Synergistic Effect and Phase Behavior of SCG-CAPB-H2O Ternary Compound System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Surface Tension Measurement
2.4. UV–Visible Spectrophotometer
2.5. Phase Behavior
2.6. Polarized Optical Microscopy (POM)
2.7. Small-Angle X-ray Scattering (SAXS)
2.8. Viscosity Measurement
3. Results and Discussion
3.1. Synergistic Effect of SCG/CAPB Ternary Compound System
3.1.1. CMC of SCG/CAPB System
3.1.2. Krafft Point of Sodium Cocoyl Glycinate (SCG)
3.1.3. Critical Packing Parameter (CPP) of Sodium Cocoyl Glycinate (SCG)
3.1.4. The Interaction Parameter (β) for the SCG/CAPB System
3.2. The Phase Behavior of SCG/CAPB System
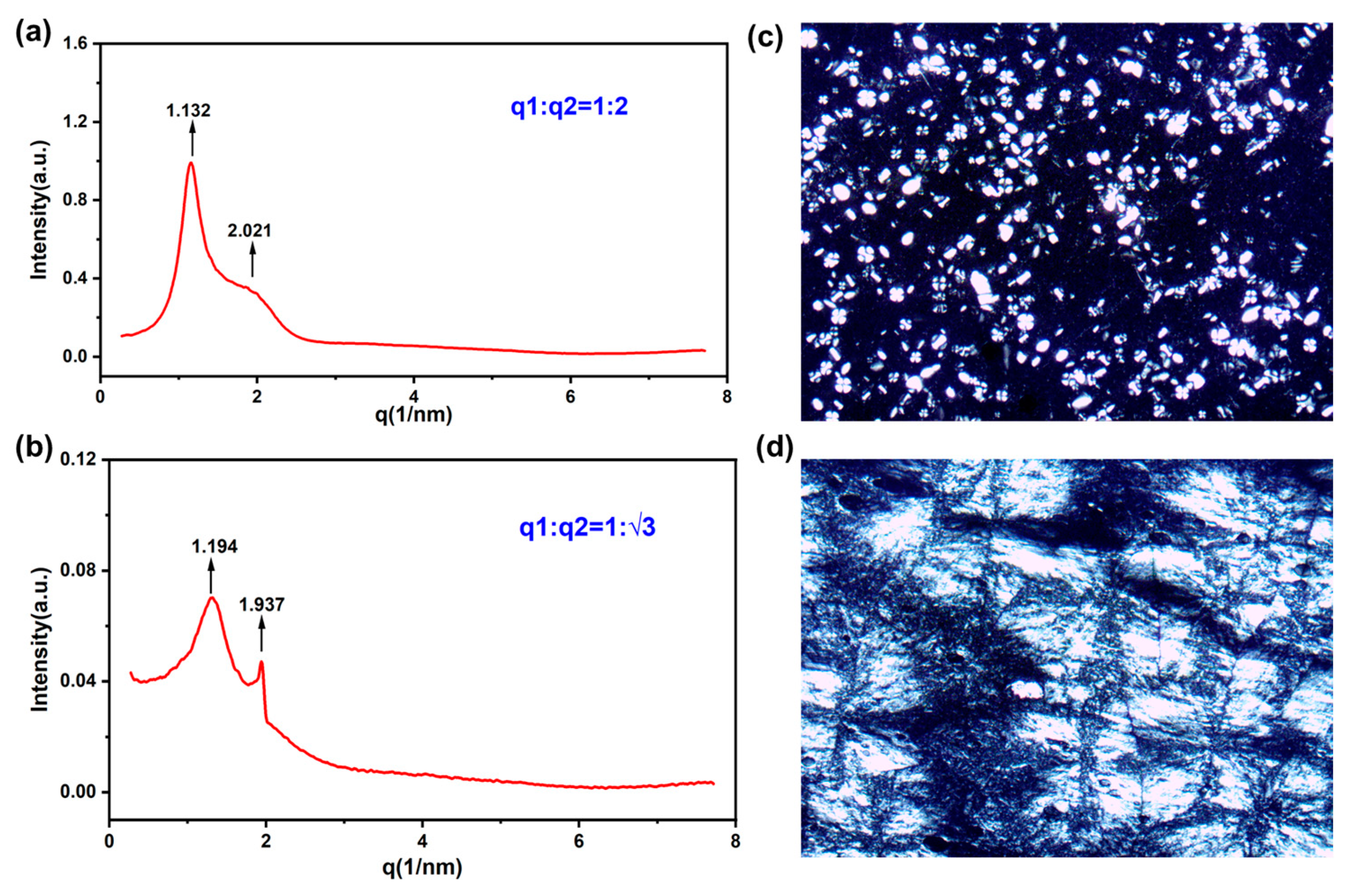
3.2.1. POM and SAXS Results
3.2.2. Phase Diagram of SCG/CAPB/H2O Ternary Compound System
3.2.3. Mechanism of Phase Transition over SCG/CAPB/H2O System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Jia, Z.L.; Sun, H.T.; Liao, L.J.; Wen, Q.Z. Rheological behavior and microstructure of an anionic surfactant micelle solution with pyroelectric nanoparticle. Colloids Surf. A 2012, 395, 267–275. [Google Scholar] [CrossRef]
- Panmai, S.; Prud’homme, R.K.; Peiffer, D.G. Rheology of hydrophobically modified polymers with spherical and rod-like surfactant micelles. Colloids Surf. A 1999, 147, 3–15. [Google Scholar] [CrossRef]
- Choi, T.S.; Shimizu, Y.; Shirai, H.; Hamada, K. Solubilization of disperse dyes in cationic gemini surfactant micelles. Dyes Pigments 2000, 45, 145–152. [Google Scholar] [CrossRef]
- Gong, L.Y.; Liao, G.Z.; Chen, Q.S.; Luan, H.X.; Feng, Y.J. Swollen surfactant micelles: Properties and applications. Acta Phys.-Chim. Sin. 2019, 35, 816–828. [Google Scholar] [CrossRef]
- Qiao, W.; Zheng, Z.; Peng, H.; Shi, L. Synthesis and properties of three series amino acid surfactants. Tenside Surfactants Deterg. 2012, 49, 161–166. [Google Scholar] [CrossRef]
- Feng, S.H.; Wang, H.X.; Zhang, G.Y.; Xie, X.L. Rheological properties of lyotropic liquid crystal. Prog. Chem. 2004, 16, 687–695. [Google Scholar]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering-ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, A.; Atef, F.; Oertli, A.G.; Stucky, G.D.; Chmelka, B.F. Alkaline lyotropic silicate-surfactant liquid crystals. J. Am. Chem. Soc. 1997, 119, 3596–3610. [Google Scholar] [CrossRef]
- Braun, P.V.; Osenar, P.; Tohver, V.; Kennedy, S.B.; Stupp, S.I. Nanostructure templating in inorganic solids with organic lyotropic liquid crystals. J. Am. Chem. Soc. 1999, 121, 7302–7309. [Google Scholar] [CrossRef]
- Li, G.Z.; Gu, Q.; Yan, L.C. Study of lyotropic liquid crystal containing nonionic surfactant of AEO-9. Acta Chim. Sin. 2001, 59, 6–10. [Google Scholar]
