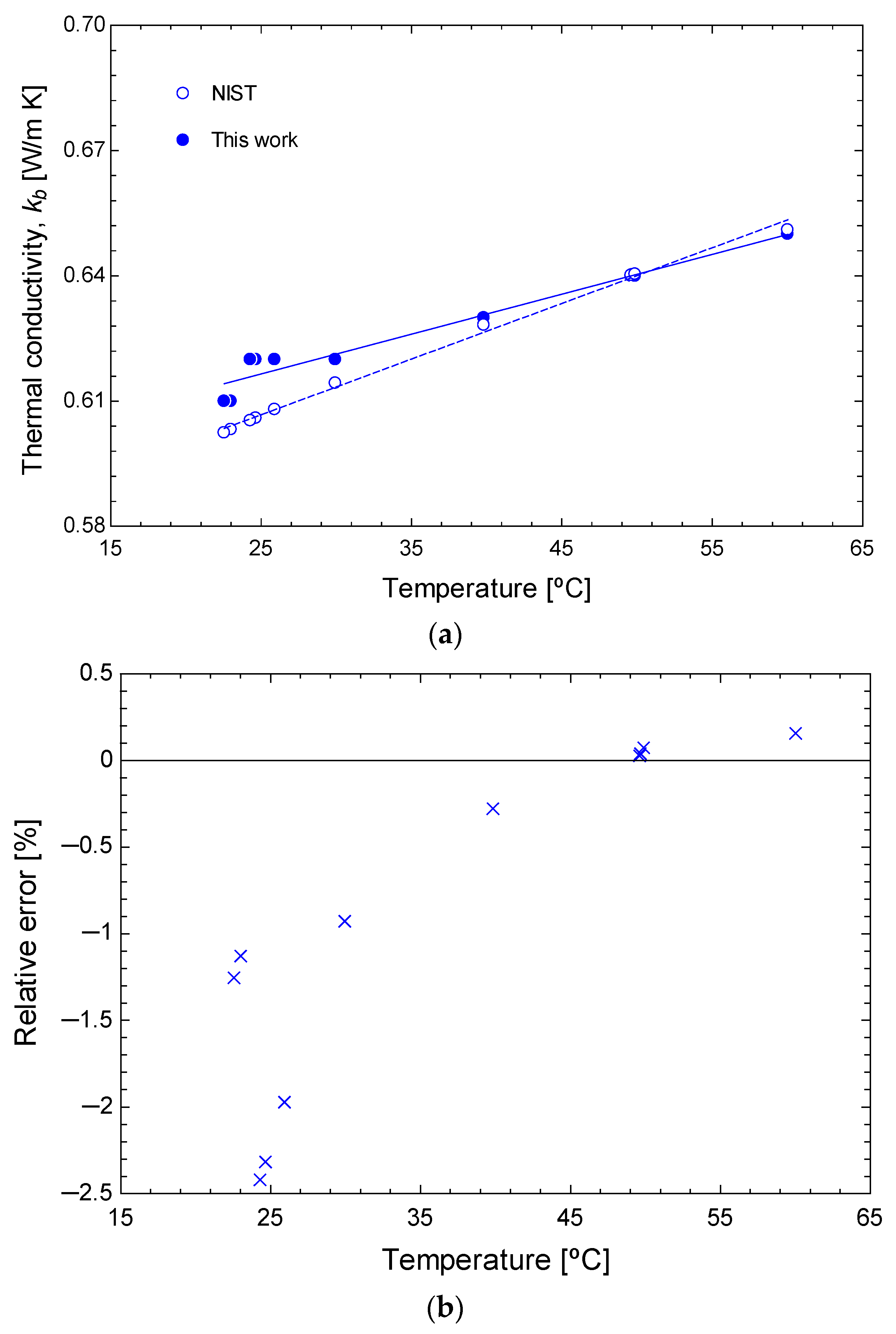
2.3. Calibration
3. Results and Discussion
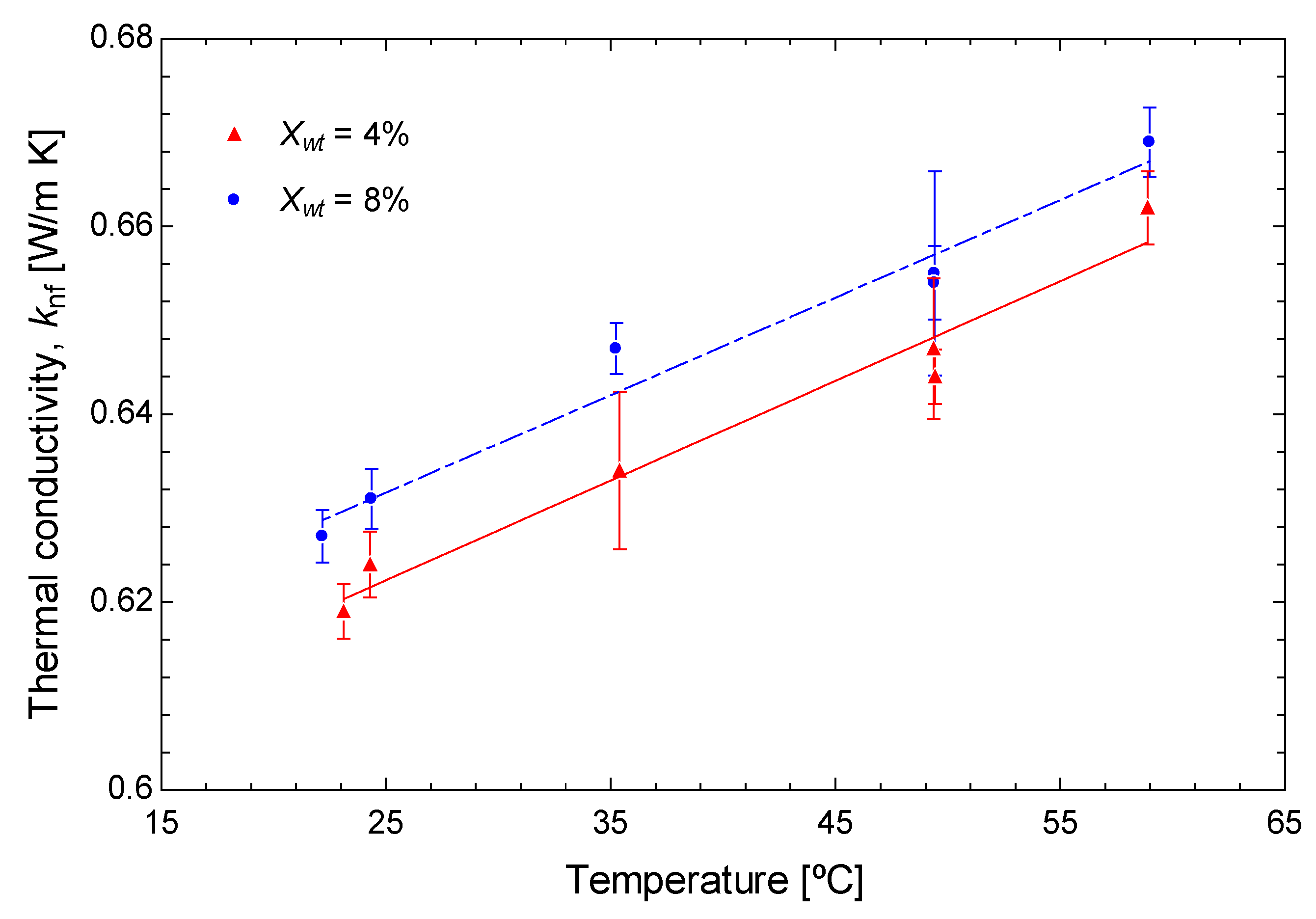
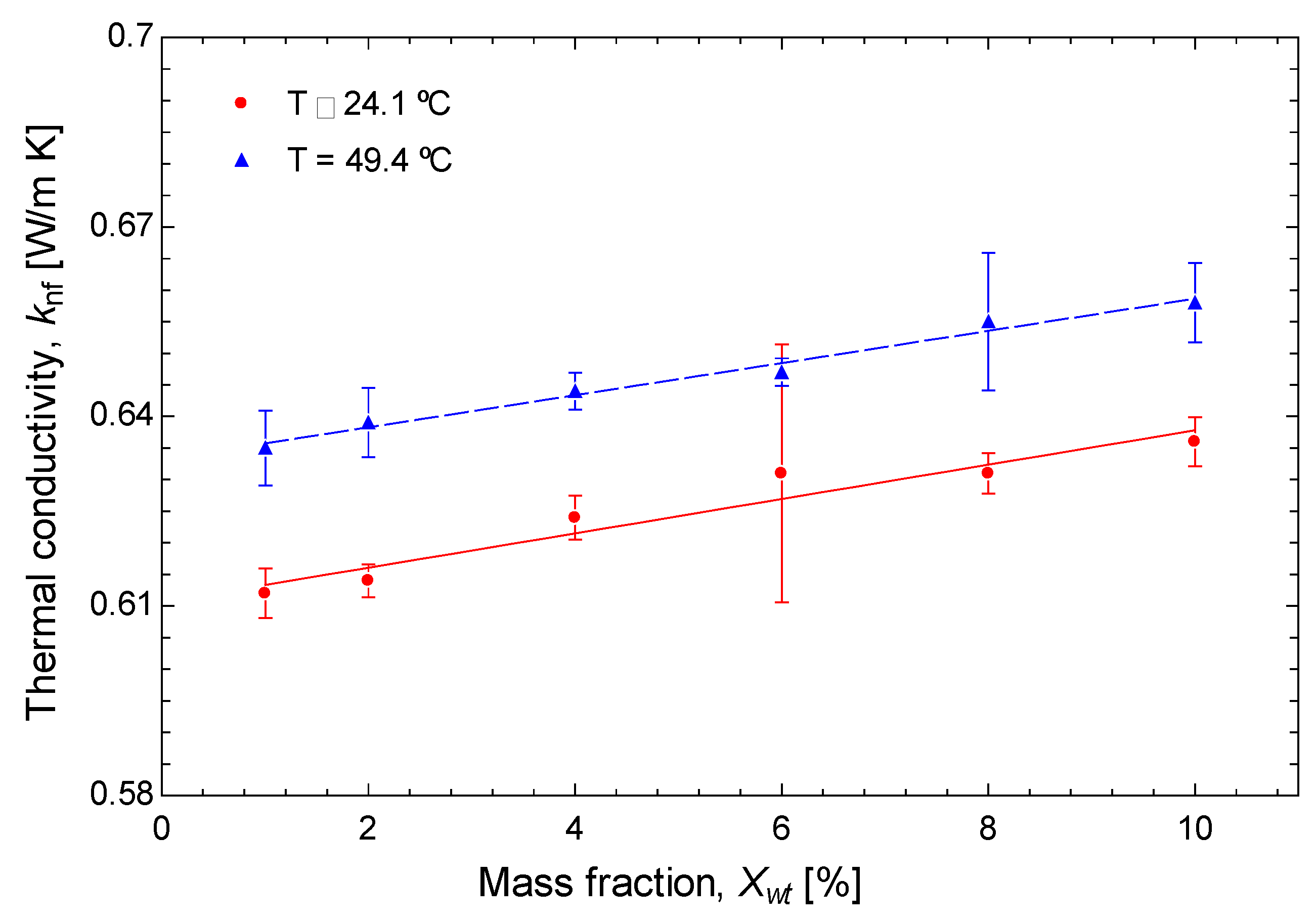
3.1. Experimental Results
3.2. Empirical Correlation
4. Conclusions
- Thermal conductivity is improved by the temperature increase (0.001 W/m·K per °C increase) and nanoparticle concentration increment (0.0027 W/m·K per unit of wt% increase).
- A high sonication time (16.5 h), not used before in the open literature, was tested. The increase in the sonication time in the range investigated (from 1.5 to 16.5 h) has a negligible effect on the enhancement of thermal conductivity.
