Development of a Thermal Separation Probe Gas Chromatography–Mass Spectrometry Method for Evaluating Wax–Resin Removal by Evolon® CR
Abstract
1. Introduction
2. Materials and Methods
3. Results
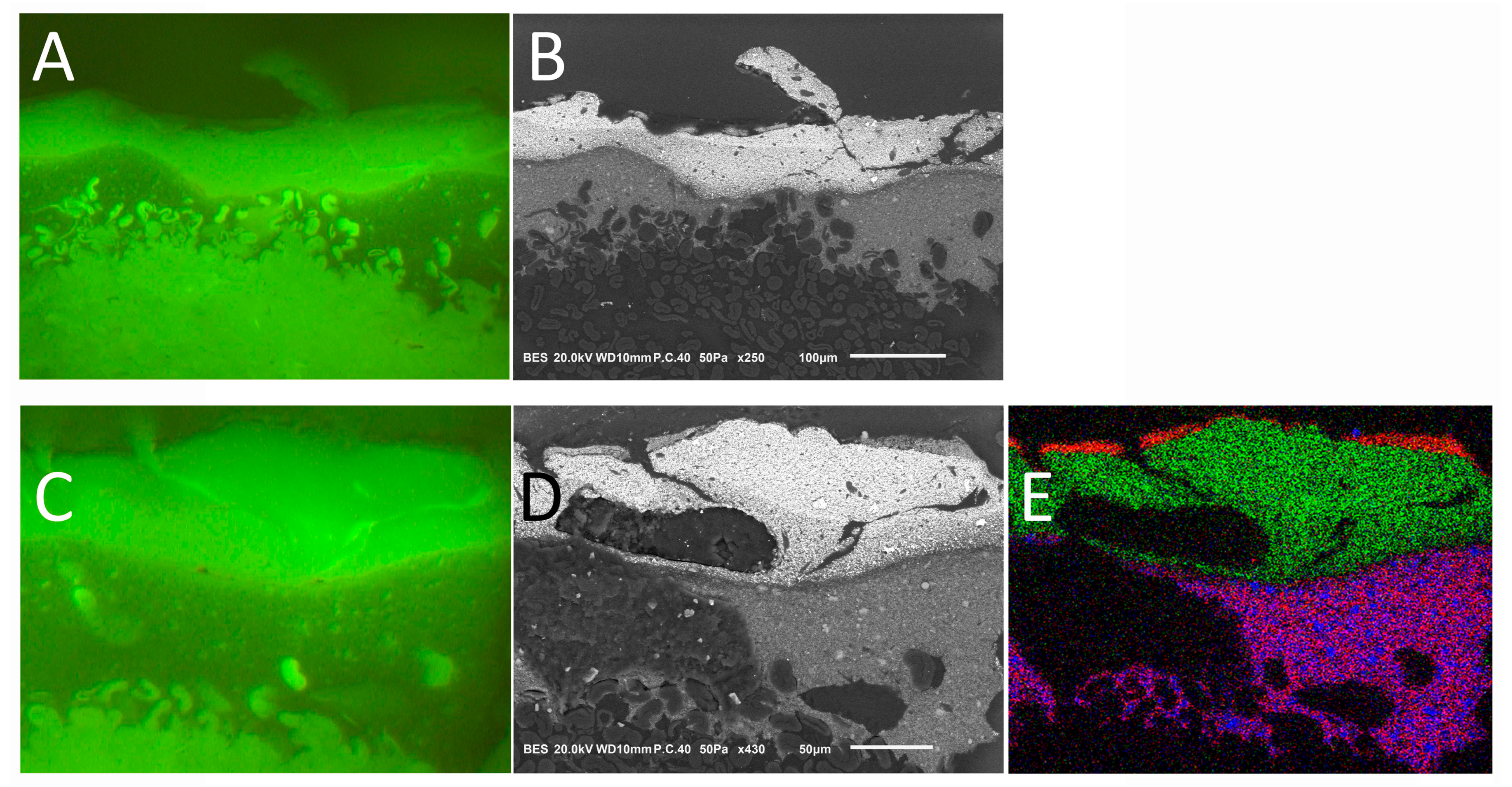