- Alam, M.M.; Matsumoto, Y.; Aramaki, K. Effects of surfactant hydrophilicity on the oil solubilization and rheological behavior of a nonionic hexagonal phase. J. Surfactants Deterg. 2014, 17, 19–25. [Google Scholar] [CrossRef]
- Funari, S.S. Induction of a hexagonal phase in phospholipid-surfactant bilayers. Eur. Biophys. J. 1998, 27, 590–594. [Google Scholar] [CrossRef]
- Linemann, R.; Lauger, J.; Schmidt, G.; Kratzat, K.; Richtering, W. Linear and nonlinear rheology of micellar solutions in the isotropic, cubic and hexagonal phase probed by rheo-small-angle light-scattering. Rheol. Acta 1995, 34, 440–449. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, X.M.; Lee, S.; Pfefferle, L.D.; Haller, G.L. Surfactant chain length effect on the hexagonal-to-cubic phase transition in mesoporous silica synthesis. Microporous Mesoporous Mater. 2012, 147, 242–251. [Google Scholar] [CrossRef]
- Gutberlet, T.; Dietrich, U.; Klose, G.; Rapp, G. X-ray diffraction study of the lamellar-hexagonal phase transition in phospholipid/surfactant mixtures. J. Colloid Interface Sci. 1998, 203, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.C.; Basri, M.; Omar, D.; Rahman, M.B.A.; Salleh, A.; Rahman, R. Self-assembly behaviour of alkylpolyglucosides (APG) in mixed surfactant-stabilized emulsions system. J. Mol. Liq. 2011, 158, 175–181. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.L.; Yan, H.; Wang, Y.; Feng, J. Self-assembly behaviors of heterogemini surfactant in aqueous solution investigated by dissipative particle dynamics. J. Dispers. Sci. Technol. 2014, 35, 1300–1307. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhao, Y.R.; Han, S.Y.; Chen, C.X.; Xu, H. Self-assembly of surfactant-like peptides and their applications. Sci. China-Chem. 2014, 57, 1634–1645. [Google Scholar] [CrossRef]
- Svenson, S. Controlling surfactant self-assembly. Curr. Opin. Colloid Interface Sci. 2004, 9, 201–212. [Google Scholar] [CrossRef]
- Alzahid, Y.A.; Mostaghimi, P.; Walsh, S.D.C.; Armstrong, R.T. Flow regimes during surfactant flooding: The influence of phase behaviour. Fuel 2019, 236, 851–860. [Google Scholar] [CrossRef]
- Zhang, K.W.; Karlstrom, G.; Lindman, B. Phase-behavior of systems of a nonionic surfactant and a nonionic polymer in aqueous-solution. Colloids Surf. 1992, 67, 147–155. [Google Scholar] [CrossRef]
- Chen, L.F.; Shang, Y.Z.; Liu, H.L.; Hu, Y. Phase behavior of n-butanol/n-octane/water/cationic gemini surfactant system. J. Dispers. Sci. Technol. 2006, 27, 317–323. [Google Scholar] [CrossRef]
- Branco, M.A.; Pinherio, L.; Faustino, C. Amino acid-based cationic gemini surfactant-protein interactions. Colloids Surf. A 2015, 480, 105–112. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Insights into the composition, structure-function relationship, and molecular organization of surfactants from spent coffee grounds. Food Hydrocoll. 2022, 124, 107204. [Google Scholar] [CrossRef]