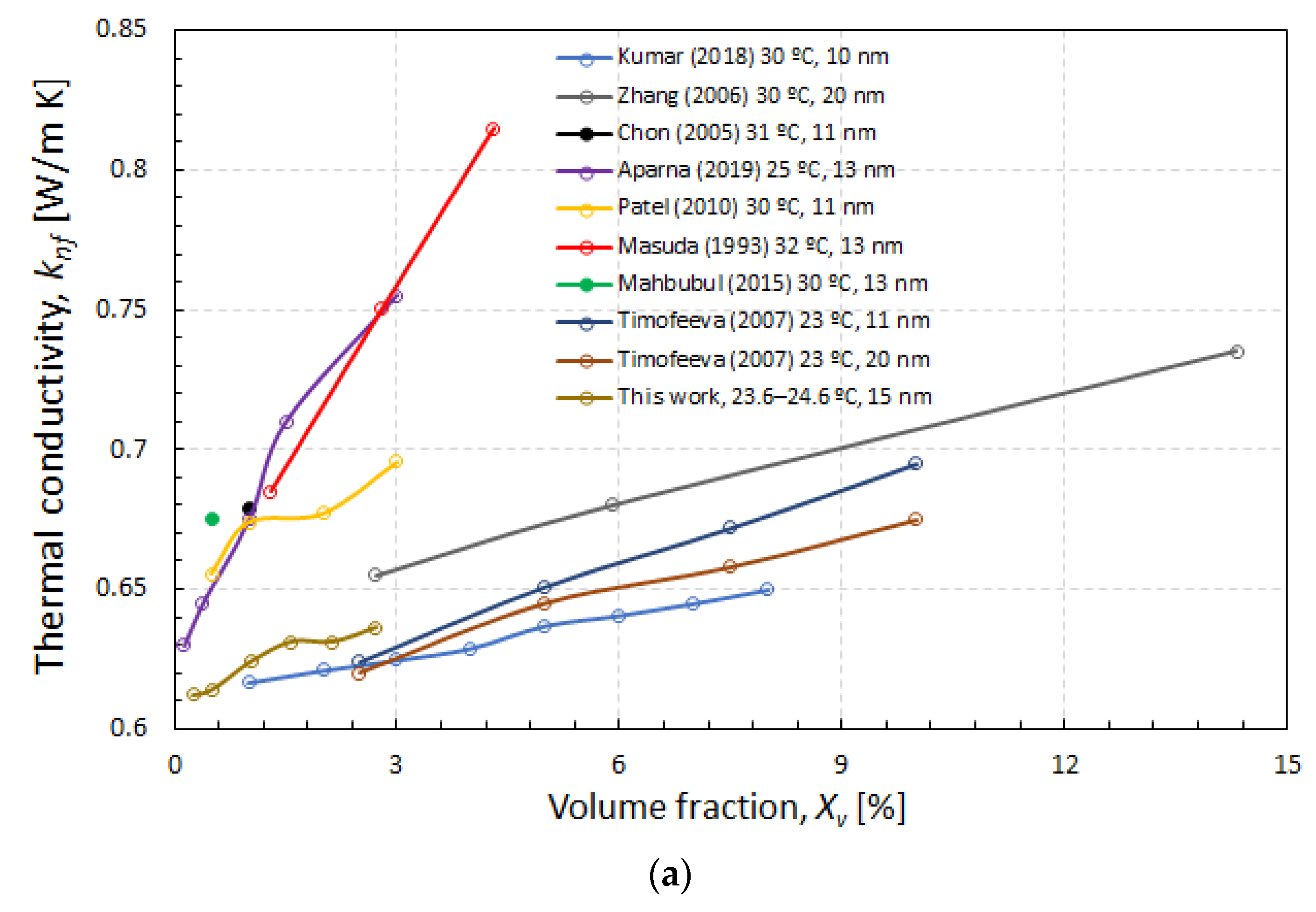
- Comparing current experimental data with values taken from the literature, it can be concluded that a high dispersion of thermal conductivity data exists, evenly using the same nanoparticle volume fraction, close temperatures, and nanoparticle sizes. The volume fraction dependence of the thermal conductivity obtained in this work seems to be approximately similar to the one obtained in some of the previous reported data (three works). In the rest of cases (five works), a much higher increase in thermal conductivity with the nanoparticle volume fraction was obtained. Two of these last studies used gamma-phase nanoparticles, while, in the rest of investigations, the phase was not specified.
- A new correlation has been derived for the thermal conductivity that provides an excellent agreement with respect to experimental data. This correlation can be used to accurately predict the thermal conductivity of the aqueous theta-phase aluminum oxide nanofluid. The correlation significantly improves predictions with respect to the empirical correlations available in the open literature.
