Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extract Preparation
2.2.1. Plant Material
2.2.2. Grapefruit Crude Polyphenol-Rich (GF-CE) Extract
2.2.3. Grapefruit Essential Oil (GF-EO) and Grapefruit Polyphenol-Rich Extract (GF-PE) Extraction
2.3. Phytochemical Analysis
2.3.1. Essential Oil (GF-EO) Composition
2.3.2. Total Phenolic Content
2.3.3. Total Flavonoid Content
Phenolic Compound Identification and Quantification
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Assay
2.4.2. Nitric Oxide Radical Scavenging Assay
2.5. Antimicrobial Activity
2.5.1. GF-EO Evaluation
2.5.2. GF Polyphenol-Rich Extracts
2.6. Anti-Inflammatory Activity of GF-EO, GF-CE, and GF-PE
2.7. Statistical Analysis
3. Results and Discussion
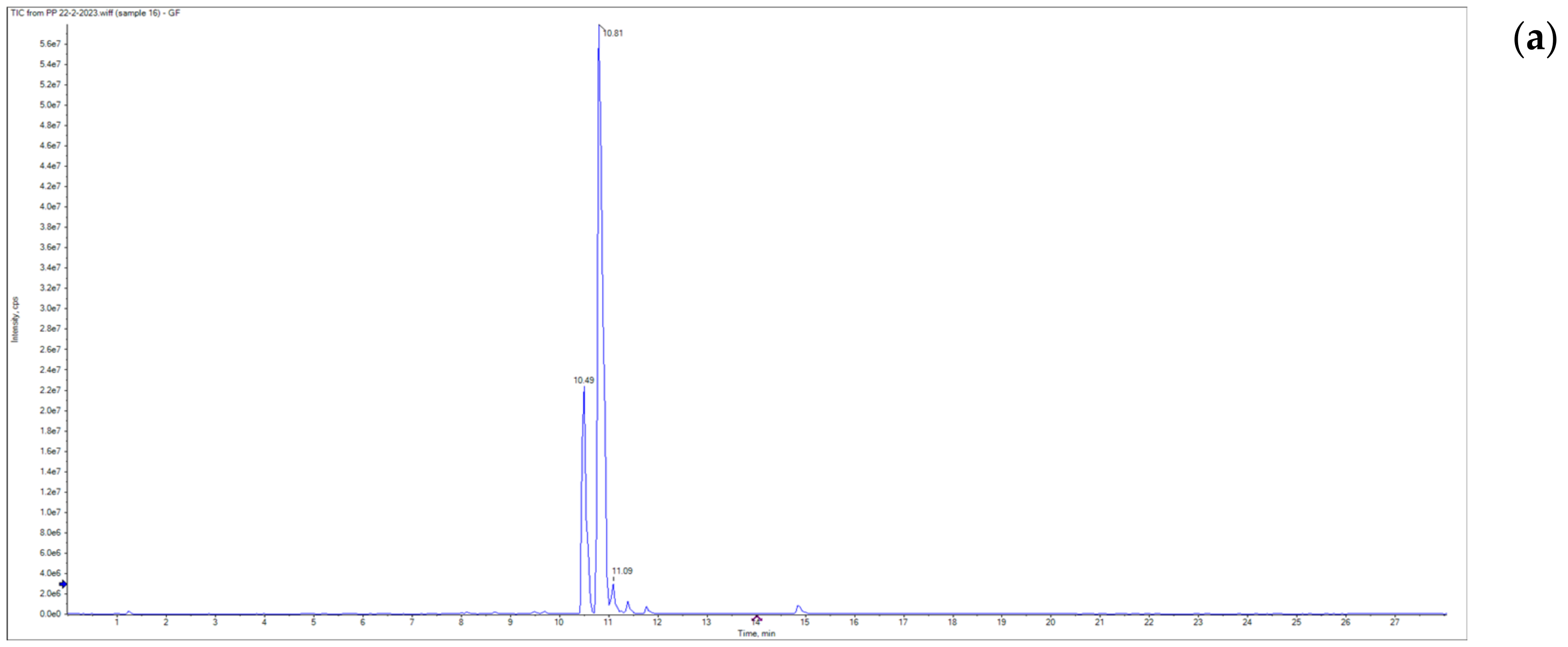
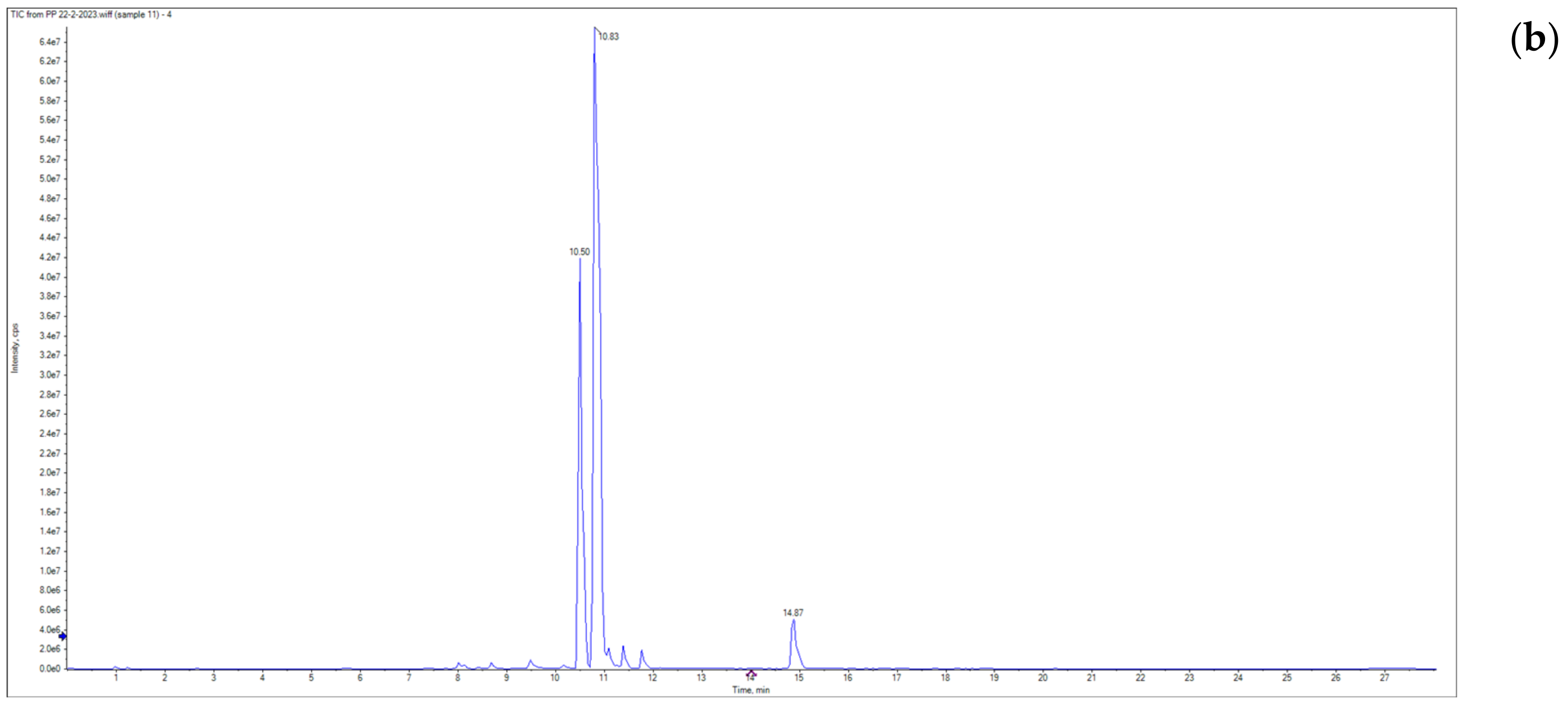
3.1. Grapefruit Essential Oil Composition

| No. | Retention Time | Molecular Weight (M+) | Base Peak | Molecular Formula | Compound | Area (%) |
|---|---|---|---|---|---|---|
| 1 | 8.07 | 136 | 93 | C10H16 | α-Pinene | 3.98 |
| 2 | 8.33 | 136 | 93 | C10H16 | Camphene | 0.44 |
| 3 | 9.33 | 136 | 93 | C10H16 | β-Pinene | 7.71 |
| 4 | 10.37 | 128 | 83 | C8H16O | 1-Octanal | 3.40 |
| 5 | 10.42 | 136 | 93 | C10H16 | D-Limonene | 24.90 |
| 6 | 12.32 | 136 | 93 | C10H16 | γ-Terpinene | 7.52 |
| 7 | 13.58 | 154 | 71 | C10H18O | Linalool | 1.10 |
| 8 | 14.49 | 152 | 67 | C10H16O | Trans-Limonene oxide | 0.29 |
| 9 | 14.91 | 154 | 69 | C10H18O | Citronellal | 0.61 |
| 10 | 15.59 | 154 | 71 | C10H18O | Terpinen-4-ol | 0.26 |
| 11 | 16.02 | 154 | 71 | C10H18O | α-Terpineol | 0.81 |
| 12 | 16.38 | 158 | 67 | C10H22O | Dihydro-citronellol | 2.65 |
| 13 | 16.85 | 152 | 109 | C10H16O | Caveol | 0.41 |
| 14 | 17.12 | 184 | 69 | C11H20O2 | Citronellyl formate | 0.93 |
| 15 | 17.38 | 152 | 69 | C10H16O | Neral | 0.36 |
| 16 | 17.47 | 150 | 82 | C10H14O | Carvone | 0.15 |
| 17 | 17.82 | 154 | 69 | C10H18O | Geraniol | 0.15 |
| 18 | 18.19 | 152 | 69 | C10H16O | Geranial (E-citral) | 1.69 |
| 19 | 20.40 | 194 | 109 | C12H18O2 | Carvyl acetate | 0.24 |
| 20 | 20.86 | 204 | 161 | C15H24 | α-Copaene | 1.12 |
| 21 | 21.10 | 196 | 69 | C12H20O2 | Geranyl acetate | 0.88 |
| 22 | 21.23 | 204 | 161 | C15H24 | β-Copaene | 0.86 |
| 23 | 21.99 | 204 | 93 | C15H24 | Trans-carophyllene | 4.40 |
| 24 | 22.80 | 204 | 93 | C15H24 | α-Humulene | 0.49 |
| 25 | 23.47 | 204 | 161 | C15H24 | Germacrene-D | 0.69 |
| 26 | 23.86 | 204 | 121 | C15H24 | Bicyclogermacrene | 0.36 |
| 27 | 24.51 | 204 | 161 | C15H24 | α-Amorphene | 2.78 |
| 28 | 25.13 | 204 | 161 | C15H24 | Elemol | 1.06 |
| 29 | 25.88 | 220 | 93 | C15H24O | Caryophllene oxide | 0.30 |
| 30 | 27.52 | 222 | 121 | C15H26O | α-Cadinol | 0.32 |
| 31 | 27.63 | 222 | 186 | C15H26O | Eudesm-7(11)en-4-ol | 0.63 |
| 32 | 28.97 | 222 | 69 | C15H26O | Farnesol | 0.65 |
| 33 | 29.32 | 206 | 135 | C15H26 | Nootkatol | 0.29 |
| 34 | 30.41 | 218 | 135 | C15H22O | Curcuphenol | 0.35 |
| 35 | 30.38 | 218 | 146 | C15H22O | Nootkatone | 24.33 |
| Total Area of the Identified Compounds | 97.11 | |||||
3.2. Grapefruit Polyphenol-Rich Extract Composition
3.2.1. TFC and TPC
3.2.2. Phenolic Compounds’ Identification and Quantification
3.3. Antioxidant Activity
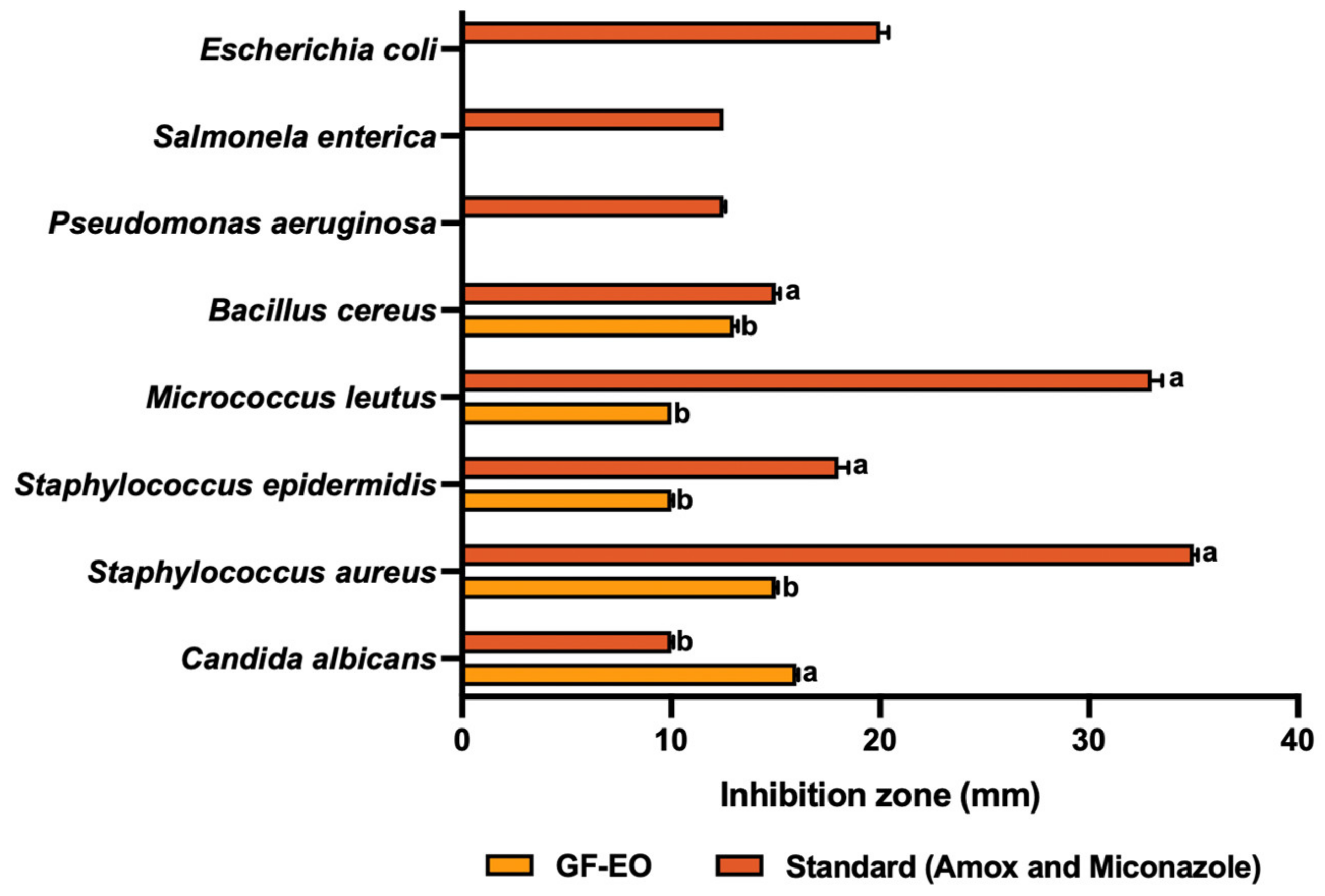