- Infante, M.R.; Pérez, L.; Pinazo, A.; Clapés, P.; Morán, M.C.; Angelet, M.; García, M.T.; Vinardell, M.P. Amino acid-based surfactants. Comptes Rendus Chim. 2004, 7, 583–592. [Google Scholar] [CrossRef]
- FAINERMAN, V.B.; MILLER, R. Dynamic surface tensions of surfactant mixtures at the water-air interface. Colloids Surf. A 1995, 97, 65–82. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic effect of mixed surfactant systems on foam behavior and surface tension. J. Surfactants Deterg. 2013, 16, 621–630. [Google Scholar] [CrossRef]
- Shalel, S.; Streichman, S.; Marmur, A. Monitoring surfactant-induced hemolysis by surface tension measurement. J. Colloid Interface Sci. 2002, 255, 265–269. [Google Scholar] [CrossRef]
- Manyala, D.L.; Varade, D. Formation and characterization of microemulsion with novel anionic sodium n-lauroylsarcosinate for personal care. J. Mol. Liq. 2021, 343, 117657. [Google Scholar] [CrossRef]
- Niu, C.; Xia, W.; Li, Y.; Bu, X.; Wang, Y.; Xie, G. Insight into the low-rank coal flotation using amino acid surfactant as a promoter. Fuel 2022, 307, 121810. [Google Scholar] [CrossRef]
- Godek, E.; Grzadka, E.; Maciolek, U.; Bastrzyk, A. Influence of zwitterionic CAPB on flocculation of the aqueous cationic guar gum/glauconite suspensions at various pH. Int. J. Mol. Sci. 2021, 22, 12157. [Google Scholar] [CrossRef]
- Tzocheva, S.S.; Kralchevsky, P.A.; Danov, K.D.; Georgieva, G.S.; Post, A.J.; Ananthapadmanabhan, K.P. Solubility limits and phase diagrams for fatty acids in anionic (SLES) and zwitterionic (CAPB) micellar surfactant solutions. J. Colloid Interface Sci. 2012, 369, 274–286. [Google Scholar] [CrossRef] [PubMed]
- El-Dossoki, F.I.; Abdalla, N.S.Y.; Gomaa, E.A.; Hamza, O.K. An insight into thermodynamic and association behaviours of cocamidopropyl betaine (CAPB) surfactant in water and water-alcohol mixed media. SN Appl. Sci. 2020, 2, 690. [Google Scholar] [CrossRef]
- Ganjoo, R.; Sharma, S.; Thakur, A.; Assad, H.; Sharma, P.K.; Dagdag, O.; Berisha, A.; Seydou, M.; Ebenso, E.E.; Kumar, A. Experimental and theoretical study of sodium cocoyl glycinate as corrosion inhibitor for mild steel in hydrochloric acid medium. J. Mol. Liq. 2022, 364, 119988. [Google Scholar] [CrossRef]
- Manna, S.; Adak, D.; Manna, S.; Maity, S.; Jana, S.; Bhattacharya, R.; Medda, S.K. Antireflection cum photocatalytic with superhydrophilic based durable single layer mesoporous TiO2-ZrO2 coating surface for efficient solar photovoltaic application. Sustain. Energy Technol. Assess. 2023, 57, 103236. [Google Scholar] [CrossRef]