- Experimental data obtained and the correlation derived can be used to predict the performance of energy systems using theta-phase alumina-water nanofluids as working fluid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chilambarasan, L.; Thangarasu, V.; Ramasamy, P. Solar flat plate collector’s heat transfer enhancement using grooved tube configuration with alumina nanofluids: Prediction of outcomes through artificial neural network modeling. Energy 2024, 289, 129953. [Google Scholar]
- Ram, S.; Yadav, S.K.; Kumar, A. Recent advancement of nanofluids in solar concentrating collectors: A brief review. Mater. Today Proc. 2023, 72, 2032–2038. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, H.; Park, J.T.; Delgado, A.; Kim, S. Experimental investigation on evaluation of thermal performance of solar heating system using Al2O3 nanofluid. Appl. Sci. 2020, 10, 5521. [Google Scholar] [CrossRef]
- Venegas, M.; García-Hernando, N.; Zacarías, A.; de Vega, M. Performance of a solar absorption cooling system using nanofluids and a membrane-based microchannel desorber. Appl. Sci. 2020, 10, 2761. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Dehaj, M.S. An experimental study on energetic performance evaluation of a parabolic trough solar collector operating with Al2O3/water and GO/water nanofluids. Energy 2021, 234, 121317. [Google Scholar]
- Ali, A.M.; Angelino, M.; Rona, A. Physically consistent implementation of the mixture model for modelling nanofluid conjugate heat transfer in minichannel heat sinks. Appl. Sci. 2022, 12, 7011. [Google Scholar] [CrossRef]
- Hatami, M.; Mosayebidorcheh, S.; Jing, D. Thermal performance evaluation of alumina-water nanofluid in an inclined direct absorption solar collector (IDASC) using numerical method. J. Mol. Liq. 2017, 231, 632–639. [Google Scholar] [CrossRef]
- Ojeda, J.A.; Messina, S. Enhancing energy harvest in a constructal solar collector by using alumina-water as nanofluid. Sol. Energy 2017, 147, 381–389. [Google Scholar] [CrossRef]
- Eastman, J.A.; Phillpot, S.R.; Choi, S.U.S.; Keblinski, P. Thermal transport in nanofluids. Annu. Rev. Mater. Res. 2004, 34, 219–246. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat transfer in nanofluids-A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Akilu, S.; Sharma, K.V.; Baheta, A.T.; Mamat, R. A review of thermophysical properties of water based composite nanofluids. Renew. Sust. Energ. Rev. 2016, 66, 654–678. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Experimental study on the effective thermal conductivity and thermal diffusivity of nanofluids. Int. J. Thermophys. 2006, 27, 569–580. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Chandra Bose, A. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp. Therm. Fluid Sci. 2010, 34, 210–216. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307. [Google Scholar] [CrossRef]
- Ho, C.J.; Liu, W.K.; Chang, Y.S.; Lin, C.C. Natural convection heat transfer of alumina-water nanofluid in vertical square enclosures: An experimental study. Int. J. Therm. Sci. 2010, 49, 1345–1353. [Google Scholar] [CrossRef]
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107. [Google Scholar] [CrossRef]
- Li, C.H.; Peterson, G.P. Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J. Appl. Phys. 2006, 99, 141–148. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Kumar, N.; Sonawane, S.S.; Sonawane, S.H. Experimental study of thermal conductivity, heat transfer and friction factor of Al2O3 based nanofluid. Int. Commun. Heat Mass Transf. 2018, 90, 1–10. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Moldoveanu, G.M.; Huminic, G.; Minea, A.A.; Huminic, A. Experimental study on thermal conductivity of stabilized Al2O3 and SiO2 nanofluids and their hybrid. Int. J. Heat Mass Transf. 2018, 127, 450–457. [Google Scholar] [CrossRef]