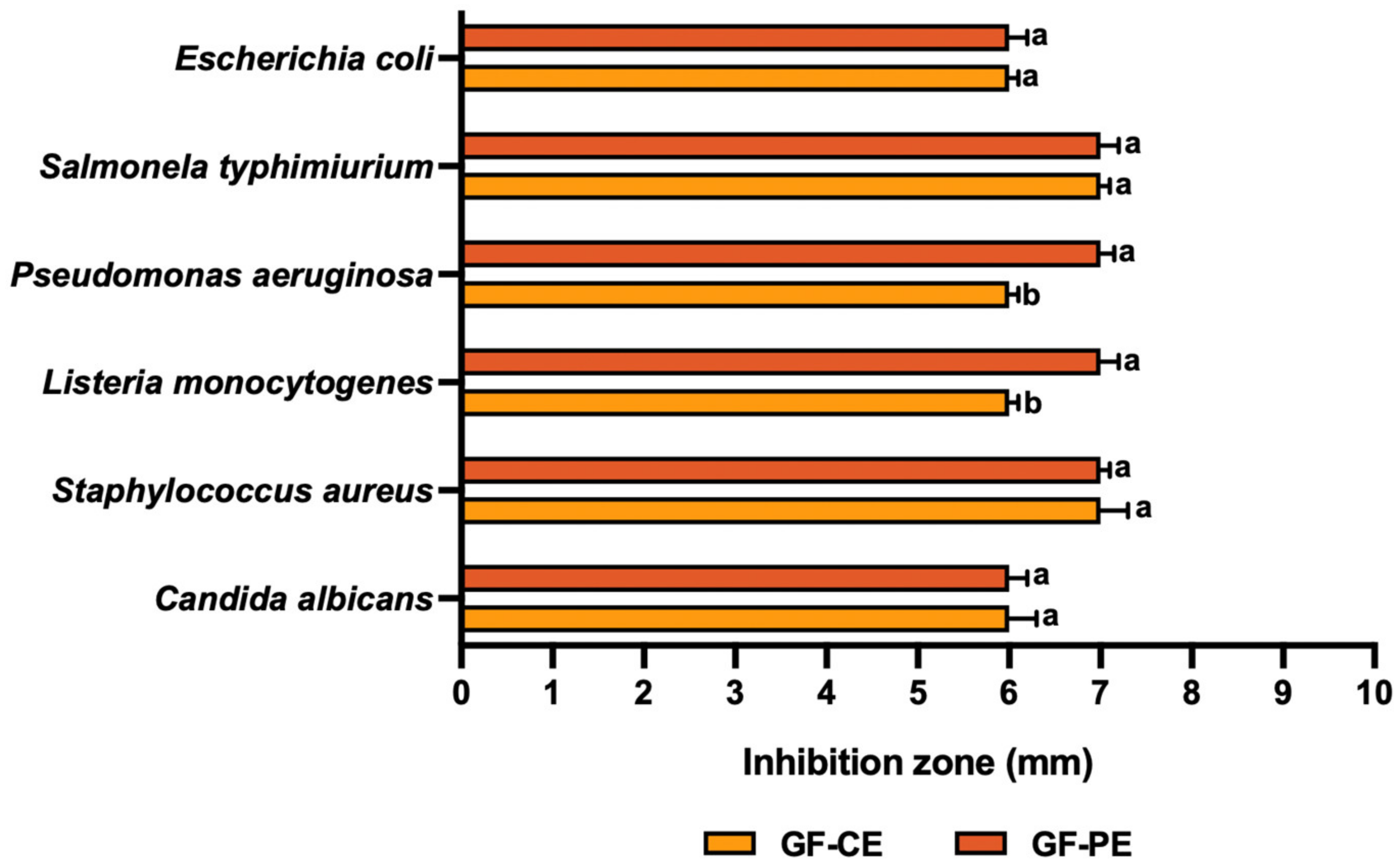
3.4. Antimicrobial Activity
3.4.1. Grapefruit Essential Oil (GF-EO)
3.4.2. Grapefruit Polyphenol-Rich Extracts (GF-CE and GF-PE)
3.5. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wedamulla, N.E.; Fan, M.; Choi, Y.-J.; Kim, E.-K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Shahbandeh, M. Grapefruit Production Worldwide from 2012/2013 to 2022/2023. Available online: https://www.statista.com/statistics/577836/world-grapefruit-production/ (accessed on 17 March 2024).
- UN Food and Agriculture Organization, C.S.D. (FAOSTAT). Grapefruit Production in 2022, Crops/Regions/World List/Production Quantity (Pick Lists). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 March 2024).
- Khalil, M.N.A.; Farghal, H.H.; Farag, M.A. Outgoing and Potential Trends of Composition, Health Benefits, Juice Production and Waste Management of the Multi-Faceted Grapefruit Citrus Χ Paradisi: A Comprehensive Review for Maximizing Its Value. Crit. Rev. Food Sci. Nutr. 2022, 62, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Gómez-García, R.; Campos, D.A.; Correia, M.; Pintado, M. Integrated Biorefinery Strategy for Orange Juice By-Products Valorization: A Sustainable Protocol to Obtain Bioactive Compounds. In Food Waste Conversion; Springer: Berlin/Heidelberg, Germany, 2023; pp. 113–124. [Google Scholar]
- Sharma, H.P.; Patel, H. Sugandha Enzymatic Added Extraction and Clarification of Fruit Juices—A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of Phenolic Compounds and Vitamin C and Antioxidant Activity in Wasted Parts of Sudanese Citrus Fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Durani, A.I.; Asari, A.; Ahmed, M.; Ahmad, M.; Yousaf, N.; Muddassar, M. Investigation of Antioxidant and Antibacterial Effects of Citrus Fruits Peels Extracts Using Different Extracting Agents: Phytochemical Analysis with in Silico Studies. Heliyon 2023, 9, e15433. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; El-Hosseiny, L.S. Comparative Phytochemical Composition and Antimicrobial Activity of Citrus Peel Essential Oils and Phenolic Compounds. Antiinfect. Agents 2023, 21, 57–68. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus Processing By-Products: An Overlooked Repository of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Park, H.; Jang, B.K.; Ju, Y.; Shin, M.H.; Oh, E.J.; Lee, E.J.; Kim, S.R. Recent Advances in the Biological Valorization of Citrus Peel Waste into Fuels and Chemicals. Bioresour. Technol. 2021, 323, 124603. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tu, Y.; Lao, S.; Wu, M.; Yin, H.; Wang, L.; Liao, W. The Role and Mechanism of Citrus Flavonoids in Cardiovascular Diseases Prevention and Treatment. Crit. Rev. Food Sci. Nutr. 2022, 62, 7591–7614. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H. A Review of the Effects of Citrus paradisi (Grapefruit) and Its Flavonoids, Naringin, and Naringenin in Metabolic Syndrome. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 515–543. [Google Scholar]