- Grundke, K.; Uhlmann, P.; Gietzelt, T.; Redlich, B.; Jacobasch, H.J. Studies on the wetting behaviour of polymer melts on solid surfaces using the wilhelmy balance method. Colloids Surf. A. 1996, 116, 93–104. [Google Scholar] [CrossRef]
- Wu, N.; Dai, J.L.; Micale, F.J. Dynamic surface tension measurement with a dynamic wilhelmy plate technique. J. Colloid Interface Sci. 1999, 215, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Zhang, D.R.; Zeng, Z.; Lytton, R.L. Effect of surface tension on the measurement of surface energy components of asphalt binders using the wilhelmy plate method. Constr. Build. Mater. 2015, 98, 900–909. [Google Scholar] [CrossRef]
- Inoue, T.; Dong, B.; Zheng, L.Q. Phase behavior of binary mixture of 1-dodecyl-3-methylimidazolium bromide and water revealed by differential scanning calorimetry and polarized optical microscopy. J. Colloid Interface Sci. 2007, 307, 578–581. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yuan, J.; Ma, H.C.; Li, N.; Zheng, L.Q.; Inoue, T. Aqueous phase behavior of ionic liquid-related gemini surfactant revealed by differential scanning calorimetry and polarized optical microscopy. Colloid Polym. Sci. 2011, 289, 213–218. [Google Scholar] [CrossRef]
- Ma, G.Q.; Yuan, X.B.; Sheng, J. Phase structure in blends of polypropylene with ethylene-propylene rubber: A study by small angle X-ray scattering. Acta Polym. Sin. 2002, 1, 63–67. [Google Scholar]
- Lang, P.; Marczuk, P.; Lermann, E.; Möller, M. Gels of semifluorinated alkanes: Structural investigations by small angle X-ray scattering. Berichte Bunsen-Ges.-Phys. Chem. Chem. Phys. 1998, 102, 1644–1647. [Google Scholar] [CrossRef]
- Nishikawa, K.; Morita, T. Fluid behavior at supercritical states studied by small-angle X-ray scattering. J. Supercrit. Fluids 1998, 13, 143–148. [Google Scholar] [CrossRef]
- Lin, T.L.; Yu, T.L.; Liu, W.J.; Tsai, Y.M. Phase segregation of crosslinked polyurethane by small angle X-ray scattering. Polym. J. 1999, 31, 120–126. [Google Scholar] [CrossRef]
- Rafique, A.S.; Khodaparast, S.; Poulos, A.S.; Sharratt, W.N.; Robles, E.S.; Cabral, J.T. Micellar structure and transformations in sodium alkylbenzenesulfonate (NaLAS) aqueous solutions: Effects of concentration, temperature, and salt. Soft Matter 2020, 16, 7835–7844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, B.; Han, F.; Zhou, Y.; Liu, H.; Li, Y.; Cui, L.; Tan, T.; Wang, N. Green synthesis, composition analysis and surface active properties of sodium cocoyl glycinate. Am. J. Anal. Chem. 2013, 4, 445–450. [Google Scholar] [CrossRef]
- Yea, D.; Lee, S.; Jo, S.; Yu, H.; Lim, J. Preparation of environmentally friendly amino acid-based anionic surfactants and characterization of their interfacial properties for detergent products formulation. J. Surfactants Deterg. 2018, 21, 541–552. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevska, S.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. Mixed solutions of anionic and zwitterionic surfactant (betaine): Surface-tension isotherms, adsorption, and relaxation kinetics. Langmuir 2004, 20, 5445–5453. [Google Scholar] [CrossRef]