- Aparna, Z.; Monisha, M.; Pabi, S.K.; Ghosh, S. Thermal conductivity of aqueous Al2O3/Ag hybrid nanofluid at different temperatures and volume concentrations: An experimental investigation and development of new correlation function. Powder Technol. 2019, 343, 714–722. [Google Scholar] [CrossRef]
- Patel, H.E.; Sundararajan, T.; Das, S.K. An experimental investigation into the thermal conductivity enhancement in oxide and metallic nanofluids. J. Nanopart. Res. 2010, 12, 1015–1031. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K.; Hishinuma, N. Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluids Nanofluids 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Shahrul, I.M.; Khaleduzzaman, S.S.; Saidur, R.; Amalina, M.A.; Turgut, A. Experimental investigation on effect of ultrasonication duration on colloidal dispersion and thermophysical properties of alumina–water nanofluid. Int. J. Heat Mass Transf. 2015, 88, 73–81. [Google Scholar] [CrossRef]
- Buonomo, B.; Manca, O.; Marinelli, L.; Nardini, S. Effect of temperature and sonication time on nanofluid thermal conductivity measurements by nano-flash method. Appl. Therm. Eng. 2015, 91, 181–190. [Google Scholar] [CrossRef]
- Das, P.K.; Islam, M.; Santra, A.K.; Ganguly, R. Experimental investigation of thermophysical properties of Al2O3–water nanofluid: Role of surfactants. J. Mol. Liq. 2017, 237, 304–312. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; Mc Closkey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.M.; Afrand, M.; Żyła, G.; Ahn, H.S.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef] [PubMed]
- Jbara, A.S.; Othaman, Z.; Ati, A.A.; Saeed, M.A. Characterization of γ-Al2O3 nanopowders synthesized by co-precipitation method. Mater. Chem. Phys. 2017, 188, 24–29. [Google Scholar] [CrossRef]
- PlasmaChem. Nanomaterials and Related Products. Catalogue & Price-List, PlasmaChem Surface and Nanotechnology, 3rd ed.; PlasmaChem GmbH: Berlin, Germany, 2018. [Google Scholar]
- Yakovlev, I.V.; Volodin, A.M.; Zaikovskii, V.I.; Stoyanovskii, V.O.; Lapina, O.B.; Vedyagin, A.A. Stabilizing effect of the carbon shell on phase transformation of the anocrystalline alumina particles. Ceram. Int. 2018, 44, 4801–4806. [Google Scholar] [CrossRef]
- Vainer, B.G.; Volodin, A.M.; Shepelin, A.V. Hydration-induced thermal behavior of crystalline and amorphous dispersed alumina. Thermochim. Acta 2021, 706, 179066. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; Mc Linden, M.O. NIST Standard Reference Database 23, Reference Fluid Thermodynamic and Transport Properties (REFPROP), version 9.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Dawood, H.K.; Mohammed, H.A.; Sidik, N.A.C.; Munisamy, K.M. Numerical investigation on heat transfer and friction factor characteristics of laminar and turbulent flow in an elliptic annulus utilizing nanofluid. Int. Commun. Heat Mass Transf. 2015, 66, 148–157. [Google Scholar] [CrossRef]
- Klein, S.A. Engineering Equation Solver © 1992–2019, Professional V10.612-3D (2019/03/06). License #5491. F-Chart Software: Madison, WI, USA, 2019.
- Buongiorno, J. Convective transport in nanofluids. J. Heat Transf. 2006, 128, 240–250. [Google Scholar] [CrossRef]




| Reference | Phase | Size (nm) | Fraction (%) | Temperature (°C) | Method |