3.1. Characterization of Radishes
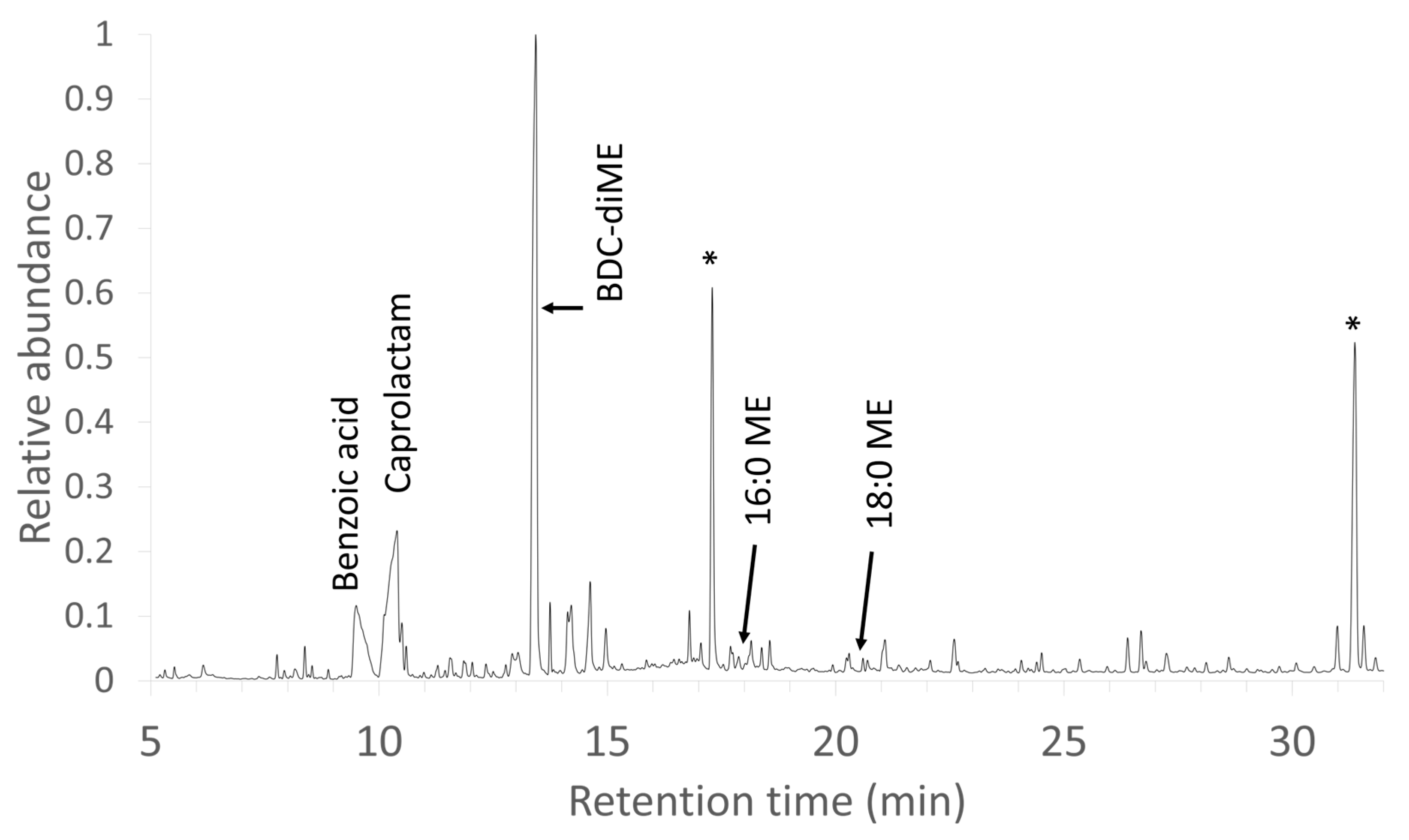
3.2. TSP-GC/MS Characterization of New, Unused Evolon
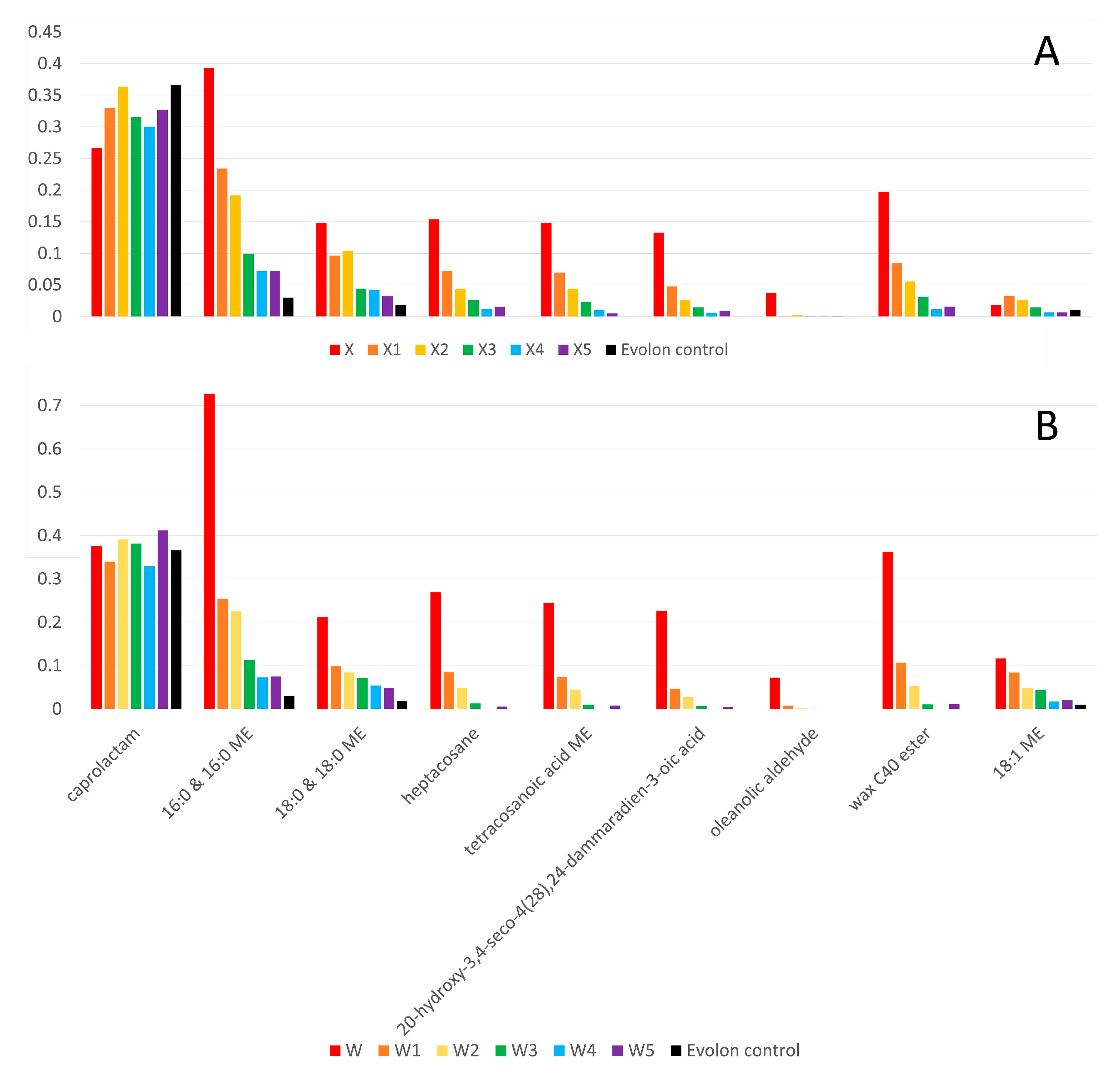
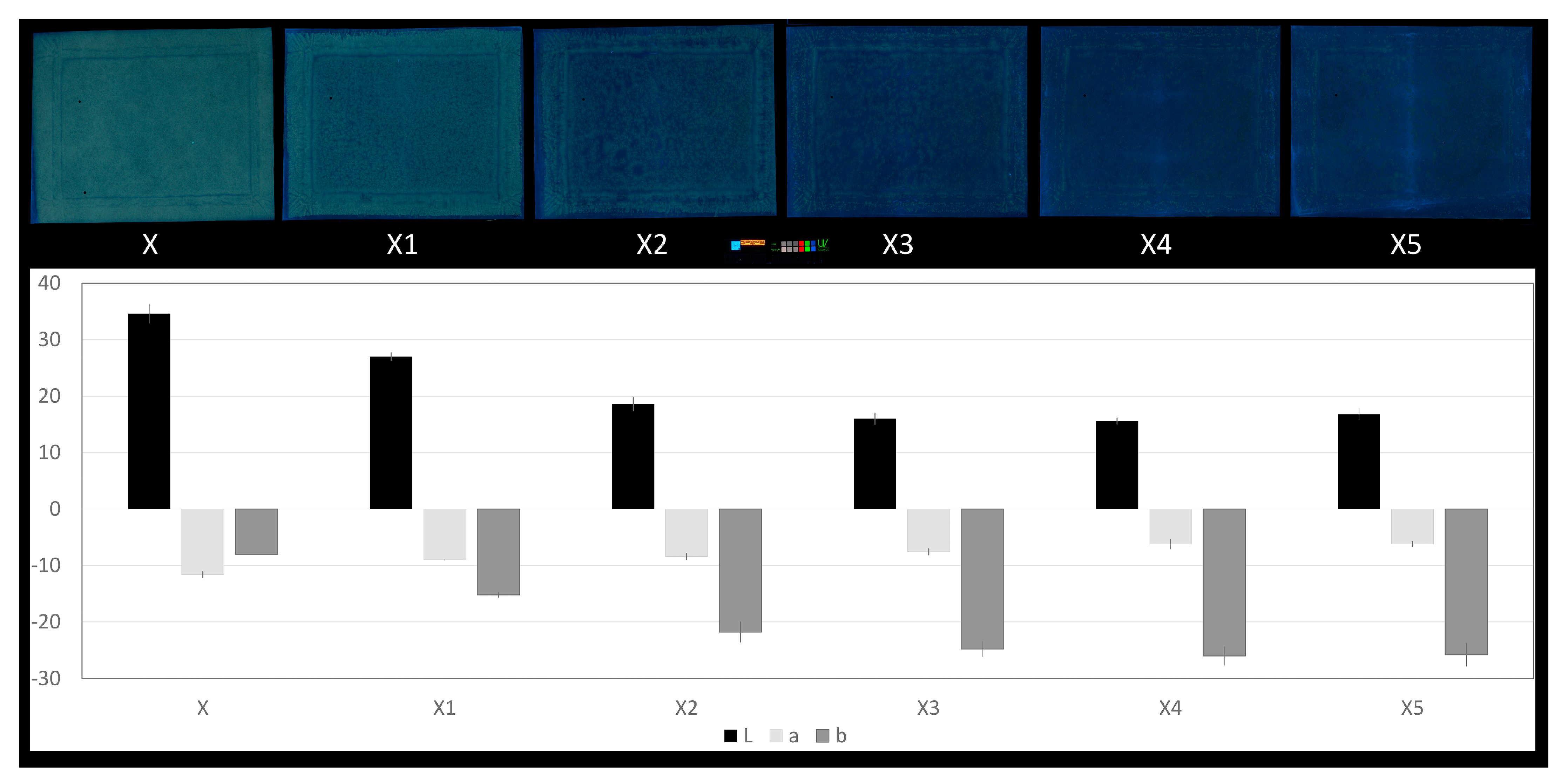
3.3. Documentation of Wax–Resin Removal by TSP-GC/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baij, L.; Liu, C.; Buijs, J.; Alvarez Martin, A.; Westert, D.; Raven, L.; Geels, N.; Noble, P.; Sprakel, J.; Keune, K. Understanding and optimizing Evolon® CR for varnish removal from oil paintings. Herit. Sci. 2021, 9, 17. [Google Scholar] [CrossRef]
- Evolon® CR. Available online: https://deffner-johann.de/pub/media/datasheets/2219005/EN/2219005_Technical%20Data%20Sheet_Evolon%20CR%20Sheet_EN_DJ.PDF (accessed on 17 October 2018).