- Chao, C.-L.; Weng, C.-S.; Chang, N.-C.; Lin, J.-S.; Kao, S.-T.; Ho, F.-M. Naringenin More Effectively Inhibits Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Expression in Macrophages than in Microglia. Nutr. Res. 2010, 30, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, O.K.; Keskin, M.; Lohani, H.; Egbuna, C.; Haider, S.Z. Bioactive Lead Compounds and Molecular Targets for the Development of Antiinflammatory Drugs. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–331. [Google Scholar]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef]
- Nikolic, D.; Bosco, L.; Moschetti, M.; Tinnirello, V.; Pucci, M.; Corleone, V.; Raimondo, S.; Alessandro, R.; Fontana, S. Anti-inflammatory Properties of an Aldehydes-enriched Fraction of Grapefruit Essential Oil. J. Food Sci. 2023, 88, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Ismail, L.C.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Bekhit, A.E.-D.; Jambrak, A.R.; Regenstein, J.M.; Chemat, F.; Morton, J.D.; Gudjónsdóttir, M.; Carpena, M.; Prieto, M.A.; Varela, P.; et al. The Fourth Industrial Revolution in the Food Industry—Part II: Emerging Food Trends. Crit. Rev. Food Sci. Nutr. 2024, 64, 407–437. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients 2023, 15, 4384. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, W.S.; Oh, S.W. Effect of Layer-by-Layer Antimicrobial Edible Coating of Alginate and Chitosan with Grapefruit Seed Extract for Shelf-Life Extension of Shrimp (Litopenaeus vannamei) Stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Durmus, M. The Effects of Nanoemulsions Based on Citrus Essential Oils (Orange, Mandarin, Grapefruit, and Lemon) on the Shelf Life of Rainbow Trout (Oncorhynchus mykiss) Fillets at 4 ± 2 °C. J. Food Saf. 2020, 40, e12718. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial Polyphenol-Rich Extracts: Applications and Limitations in the Food Industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Cioni, E.; Migone, C.; Ascrizzi, R.; Muscatello, B.; De Leo, M.; Piras, A.M.; Zambito, Y.; Flamini, G.; Pistelli, L. Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their in Vitro Antioxidant Activity. Antioxidants 2022, 11, 2047. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Khade, M.; Savant, S.; Musale, A.; Chelliah, M.S.; Dasgupta, S. Empowering Blue Economy: From Underrated Ecosystem to Sustainable Industry. J. Environ. Manag. 2021, 291, 112697. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining Upcycled Food: The Dual Role of Upcycling in Reducing Food Loss and Waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Some, S.; Roy, J.; Chatterjee, J.S.; Butt, M.H. Low Demand Mitigation Options for Achieving Sustainable Development Goals: Role of Reduced Food Waste and Sustainable Dietary Choice. J. Clean. Prod. 2022, 369, 133432. [Google Scholar] [CrossRef]
- Lemaire, A.; Limbourg, S. How Can Food Loss and Waste Management Achieve Sustainable Development Goals? J. Clean. Prod. 2019, 234, 1221–1234. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1. ed.; Allured Pub Corp: Carol Stream, IL, USA, 2017; ISBN 978-1-932633-21-4. [Google Scholar]
- Farid, M.M.; Ibrahim, F.M.; Ragheb, A.Y.; Mohammed, R.S.; Hegazi, N.M.; Shabrawy, M.O.E.L.; Kawashty, S.A.; Marzouk, M.M. Comprehensive Phytochemical Characterization of Raphanus raphanistrum L.: In Vitro Antioxidant and Antihyperglycemic Evaluation. Sci. Afr. 2022, 16, e01154. [Google Scholar] [CrossRef]