|---|---|---|---|---|---|
| [12] | n/a | 38.4 | 0–4.3 vol | Room temperature | THW |
| [13] | γ | 28 | 2.4–5.5 vol | 24 | SS |
| [14] | n/a | 20 | 0–40 wt | 10–50 | THW |
| [15] | n/a | 43 | 0–3 vol | Room temperature | THW |
| [16] | n/a | 38 | 0.3–3 vol | n/a | THW |
| [17] | n/a | 33 | 0–4 vol | 5–40 | THW |
| [18] | n/a | 11, 47, 150 | 1–4 vol | 21–71 | THW |
| [19] | n/a | 36 | 2–10 vol | 27.2–35.7 | SS |
| [20] | n/a | 80, 150 | 1 vol | 20–60 | THW |
| [21] | n/a | 10 | 1–8 vol | 30–50 | THW |
| [22] | n/a | 38.4 | 0–4 vol | 21–51 | TO |
| [23] | n/a | 43 | 1–3 vol | 20–50 | THW |
| [24] | γ | 13 | 0.1–3 vol | 25–52 | THW |
| [25] | n/a | 11, 45, 150 | 0–3 vol | 20–50 | THW, TO |
| [26] | γ | 13 | 1.2–4.3 vol | 32–67 | THW |
| [27] | n/a | 36, 47 | 0–18 vol | 20–48 | THW |
| [28] | γ | 25 | 2–3 vol | 15–45 | THW |
| [29] | n/a | 13 | 0.5 vol | 10–50 | THW |
| [30] | n/a | 40 | 0.1–20 vol | 25–65 | NF |
| [31] | n/a | ~50 | 0.1–2 vol | 20–60 | THW |
| [32] | n/a | 11, 20, 40 | 0–10 vol | 23 | THW |
| Component | CAS Reg. No. | Supplier | Purity | Purification Method |
|---|---|---|---|---|
| Alumina | 1344-28-1 | PlasmaChem GmbH (Berlin, Germany) | >99.8% | Ignition |
| Variable | Range | Accuracy |
|---|---|---|
| Thermal conductivity, k (W/m K) | 0.01–2 | ±5% |
| Temperature, T (°C) | −50–100 | ±5% |
| Nanoparticle Mass Fraction (%) | Temperature (°C) | Thermal Conductivity (W/m K) | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| 2 | 23.50 | 0.40 | 0.615 | 0.003 |
| 2 | 49.38 | 0.08 | 0.643 | 0.007 |
| 4 | 23.7 | 0.62 | 0.622 | 0.004 |
| 4 | 49.39 | 0.08 | 0.645 | 0.005 |
| 6 | 49.41 | 0.08 | 0.651 | 0.007 |
| 8 | 23.27 | 1.14 | 0.629 | 0.003 |
| 8 | 49.41 | 0.08 | 0.655 | 0.007 |
| 10 | 23.37 | 1.31 | 0.634 | 0.004 |
| 10 | 49.43 | 0.07 | 0.659 | 0.004 |
| Reference | Correlation | Particle Size (nm) | Fraction, Xv (vol%) | Temperature (°C) |
|---|---|---|---|---|
| [17] | 33 | 1–4 | 26 | |
| [19] | 36 | 2–10 | 27.2–35.7 | |
| [23] | 43 | 1–3 | 25 | |
| [25] | 10–150 | 0.1–3 | 20–50 | |
| [27] | 36, 47 | 0–18 | 21–23 | |
| [41] | 13 | 1.34–4.33 | 27 |
| Reference | Relative Error (%) | |
|---|---|---|
| Average of Absolute Values | Range | |
| [17] | 21.7 | 1.8–45 |
| [19] | 31.4 | −3.5–65.8 |
| [23] | 23.6 | 4.3–42.5 |
| [25] | 20.2 | 7–32.2 |
| [27] | 7.0 | 0.4–14 |
| [41] | 38.7 | 6.1–69.9 |
| i | c | d |
|---|---|---|
| 1 | 2.72 × 10−3 | 5.54 × 10−4 |
| 2 | 4.80 × 10−6 | 7.20 × 10−6 |
| a = 5.94 × 10−1 | b = −8.28 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacarías, A.; de Vega, M.; García-Hernando, N.; Venegas, M. Aqueous Theta-Phase Aluminum Oxide Nanofluid for Energy Applications: Experimental Study on Thermal Conductivity. Appl. Sci. 2024, 14, 3225. https://doi.org/10.3390/app14083225
Zacarías A, de Vega M, García-Hernando N, Venegas M. Aqueous Theta-Phase Aluminum Oxide Nanofluid for Energy Applications: Experimental Study on Thermal Conductivity. Applied Sciences. 2024; 14(8):3225. https://doi.org/10.3390/app14083225
Chicago/Turabian StyleZacarías, Alejandro, Mercedes de Vega, Néstor García-Hernando, and María Venegas. 2024. "Aqueous Theta-Phase Aluminum Oxide Nanofluid for Energy Applications: Experimental Study on Thermal Conductivity" Applied Sciences 14, no. 8: 3225. https://doi.org/10.3390/app14083225
APA StyleZacarías, A., de Vega, M., García-Hernando, N., & Venegas, M. (2024). Aqueous Theta-Phase Aluminum Oxide Nanofluid for Energy Applications: Experimental Study on Thermal Conductivity. Applied Sciences, 14(8), 3225. https://doi.org/10.3390/app14083225