- Tauber, G.; Smelt, S.; Noble, P.; Kirsch, K.; Siejek, A.; Keune, K.; van Keulen, H.; Smulders-De Jong, S.; Erdman, R. Evolon CR: Its use from a scientific and practical conservation perspective. AIC Paint. Spec. Group Postprints 2018, 31, 45–50. [Google Scholar]
- Vergeer, M.; van den Berg, K.J.; van Oudheusden, S.; Stols-Witlox, M. Evolon® CR Microfibre Cloth as a Tool for Varnish Removal. In Conservation of Modern Oil Paintings; van den Berg, K.J., Bonaduce, I., Burnstock, A., Ormsby, B., Scharff, M., Carlyle, L., Heydenreich, G., Keune, K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 587–596. [Google Scholar]
- Hackney, S.; Reifsnyder, J.; te Marvelde, M.; Scharff, M. Lining easel paintings. In Conservation of Easel Paintings; Stoner, J.H., Rushfield, R., Eds.; Routledge: New York, NY, USA, 2012; pp. 415–452. [Google Scholar]
- Kerr, A.; Manthey, G.; Teeter, K.; DeGhetaldi, K.; Baade, B.; Petersen, W.C.; Matsen, C. Chronicles in Wax-Resin Lining: A Historic Look at Lining Practices and Their Effectual Legacy on Paintings in the Smithsonian American Art Museum Collection. In Conserving Canvas; Schwarz, C., McClure, I., Coddington, J., Eds.; Getty Publications: Los Angeles, CA, USA, 2023; pp. 137–143. [Google Scholar]
- Bomford, D.; Staniforth, S. Wax–resin lining and colour change: An evaluation. Natl. Gallery Tech. Bull. 1981, 5, 58–65. [Google Scholar]
- Ly, J.; Liu, C.; Qu, J.; Poirier, G.; Cushman, M. Analysis of Evolon CR as a Poulticing Agent for Wax-Resin Lining Adhesives: Py-GCMS, BET, and SEM Analyses of Used Evolon CR Tissues. In Conserving Canvas; Schwarz, C., McClure, I., Coddington, J., Eds.; Getty Publications: Los Angeles, CA, USA, 2023; pp. 376–381. [Google Scholar]
- Dijkema, D.; Epley, B. Wax-Resin Extraction Traction on a Late Georges Braque Still Life. In Conserving Canvas; Schwarz, C., McClure, I., Coddington, J., Eds.; Getty Publications: Los Angeles, CA, USA, 2023; pp. 321–330. [Google Scholar]
- Colombini, M.P.; Modugno, F. (Eds.) Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Chinchester, UK, 2009. [Google Scholar]
- Sutherland, K. Gas chromatography/mass spectrometry techniques for the characterisation of organic materials in works of art. Phys. Sci. Rev. 2019, 4, 20180010. [Google Scholar] [CrossRef]
- Chiavari, G.; Fabbri, D.; Mazzeo, R.; Bocchini, P.; Galletti, G. Pyrolysis gas chromatography-mass spectrometry of natural resins used for artistic objects. Chromatographia 1995, 41, 273–281. [Google Scholar] [CrossRef]
- Asperger, A.; Engewald, W.; Fabian, G. Analytical characterization of natural waxes employing pyrolysis–gas chromatography–mass spectrometry. J. Anal. Appl. Pyrolysis 1999, 50, 103–115. [Google Scholar] [CrossRef]
- Moffatt, E.; Salmon, A.; Poulin, J.; Fox, A.; Hay, J. Characterization of varnishes on nineteenth-century Canadian furniture. J. Can. Assoc. Conserv. 2015, 40, 3–18. [Google Scholar]
- Poulin, J.; Kearney, M.; Veall, M.-A. Direct Inlet Py-GC-MS analysis of cultural heritage materials. J. Anal. Appl. Pyrolysis 2022, 164, 16. [Google Scholar] [CrossRef]