- Ibrahim, F.M.; Fouad, R.; EL-Hallouty, S.; Hendawy, S.F.; Omer, E.A.; Mohammed, R.S. Egyptian Myrtus communis L. Essential Oil Potential Role as Invitro Antioxidant, Cytotoxic and α-Amylase Inhibitor. Egypt. J. Chem. 2021, 64, 3005–3017. [Google Scholar] [CrossRef]
- Mostafa, F.A.; Abd El Aty, A.A.; Hamed, E.R.; Eid, B.M.; Ibrahim, N.A. Enzymatic, Kinetic and Anti-Microbial Studies on Aspergillus Terreus Culture Filtrate and Allium Cepa Seeds Extract and Their Potent Applications. Biocatal. Agric. Biotechnol. 2016, 5, 116–122. [Google Scholar] [CrossRef]
- Visan, D.-C.; Oprea, E.; Radulescu, V.; Voiculescu, I.; Biris, I.-A.; Cotar, A.I.; Saviuc, C.; Chifiriuc, M.C.; Marinas, I.C. Original Contributions to the Chemical Composition, Microbicidal, Virulence-Arresting and Antibiotic-Enhancing Activity of Essential Oils from Four Coniferous Species. Pharmaceuticals 2021, 14, 1159. [Google Scholar] [CrossRef] [PubMed]
- McFarland, J. The Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar] [CrossRef]
- El-Liethy, M.A.; Hemdan, B.A.; El-Taweel, G.E. Phenotyping Using Semi-Automated BIOLOG and Conventional PCR for Identification of Bacillus Isolated from Biofilm of Sink Drainage Pipes. Acta Ecol. Sin. 2018, 38, 334–338. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and Functional Basis of Cyclooxygenase Inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Dao, T.P.; Le, X.T.; Huynh, B.L.; Minh, L.T.N. Volatile Compounds of Grapefruit (Citrus grandis (L.) Osbeck) Peel Essential Oil by Cold Pressing and Hydrodistillation Methods. In IOP Conference Series: Earth and Environmental Science; Institute of Physics; IOP Publishing: Bristol, UK, 2023; Volume 1241. [Google Scholar]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S. Safety Evaluation and Risk Assessment of D-Limonene. J. Toxicol. Environ. Health Part. B 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rattanpal, H.S.; Gul, K.; Dar, R.A.; Sharma, A. Chemical Composition, Antioxidant Activity and GC-MS Analysis of Juice and Peel Oil of Grapefruit Varieties Cultivated in India. J. Integr. Agric. 2019, 18, 1634–1642. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef]
- Kirbaşlar, F.G.; Boz, I. Composition of Turkish Lemon and Grapefruit Peel Oils. J. Essent. Oil Res. 2006, 18, 525–543. [Google Scholar] [CrossRef]
- Kirbaşlar, F.G.; Tavman, A.; Dülger, B.; Türker, G. Antimicrobial Activity of Turkish Citrus Peel Oils. Pak. J. Bot. 2009, 41, 3207–3212. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective Effects of D-limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-induced Diabetic Rats. Basic. Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.-J.; Lee, N.H.; Hyun, C.-G. Limonene Suppresses Lipopolysaccharide-Induced Production of Nitric Oxide, Prostaglandin E2, and pro-Inflammatory Cytokines in RAW 264.7 Macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, J.-N.; Fan, G.; Zhang, L.-L.; Pan, S.-Y. Advances on (+)-Nootkatone Microbial Biosynthesis and Its Related Enzymes. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab046. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Rodrigues Dantas, L.; Silva, A.L.M.; da Silva Júnior, C.P.; Alcântara, I.S.; de Oliveira, M.R.; Oliveira Brito Pereira Bezerra Martins, A.