Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.1.1. Lithium Mica Slag
2.1.2. Cement
2.1.3. Sand
2.1.4. Mixing Water
2.1.5. Calcium Hydroxide
2.2. Methods
2.2.1. Specimen Molding
2.2.2. SO42− Dissolution Mechanism
2.2.3. Mass Change
2.2.4. Expansion Test on Mortar Specimens
2.2.5. Flexural and Compressive Strengths
2.2.6. X-ray Diffraction (XRD) Analysis on Mortar Specimens
2.2.7. Thermogravimetry–Differential Scanning Calorimetry (TG–DSC) Analysis on Mortar Specimens
2.2.8. Mercury Intrusion Porosimetry (MIP) Analysis on Mortar Specimens
2.2.9. Scanning Electron Microscope (SEM) Analysis on Mortar Specimens
3. Results and Discussion
3.1. Dissolution of SO42− from Lithium Mica Slag
3.2. Mass Change
3.3. Flexural and Compressive Strengths of Mortar Specimens
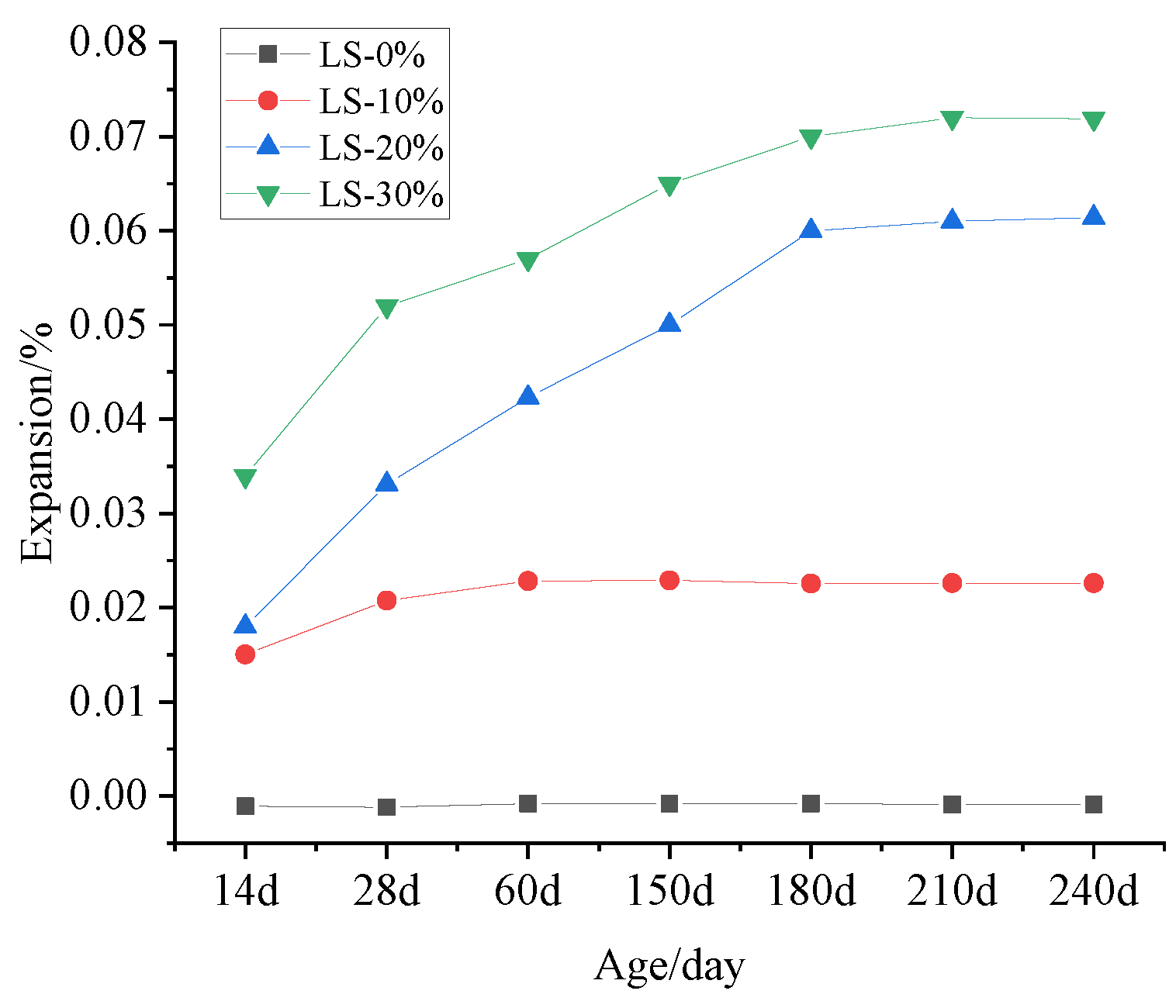
3.4. Expansion Measurement
3.5. XRD Examination
3.6. TG–DSC Examination
3.7. MIP Examination
3.8. SEM Examination
4. Conclusions
- (1)
- Since the pH value is 12.5 in the pore solution of concrete, the mortar specimens with lithium mica slag produce a large amount of sulfate ions (SO42−).