- Sutherland, K.R.; Kokkori, M. Investigating formulations of cellulose acetate plastics in the collections of the Art Institute of Chicago using pyrolysis gas chromatography mass spectrometry. J. Anal. Appl. Pyrolysis 2022, 163, 9. [Google Scholar] [CrossRef]
- Poulin, J.; Helwig, K. Inside amber: The structural role of succinic acid in Class Ia and Class Id resinite. Anal. Chem. 2014, 86, 7428–7435. [Google Scholar] [CrossRef]
- Rogge, C.E.; Mazurek, J.; Schilling, M. The Nucleus of Color: Analysis of Hélio Oiticica’s Studio Materials. Stud. Conserv. 2023, 68, 627–656. [Google Scholar] [CrossRef]
- Van Keulen, H.; Schilling, M. AMDIS & EXCEL: A Powerful Combination for Evaluating THM-Py-GC/MS Results from European Lacquers. Stud. Conserv. 2019, 64, S74–S80. [Google Scholar] [CrossRef]
- UV Innovations. Setup Workflow Adobe Photoshop Creative Cloud; UV Innovations: Fremont, CA, USA, 2018; Volume 7. [Google Scholar]
- Scalarone, D.; Chiantore, O. Separation techniques for the analysis of artists’ acrylic emulsion paints. J. Sep. Sci. 2004, 27, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H. Polymerization of drying oils. Chem. Rev. 1964, 64, 591–611. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- van den Berg, J.D.J. Analytical Chemical Studies on Traditional Linseed Oil Paints; University of Amsterdam: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Van der Doelen, G.A.; van den Berg, K.J.; Boon, J.J.; Shibayama, N.; De La Rie, E.R.; Genuit, W.J.L. Analysis of fresh triterpenoid resins and aged triterpenoid varnishes by high-performance liquid chromatography–atmospheric pressure chemical ionisation (tandem) mass spectrometry. J. Chromatogr. A 1998, 809, 21–37. [Google Scholar] [CrossRef]
- Watts, S.; De La Rie, E.R. Gcms Analysis of Triterpenoid Resins: In Situ Derivatization Procedures Using Quaternary Ammonium Hydroxides. Stud. Conserv. 2002, 47, 257–272. [Google Scholar] [CrossRef]
- Bonaduce, I.; Colombini, M.P. Characterisation of beeswax in works of art by gas chromatography–mass spectrometry and pyrolysis–gas chromatography–mass spectrometry procedures. J. Chromatogr. A 2004, 1028, 297–306. [Google Scholar] [CrossRef]
- Maines, C.A.; Rogala, D.; Lake, S.; Mecklenburg, M. Deterioration in Abstract Expressionist Paintings: Analysis of Zinc Oxide Paint Layers in Works from the Collection ofthe Hirshhorn Museum and Sculpture Garden, Smithsonian Institution. In Proceedings of the Materials Issues in Art and Archaeology IX, Boston, MA, USA, 29 November–3 December 2010. [Google Scholar]
- Rogala, D.; Lake, S.; Maines, C.; Mecklenburg, M. Condition problems related to zinc oxide underlayers: Examination of selected abstract expressionist paintings from the collection of the Hirshhorn Museum and sculpture garden, Smithsonian Institution. J. Am. Inst. Conserv. 2010, 29, 96–113. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Bernazzani, L.; Tine, M.R.; Treil, C.; Duce, C.; Bonaduce, I. Oxidation and Cross-Linking in the Curing of Air-Drying Artists’ Oil Paints. ACS Appl. Polym. Mater. 2021, 3, 1912–1922. [Google Scholar] [CrossRef]
- Izzo, F.C. 20th Century Artists’ Oil Paints: A Chemical-Physical Survey; Università Ca’ Foscari Venezia: Venice, Italy, 2011. [Google Scholar]