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Rocha Santos Passos, F.; Quintans-Junior, L.J.; et al. Nootkatone Inhibits Acute and Chronic Inflammatory Responses in Mice. Molecules 2020, 25, 2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Fan, G.; Li, X. Production, Function, and Applications of the Sesquiterpenes Valencene and Nootkatone: A Comprehensive Review. J. Agric. Food Chem. 2022, 71, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Schulz, B.; Dickschat, J.S. Pogostol Biosynthesis by the Endophytic Fungus Geniculosporium. ChemBioChem 2014, 15, 2379–2383. [Google Scholar] [CrossRef] [PubMed]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Babaoğlu, A.S.; Ainiwaer, T.; Özkan, H.; Karakaya, M. Grapefruit and Pomelo Peel Extracts as Natural Antioxidants for Improved Storage Stability of Turkey Patties during Refrigerated Storage. J. Food Sci. Technol. 2022, 59, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Malakar, S.; Dwivedi, U.; Kumar, N.; Prabakar, P.K.; Kishore, A.; Kumar, A. Impact of Different Drying Techniques on Grapefruit Peels and Subsequent Optimization of Ultrasonic Extraction Conditions for Bioactive Compounds. J. Food Process Eng. 2023, 46, e14331. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of Conventional and Ultrasound Assisted Extraction of Flavonoids from Grapefruit (Citrus paradisi L.) Solid Wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Singh, V.; Chahal, T.S.; Grewal, S.K.; Gill, P.S. Effect of Fruit Development Stages on Antioxidant Properties and Bioactive Compounds in Peel, Pulp and Juice of Grapefruit Varieties. J. Food Meas. Charact. 2021, 15, 2531–2539. [Google Scholar] [CrossRef]
- Bağdatli, İ.; Khalily, F. Changes in Total Phenolic Content and Antioxidant Activity of Grapefruit and Mandarin Peels Extracted with Different Solvents. Eurasian J. Food Sci. Technol. 2022, 6, 88–93. [Google Scholar]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A.A.; Athayde, M.L. Phenolics from Grapefruit Peels Inhibit HMG-CoA Reductase and Angiotensin-I Converting Enzyme and Show Antioxidative Properties in Endothelial EA. Hy 926 Cells. Food Sci. Hum. Wellness 2015, 4, 80–85. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jimenez, M.D.M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxidative Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Gan, J.; Dong, R.; Chen, Y.; Xie, J.; Huang, Z.; Gu, Y.; Huang, D.; Yu, Q. Combined Microwave and Enzymatic Treatment Improve the Release of Insoluble Bound Phenolic Compounds from the Grapefruit Peel Insoluble Dietary Fiber. LWT 2021, 149, 111905. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of Bioactive Compounds from Mango (Mangifera indica L. Var. Carabao) Seed Kernel with Ethanol–Water Binary Solvent Systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Dibacto, R.E.K.; Tchuente, B.R.T.; Nguedjo, M.W.; Tientcheu, Y.M.T.; Nyobe, E.C.; Edoun, F.L.E.; Kamini, M.F.G.; Dibanda, R.F.; Medoua, G.N. Total Polyphenol and Flavonoid Content and Antioxidant Capacity of Some Varieties of Persea americana Peels Consumed in Cameroon. Sci. World J. 2021, 2021, 8882594. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in Vitro of Quercetin Glycoside Obtained in Beauveria Bassiana Culture and Its Interaction with Liposome Membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, E.R.; Chiţescu, C.L.; Geană, E.-I.; Gird, C.E.; Socoteanu, R.P.; Boscencu, R. Advanced Analytical Approaches for the Analysis of Polyphenols in Plants Matrices—A Review. Separations 2021, 8, 65. [Google Scholar] [CrossRef]