- (2)
- It was observed that the mortar specimens with lithium mica slag initially formed new ettringite, according to the results from microscopic analyses. In addition, with an increase in the content of lithium mica slag, the mass, expansion rate, and strength of the mortar specimens increased as testing progressed, which indicates that internal sulfate reactions occurred within the mortar specimens.
- (3)
- When the content of lithium mica slag is below 30%, the content of AFt decreases in the later stages. Furthermore, the occurrence of internal sulfate attacks did not inflict cracking damage to the mortar specimens; instead, the strength of the mortar specimens was enhanced in the later stages.
- (4)
- This research study has proven that sulfates in lithium mica slag do not damage mortar specimens; thus, lithium mica slag is a good alternative material, as it not only reduces land resource waste, but also conserves resources and promotes economic recycling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poetschke, L.L.; Miller, J.P. Assessing future lithium demand for electric vehicle batteries. J. Ind. Ecol. 2019, 23, 808–819. [Google Scholar]
- Bide, T.; Smidt, E.; Ulseth, R. Environmental and social impacts of tantalum mining. J. Clean. Prod. 2019, 226, 708–719. [Google Scholar]
- Selvaraj, M.; Ramesha, K.; Watanabe, Y.; Honma, I. Li-extraction from lithium mica by sulfuric acid and its electrochemical behavior in lithium cells. Chem. Eng. J. 2017, 319, 47–52. [Google Scholar]
- Sun, S.; Jiang, M.; Zou, P.; Yang, H. Recovery of lithium from Yichun spodumene ore by bioleaching. Minerals 2018, 8, 247–259. [Google Scholar]
- Wang, Y.; Wang, D.; Cui, Y.; Zheng, D.; Liu, Z. Micro-morphology and phase composition of lithium slag from lithiumcarbonate production by sulphuric acid process. Constr. Build. Mater. 2019, 203, 304–313. [Google Scholar]
- Wu, F.F.; Shi, K.B.; Dong, S.K. Influence of concrete with lithium-slag and steel slag by early curing conditions. Key Eng. Mater. 2014, 599, 52–55. [Google Scholar] [CrossRef]
- Li, B.; Cao, R.; You, N.; Chen, C.; Zhang, Y. Products and properties of steam cured cement mortar containing lithium slag under partial immersion in sulfate solution. Constr. Build. Mater. 2019, 220, 596–606. [Google Scholar] [CrossRef]
- He, Z.H.; Du, S.G.; Chen, D. Microstructure of ultra high performance concretecontaining lithium slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, X.; He, C.; Ma, B.; Bai, Y.; Luo, Z. Utilization of lithium slag as an admixture in blended cements: Physico-mechanical and hydration characteristics. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 129–133. [Google Scholar] [CrossRef]
- Dong, P.; Ahmad, M.R.; Chen, B.; Kazmi, S.; Munir, M. Preparation and study of magnesium ammonium phosphate cement from waste lithium slag. J. Clean. Prod. 2021, 316, 128371. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, J.; Fan, J.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Lu, Z. Insight to workability, compressive strength and microstructure of lithium slag-steel slag based cement under standard condition. J. Build. Eng. 2023, 75, 107076. [Google Scholar] [CrossRef]