- Tulloch, A.P. Beeswax: Structure of the esters and their component hydroxy acids and diols. Chem. Phys. Lipids 1971, 6, 235–265. [Google Scholar] [CrossRef]
- van der Doelen, G.A.; van den Berg, K.J.; Boon, J.J. Comparative chromatographic and mass-spectrometric studies of triterpenoid varnishes: Fresh material and aged samples from paintings. Stud. Conserv. 1998, 43, 249–264. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Chiantore, O. Ageing behaviour and analytical pyrolysis characterisation of diterpenic resins used as art materials: Manila copal and sandarac. J. Anal. Appl. Pyrolysis 2003, 68–69, 115–136. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Chiantore, O. Ageing behaviour and pyrolytic characterisation of diterpenic resins used as art materials: Colophony and Venice turpentine. J. Anal. Appl. Pyrolysis 2002, 64, 345–361. [Google Scholar] [CrossRef]
- Dietemann, P.; Higgitt, C.; Kälin, M.; Edelmann, M.J.; Knochenmuss, R.; Zenobi, R. Aging and yellowing of triterpenoid resin varnishes—Influence of aging conditions and resin composition. J. Cult. Herit. 2009, 10, 30–40. [Google Scholar] [CrossRef]
- Dietemann, P.; Kälin, M.; Zumbühl, S.; Knochenmuss, R.; Wülfert, S.; Zenobi, R. A Mass Spectrometry and Electron Paramagnetic Resonance Study of Photochemical and Thermal Aging of Triterpenoid Varnishes. Anal. Chem. 2001, 73, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, I.D.; van den Berg, K.J.; Schmitt, S.; Boon, J.J. Molecular Characterization of Copaiba Balsam as Used in Painting Techniques and Restoration Procedures. Stud. Conserv. 2000, 45, 1–18. [Google Scholar] [CrossRef]
- De la Cruz-Cañizares, J.; Doménech-Carbó, M.-T.; Gimeno-Adelantado, J.-V.; Mateo-Castro, R.; Bosch-Reig, F. Study of Burseraceae resins used in binding media and varnishes from artworks by gas chromatography–mass spectrometry and pyrolysis-gas chromatography–mass spectrometry. J. Chromatogr. A 2005, 1093, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Baij, L.; Astefanei, A.; Hermans, J.; Brinkhuis, F.; Groenewegen, H.; Chassouant, L.; Johansson, S.; Corthals, G.; Tokarski, C.; Iedema, P.; et al. Solvent-mediated extraction of fatty acids in bilayer oil paint models: A comparative analysis of solvent application methods. Herit. Sci. 2019, 7, 31–39. [Google Scholar] [CrossRef]
- Regert, M.; Colinart, S.; Degrand, L.; Decavallas, O. Chemical Alteration and Use of Beeswax Through Time: Accelerated Ageing Tests and Analysis of Archaeological Samples from Various Environmental Contexts. Archaeometry 2001, 43, 549–569. [Google Scholar] [CrossRef]
- Scalarone, D.; van der Horst, J.; Boon, J.J.; Chiantore, O. Direct-temperature mass spectrometric detection of volatile terpenoids and natural terpenoid polymers in fresh and artificially aged resins. J. Mass Spectrom. 2003, 38, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Baij, L.; Hermans, J.; Ormsby, B.; Noble, P.; Iedema, P.; Keune, K. A review of solvent action on oil paint. Herit. Sci. 2020, 8, 43–66. [Google Scholar] [CrossRef]
- Tulloch, A.P. Beeswax—Composition and analysis. Bee World 1980, 61, 47–62. [Google Scholar] [CrossRef]