- Kubra, I.R.; Jagan Mohan Rao, L. Microwave Drying of Ginger (Zingiber Officinale Roscoe) and Its Effects on Polyphenolic Content and Antioxidant Activity. Int. J. Food Sci. Technol. 2012, 47, 2311–2317. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mandal, N.; Dey, A.; Mondal, B. An Approach towards Optimization of the Extraction of Polyphenolic Antioxidants from Ginger (Zingiber Officinale). J. Food Sci. Technol. 2014, 51, 3301–3308. [Google Scholar] [CrossRef]
- da Silveira, T.F.F.; Meinhart, A.D.; Ballus, C.A.; Godoy, H.T. The Effect of the Duration of Infusion, Temperature, and Water Volume on the Rutin Content in the Preparation of Mate Tea Beverages: An Optimization Study. Food Res. Int. 2014, 60, 241–245. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical Characteristics of Citrus Peel and Effect of Conventional and Nonconventional Processing on Phenolic Compounds: A Review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.-N.; Fan, G.; Pan, S.-Y. Changes of Aroma Compounds and Qualities of Freshly-Squeezed Orange Juice during Storage. J. Food Sci. Technol. 2018, 55, 4530–4543. [Google Scholar] [CrossRef] [PubMed]
- Bozkir, H.; Kola, O.; Duran, H.; Şimşek, M.; Kelebek, H. Effect of Thermal Processing on Carotenoids of Some Orange Juices. J. Food Agric. Environ. 2015, 13, 52–57. [Google Scholar]
- Oikeh, E.I.; Oriakhi, K.; Omoregie, E.S. Proximate Analysis and Phytochemical Screening of Citrus Sinensis Fruit Wastes. Biosci. J. 2013, 1, 164–170. [Google Scholar]
- Oikeh, E.I.; Omoregie, E.S.; Oviasogie, F.E.; Oriakhi, K. Phytochemical, Antimicrobial, and Antioxidant Activities of Different Citrus Juice Concentrates. Food Sci. Nutr. 2016, 4, 103–109. [Google Scholar] [CrossRef]
- Moran, E.P.; Wang, Z.; Chen, J.; Sapieha, P.; Smith, L.E.H.; Ma, J. Neurovascular Cross Talk in Diabetic Retinopathy: Pathophysiological Roles and Therapeutic Implications. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H738–H749. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, C.; Zang, Y.; Huang, Z.; Liu, G. The Flavonoid Composition of Flavedo and Juice from the Pummelo Cultivar (Citrus grandis (L.) Osbeck) and the Grapefruit Cultivar (Citrus paradisi) from China. Food Chem. 2011, 129, 1530–1536. [Google Scholar] [CrossRef]
- Al-Ogaili, N.A.; Yasin, Z. Qualitative and Quantitative Investigation of Iraqi Grapefruit (Citrus padisi) Flavonoids From Peel and Seeds and Comparing Their Aqueous Extracts for Antimicrobial Activity. Iraqi J. Sci. 2016, 57, 2627–2633. [Google Scholar]
- Can, Z.; Keskin, B.; Üzer, A.; Apak, R. Detection of Nitric Oxide Radical and Determination of Its Scavenging Activity by Antioxidants Using Spectrophotometric and Spectrofluorometric Methods. Talanta 2022, 238, 122993. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic Acid, Total Phenol Content and Antioxidant Activity of Fresh Juices of Four Ripe and Unripe Citrus Fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 5 February 2024).