- Ouverney Ferreira, M.; Câmara de Assis, H.F.; Percebom, A.M. Cocamidopropyl betaine can behave as a cationic surfactant and electrostatically associate with polyacids of high molecular weight. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 654, 130123. [Google Scholar] [CrossRef]
- Wilson, K.M.; Danielson, N.D. Micellar and sub-micellar chromatography with a cocamidopropyl betaine surfactant. J. Chromatogr. A 2022, 1681, 463442. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Pal, A.; Rakshit, A.; Saha, B. Properties and applications of amphoteric surfactant: A concise review. J. Surfactants Deterg. 2021, 24, 709–730. [Google Scholar] [CrossRef]
- Yavrukova, V.I.; Radulova, G.M.; Danov, K.D.; Kralchevsky, P.A.; Xu, H.; Ung, Y.W.; Petkov, J.T. Rheology of mixed solutions of sulfonated methyl esters and betaine in relation to the growth of giant micelles and shampoo applications. Adv. Colloid Interface Sci. 2020, 275, 102062. [Google Scholar] [CrossRef] [PubMed]
- Staszak, K.; Wieczorek, D.; Michocka, K. Effect of sodium chloride on the surface and wetting properties of aqueous solutions of cocamidopropyl betaine. J. Surfactants Deterg. 2015, 18, 321–328. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, J.; Yan, L.; Zhao, M. Adsorption behavior of cocamidopropyl betaine under conditions of high temperature and high salinity. J. Appl. Polym. Sci. 2014, 131, 40424. [Google Scholar] [CrossRef]
- Kareem, S.H.; Sattar, B. Adsorption properties for aqueous solution of binary mixture of cocamidopropyl betaine-sodiumdodecyl sulfate surfactants on air-liquid interface. Int. J. Sci. 2015, 24, 50–58. [Google Scholar]
- Kamrath, R.F.; Franses, E.I. Thermodynamics of mixed micellization. Pseudo-phase separation models. Ind. Eng. Chem. Fundam. 1983, 22, 230–239. [Google Scholar] [CrossRef]
- Shinoda, K.; Hutchinson, E. Pseudo-phase separation model for thermodynamic calculations on micellar solutions1. J. Phys. Chem. 1962, 66, 577–582. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Deng, S.-P.; Li, X.-K. Micellization and synergistic interaction of binary surfactant mixtures based on sodium nonylphenol polyoxyethylene ether sulfate. J. Colloid Interface Sci. 2008, 318, 389–396. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. A new classification of isotherms for gibbs adsorption of gases on solids. Fluid Phase Equilib. 1999, 158, 557–563. [Google Scholar] [CrossRef]