- Keck, S. Some picture cleaning controversies: Past and present. J. Am. Inst. Conserv. 1984, 23, 73–87. [Google Scholar] [CrossRef]
- Erhardt, D.; Cunningham, R.H.; Räsänen, S. Extraction of Material from Oil Paints by Solvents. MRS Proc. 2002, 712, II1.7. [Google Scholar] [CrossRef]
- Erhardt, D.; Tsang, J.-S. The extractable components of oil paint films. Stud. Conserv. 1990, 35, 93–97. [Google Scholar] [CrossRef]
- Tsang, J.-S.; Erhardt, D. Current research on the effects of solvents and gelled and aqueous cleaning systems on oil paint films. J. Am. Inst. Conserv. 1992, 31, 87–94. [Google Scholar] [CrossRef][Green Version]
- White, R.; Roy, A. GC-MS and SEM studies on the effects of solvent cleaning on old master paintings from the National Gallery, London. Stud. Conserv. 1998, 43, 159–176. [Google Scholar] [CrossRef]
- Sutherland, K. Measurements of Solvent Cleaning Effects on Oil Paintings. J. Am. Inst. Conserv. 2006, 45, 211–226. [Google Scholar] [CrossRef]
- Sutherland, K.R. Solvent Extractable Components Paint Films; University of Amsterdam: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Mecklenburg, M.F.; Charola, A.E.; Koestler, R.J. (Eds.) New Insights into the Cleaning of Paintings Proceedings from the Cleaning 2010 International Conference Universidad Politécnica de Valencia and Museum Conservation Institute; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2010; p. 257. [Google Scholar]
- Nardelli, F.; Martini, F.; Lee, J.; Lluvears-Tenorio, A.; La Nasa, J.; Duce, C.; Ormsby, B.; Geppi, M.; Bonaduce, I. The stability of paintings and the molecular structure of the oil paint polymeric network. Sci. Rep. 2021, 11, 13. [Google Scholar] [CrossRef]
- Phenix, A.; Sutherland, K.R. The cleaining of paintings: Effects of organic solvents on oil paint films. Stud. Conserv. 2001, 46, 47–60. [Google Scholar] [CrossRef]
- Tumosa, C.S.; Millard, J.; Erhardt, D.; Mecklenburg, M.F. Effects of solvents on the physical properties of paint films. In Proceedings of the ICOM Committee for Conservation, 12th Triennial Meeting, Lyon, France, 29 August–3 September 1999; pp. 347–352. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogge, C.E.; Dijkema, D.; Epley, B.A. Development of a Thermal Separation Probe Gas Chromatography–Mass Spectrometry Method for Evaluating Wax–Resin Removal by Evolon® CR. Appl. Sci. 2024, 14, 2941. https://doi.org/10.3390/app14072941
Rogge CE, Dijkema D, Epley BA. Development of a Thermal Separation Probe Gas Chromatography–Mass Spectrometry Method for Evaluating Wax–Resin Removal by Evolon® CR. Applied Sciences. 2024; 14(7):2941. https://doi.org/10.3390/app14072941
Chicago/Turabian StyleRogge, Corina E., Desirae Dijkema, and Bradford A. Epley. 2024. "Development of a Thermal Separation Probe Gas Chromatography–Mass Spectrometry Method for Evaluating Wax–Resin Removal by Evolon® CR" Applied Sciences 14, no. 7: 2941. https://doi.org/10.3390/app14072941
APA StyleRogge, C. E., Dijkema, D., & Epley, B. A. (2024). Development of a Thermal Separation Probe Gas Chromatography–Mass Spectrometry Method for Evaluating Wax–Resin Removal by Evolon® CR. Applied Sciences, 14(7), 2941. https://doi.org/10.3390/app14072941