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, T.; Gülnaz, O.; Kaçar, Y.A. Chemical Composition of the Essential Oils from Some Citrus Species and Evaluation of the Antimicrobial Activity. J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 29–33. [Google Scholar]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical Composition of Dietary Fiber and Polyphenols of Five Different Varieties of Wine Grape Pomace Skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit Essential Oils Inhibit Quorum Sensing of Pseudomonas Aeruginosa. Food Sci. Technol. Int. 2020, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Stevens, C.M.; Zhang, L.; Zgurskaya, H.I.; Niederweis, M. Transporters Involved in the Biogenesis and Functionalization of the Mycobacterial Cell Envelope. Chem. Rev. 2020, 121, 5124–5157. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Podoprigora, I.V.; Davares, A.K.L.; Razan, M.; Das, M.S.; Senyagin, A.N. Antibacterial Activity of Grapefruit Peel Extracts and Green-Synthesized Silver Nanoparticles. Vet. World 2021, 14, 1330. [Google Scholar] [CrossRef]
- Yaldiz, B.; Saglam-Metiner, P.; Cakmak, B.; Kaya, E.; Deliogullari, B.; Yesil-Celiktas, O. Essential Oil and Supercritical Carbon Dioxide Extract of Grapefruit Peels Formulated for Candida Albicans Infections: Evaluation by an in Vitro Model to Study Fungal–Host Interactions. ACS Omega 2022, 7, 37427–37435. [Google Scholar] [CrossRef] [PubMed]
- Côté, H.; Pichette, A.; Simard, F.; Ouellette, M.-E.; Ripoll, L.; Mihoub, M.; Grimard, D.; Legault, J. Balsacone C, a New Antibiotic Targeting Bacterial Cell Membranes, Inhibits Clinical Isolates of Methicillin-Resistant Staphylococcus Aureus (MRSA) without Inducing Resistance. Front. Microbiol. 2019, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.-H.; Wang, R.; Zhao, S.-Q.; Chen, B.-R.; Zeng, X.-A. Inhibition of Biofilm Formation of Foodborne Staphylococcus Aureus by the Citrus Flavonoid Naringenin. Foods 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-P.; He, Y.-H.; Zhang, S.-Y.; Shi, Y.-P. Antibacterial Activity and Action Mechanism of Flavonoids against Phytopathogenic Bacteria. Pestic. Biochem. Physiol. 2022, 188, 105221. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.K.; Negi, D.S. The Development of COX-1 and COX-2 Inhibitors: A Review. Int. J. Pharm. Sci. Res. 2020, 11, 3544. [Google Scholar]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-Inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef] [PubMed]
- Ambati, G.G.; Jachak, S.M. Natural Product Inhibitors of Cyclooxygenase (COX) Enzyme: A Review on Current Status and Future Perspectives. Curr. Med. Chem. 2021, 28, 1877–1905. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Non-Steroidal Anti-Inflammatory Drugs: Recent Advances in the Use of Synthetic COX-2 Inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids Inhibit COX-1 and COX-2 Enzymes and Cytokine/Chemokine Production in Human Whole Blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Ho, S.-C. Polymethoxy Flavones Are Responsible for the Anti-Inflammatory Activity of Citrus Fruit Peel. Food Chem. 2010, 119, 868–873. [Google Scholar] [CrossRef]
- Miya, G.; Nyalambisa, M.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Profiling, Toxicity and Anti-Inflammatory Activities of Essential Oils from Three Grapefruit Cultivars from KwaZulu-Natal in South Africa. Molecules 2021, 26, 3387. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S. Multi-Therapeutic Potential of Naringenin (4′, 5, 7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Dayarathne, L.A.; Ranaweera, S.S.; Natraj, P.; Rajan, P.; Lee, Y.J.; Han, C.-H. Restoration of the Adipogenic Gene Expression by Naringenin and Naringin in 3T3-L1 Adipocytes. J. Vet. Sci. 2021, 22, e55. [Google Scholar] [CrossRef]
- Kampschulte, N.; Alasmer, A.; Empl, M.T.; Krohn, M.; Steinberg, P.; Schebb, N.H. Dietary Polyphenols Inhibit the Cytochrome P450 Monooxygenase Branch of the Arachidonic Acid Cascade with Remarkable Structure-Dependent Selectivity and Potency. J. Agric. Food Chem. 2020, 68, 9235–9244. [Google Scholar] [CrossRef]