- Zhang, S.M. Gibbs adsorption isotherm for concentration as variable. Surf. Rev. Lett. 2005, 12, 379–389. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76, 137–152. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2004; pp. 60–62. [Google Scholar]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Tanford, C. Micelle shape and size. J. Phys. Chem. 1972, 76, 3020–3024. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Rubingh, D.N. Mixed Micelle Solutions. In Solution Chemistry of Surfactants; Mittal, K.L., Ed.; Springer New York: Boston, MA, USA, 1979; Volume 1, pp. 337–354. [Google Scholar]
- Park, B.D.; Youm, J.K.; Jeong, S.K.; Choi, E.H.; Ahn, S.K.; Lee, S.H. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type iii synthetic ceramide. J. Investig. Dermatol. 2003, 121, 794–801. [Google Scholar] [CrossRef]






| X1 | CMC (mmol·L−1) | CMC* (mmol·L−1) | γCMC (mN·m−1) | pC20 |
|---|---|---|---|---|
| 0 | 0.974 | - | 32.8 | 4.69 |
| 0.2 | 0.172 | 2.124 | 25.9 | 4.21 |
| 0.4 | 0.148 | 1.640 | 26.8 | 4.36 |
| 0.6 | 0.152 | 1.336 | 26.7 | 4.38 |
| 0.8 | 0.185 | 1.127 | 22.4 | 4.41 |
| 1 | 3.013 | - | 29.3 | 3.62 |
| Parameter | Value |
|---|---|
| Krafft point | 36 |
| 3.085 | |
| 0.538 | |
| CPP | 0.7935 |
| 0.2 | 0.490 | 0.529 | −10.112 | −12.531 | −1.124 | −0.902 | −2.419 | 0.222 |
| 0.4 | 0.531 | 0.558 | −9.854 | −12.152 | −2.298 | |||
| 0.6 | 0.567 | 0.583 | −9.606 | −11.685 | −2.079 | |||
| 0.8 | 0.612 | 0.631 | −9.254 | −11.012 | −1.758 |
| Sample No. | SCG (wt%) | CAPB (wt%) | H2O (wt%) |
|---|---|---|---|
| 1 | 5 | 10 | 85 |
| 2 | 5 | 40 | 55 |
| 3 | 15 | 45 | 40 |
| 4 | 15 | 25 | 60 |
| Sample No. | SCG (wt%) | CAPB (wt%) | H2O (wt%) | Phase | Viscosity (Pa·S) |
|---|---|---|---|---|---|
| 5 | 15 | 5 | 80 | S-L1 | 0.5168 |
| 6 | 5 | 15 | 80 | L1 | 0.0072 |
| 7 | 5 | 25 | 70 | L1 | 0.0176 |
| 8 | 10 | 30 | 60 | Lα | 1.1907 |
| 9 | 10 | 35 | 55 | Lα | 6.7612 |
| 10 | 15 | 35 | 50 | H | 16.3508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhang, J.; Wang, F.; Feng, W.; Dang, L.; Wei, H. Synergistic Effect and Phase Behavior of SCG-CAPB-H2O Ternary Compound System. Appl. Sci. 2024, 14, 3081. https://doi.org/10.3390/app14073081
Zhu Z, Zhang J, Wang F, Feng W, Dang L, Wei H. Synergistic Effect and Phase Behavior of SCG-CAPB-H2O Ternary Compound System. Applied Sciences. 2024; 14(7):3081. https://doi.org/10.3390/app14073081
Chicago/Turabian StyleZhu, Zhendong, Jiahao Zhang, Feihong Wang, Wenhui Feng, Leping Dang, and Hongyuan Wei. 2024. "Synergistic Effect and Phase Behavior of SCG-CAPB-H2O Ternary Compound System" Applied Sciences 14, no. 7: 3081. https://doi.org/10.3390/app14073081
APA StyleZhu, Z., Zhang, J., Wang, F., Feng, W., Dang, L., & Wei, H. (2024). Synergistic Effect and Phase Behavior of SCG-CAPB-H2O Ternary Compound System. Applied Sciences, 14(7), 3081. https://doi.org/10.3390/app14073081