- Cheng, L.; Zheng, W.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus Fruits Are Rich in Flavonoids for Immunoregulation and Potential Targeting ACE2. Nat. Prod. Bioprospect. 2020, 12, 4. [Google Scholar]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1β Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]


| TPC (mg GAE/g DE) | TFC (mg QE/g DE) | |
|---|---|---|
| GF-CE | 20.18 ± 0.02 a | 13.09 ± 0.06 b |
| GF-EO | 13.21 ± 0.00 c | -- |
| GF-PE | 51.27 ± 0.01 b | 29.31 ± 0.01 a |
| Phenolic Compound | GF-CE | GF-PE |
|---|---|---|
| Hydroxycinnamic acids | ||
| Chlorogenic acid | 3.88 ± 0.21 a | 11.22 ± 0.09 b |
| Caffeic acid | 7.48 ± 0.40 b | 43.83 ± 0.61 a |
| p-Coumaric acid | 12.92 ± 0.52 b | 52.50 ± 0.45 a |
| Ferulic acid | 50.99 ± 1.03 b | 227.89 ± 2.15 a |
| Hydroxybenzoic acids | ||
| Gallic acid | 2.86 ± 0.01 | n.d. |
| 3.4-Dihydroxybenzoic acid | 11.63 ± 0.18 b | 24.52 ± 0.08 a |
| Methyl gallate | 0.43 ± 0.00 a | 0.12 ± 0.01 b |
| Ellagic acid | 2.32 ± 0.00 b | 3.15 ± 0.03 a |
| Saponarin | 1.29 ± 0.00 | n.d |
| Flavonoids | ||
| Rutin | 6.43 ± 0.24 a | 3.71 ± 0.23 b |
| Narirutin | 15,026.94 ± 6.05 b | 28,729.79 ± 7.41 a |
| Hesperidin | 667.73 ± 1.53 a | 285.58 ± 1.98 b |
| Diosmin | 62.34 ± 0.81 b | 80.80 ± 0.78 a |
| Quercetin | 0.62 ± 0.01 b | 1.14 ± 0.11 a |
| Naringenin | 7716.13 ± 2.36 b | 48,609.40 ± 9.16 a |
| Hesperitin | 3.00 ± 0.02 b | 4.08 ± 0.31 a |
| DPPH | Nitric Oxide | |||
|---|---|---|---|---|
| Concentration (µg/mL) | GP-EO | Vit. C | GP-EO | Vit. C |
| 250 | 21.8 ± 0.69 e | 23.73 ± 0.53 e | 9.85 ± 0.047 e | 43.32 ± 0.32 e |
| 500 | 35.7 ± 0.37 d | 41.08 ± 0.52 d | 14.63 ± 0.18 d | 63.61± 0.42 d |
| 1000 | 46.7 ± 1.27 c | 64.57 ± 0.59 c | 29.37 ± 0.33 c | 77.83 ± 0.51 c |
| 1500 | 54.8 ± 0.46 b | 81.85 ± 0.46 b | 39.86 ± 0.53 b | 85.25 ± 0.32 b |
| 2000 | 65.8 ± 0.28 a | 96.09 ± 0.14 a | 64.71 ± 0.52 a | 92.94 ± 0.28 a |
| IC50 | 1271.24 ± 0.85 b | 734.42 ± 0.43 c | 1656.19 ± 0.71 a | 263.60 ± 0.52 b |
| DPPH | Nitric Oxide | |||||
|---|---|---|---|---|---|---|
| Concentration (µg/mL) | GP-CE | GF-PE | Vit. C | GP-CE | GF-PE | Vit. C |
| 31.25 | 31.20 ± 0.72 f | 2.97 ± 0.82 f | 23.73 ± 0.471 f | 27 ± 1.31 f | 2.60 ± 0.25 e | 43.32 ± 0.32 f |
| 62.5 | 46.06 ± 0.53 e | 13.80 ± 0.76 e | 41.08 ± 0.68 e | 45.33 ± 0.88 e | 9.62 ± 0.71 d | 63.61 ± 0.42 e |
| 125 | 62.23 ± 0.28 d | 21.27 ± 0.44 e | 64.57 ± 0.53 d | 65.67 ± 0.33 d | 10.5 ± 0.40 d | 77.83 ± 0.51 d |
| 250 | 71.13 ± 0.33 c | 25.83 ± 0.75 c | 71.85 ± 0.52 c | 75.33 ± 0.67 c | 28.86 ± 0.52 c | 85.25 ±0.32 c |
| 500 | 88.96 ± 0.52 b | 31.42 ± 1.47 b | 86.09 ± 0.59 b | 84.33 ± 0.33 b | 44.04 ± 0.76 b | 88.12 ± 0.64 b |
| 1000 | 92.26 ± 0.54 a | 42.87 ± 0.62 a | 96.09 ± 46 a | 90 ± 0.58 a | 55.30 ± 0.51 a | 94.89 ± 0.78 a |
| IC50 | 75.69 ± 0.81 e | 1069.00 ± 0.56 a | 118.16 ± 0.78 c | 113.45 ± 0.71 d | 791.40 ± 0.52 b | 59.61 ± 0.65 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, F.M.; Abdelsalam, E.; Mohammed, R.S.; Ashour, W.E.S.; Vilas-Boas, A.A.; Pintado, M.; El Habbasha, E.S. Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives. Appl. Sci. 2024, 14, 2776. https://doi.org/10.3390/app14072776
Ibrahim FM, Abdelsalam E, Mohammed RS, Ashour WES, Vilas-Boas AA, Pintado M, El Habbasha ES. Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives. Applied Sciences. 2024; 14(7):2776. https://doi.org/10.3390/app14072776
Chicago/Turabian StyleIbrahim, Faten Mohamed, Eman Abdelsalam, Reda Sayed Mohammed, Wedian El Sayed Ashour, Ana A. Vilas-Boas, Manuela Pintado, and El Sayed El Habbasha. 2024. "Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives" Applied Sciences 14, no. 7: 2776. https://doi.org/10.3390/app14072776
APA StyleIbrahim, F. M., Abdelsalam, E., Mohammed, R. S., Ashour, W. E. S., Vilas-Boas, A. A., Pintado, M., & El Habbasha, E. S. (2024). Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives. Applied Sciences, 14(7), 2776. https://doi.org/10.3390/app14072776