- Tan, H.B.; Li, M.G.; He, X.Y.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-ithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2019, 250, 119528. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive utilization of waste residue from lithium extraction process of spodumene. Miner. Eng. 2021, 170, 106986. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization ofzeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh FU, A.; Sarker, P.K. Microstructural investigation of thermo-mechanically processed lithium slag for geopolymer precursor using various characterization techniques. Constr. Build. Mater. 2022, 342, 127952. [Google Scholar] [CrossRef]
- Yang, H.; He, Y.; Li, Z.; Wu, L. Lithium extraction from β-spodumene using sodium salts and Na2SO4 molten salt roasting. J. Electrochem. Soc. 2019, 166, D568–D573. [Google Scholar]
- Li, Z.; Luo, T.; Yuan, L.; Song, B.; Guan, H.; Yuan, J.; Tang, A.; Tao, S. Lithium extraction from β-spodumene using sodium carbonate and sodium chloride roasting followed by water leaching. Minerals 2017, 7, 239. [Google Scholar]
- Moriguchi, I.; Tsuruta, T.; Sasaki, K.; Kunii, D.; Ohtani, K.; Tamaura, Y. Efficient lithium recovery from lithium-rich mica using sulphuric acid leaching. Miner. Eng. 2018, 116, 135–140. [Google Scholar]
- Pausch, M.; Weiß, R.; Ditze, A.; Bertau, M.; Friedrich, B. A new process to upgrade lithium from spodumene using thermal treatment. Miner. Eng. 2019, 143, 106087. [Google Scholar]
- Zhang, J.; Li, G.; Li, C.; Zhu, Z.; Mei, G.; Sun, Z. Study on the lithium extraction from β-spodumene through chlorination roasting followed by water leaching. Miner. Eng. 2017, 100, 67–71. [Google Scholar]
- GB/T 749-2008; Test Method for Cement Resistance to Sulfate Attack. National Standard of the People’s Republic of China: Beijing, China, 2008.
- GB/T 34672-2017; Chemical Reagent-General Rules for the Ion Chromatography. National Standard of the People’s Republic of China: Beijing, China, 2017.
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. National Standard of the People’s Republic of China: Beijing, China, 2009.
- GB/T 29417-2012; Standard Test Methods for Drying Shrinkage Stress and Cracking Possibility of Cement Mortar and Concrete. National Standard of the People’s Republic of China: Beijing, China, 2012.
- GB∕T17671-2021; Testing Method for Cement Mortar Strength. National Standard of the People’s Republic of China: Beijing, China, 2021.









| SiO2 | SO3 | CaO | Al2O3 | Na2O | K2O | F | Fe2O3 | LOI |
|---|---|---|---|---|---|---|---|---|
| 27.57 | 20.63 | 15.35 | 15.18 | 5.50 | 4.20 | 2.33 | 1.69 | 5.93 |
| SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|
| 18.83 | 65.29 | 4.62 | 0.86 | 3.30 | 1.71 | 0.63 | 0.17 | 3.55 |
| Manufacturer | Specification | Quality Indicators | ||
|---|---|---|---|---|
| SiO2/% | LOI/% | Silt Content/% | ||
| Xiamen Aceo | Medium Sand | >96 | ≤0.40 | ≤0.20 |
| Sample Code | W/C | Water/g | Standard Sand/g | Cement/g | Lithium Mica Slag/g |
|---|---|---|---|---|---|
| S0 | 0.5 | 225 | 1350 | 500 | 0 |
| S1 | 450 | 50 | |||
| S2 | 400 | 100 | |||
| S3 | 350 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Huang, B.; Dong, Z. Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar. Appl. Sci. 2024, 14, 2723. https://doi.org/10.3390/app14072723
Liu N, Huang B, Dong Z. Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar. Applied Sciences. 2024; 14(7):2723. https://doi.org/10.3390/app14072723
Chicago/Turabian StyleLiu, Na, Bei Huang, and Zebo Dong. 2024. "Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar" Applied Sciences 14, no. 7: 2723. https://doi.org/10.3390/app14072723
APA StyleLiu, N., Huang, B., & Dong, Z. (2024). Effect of Lithium Mica Slag on the Internal Sulfate Attack of Cement Mortar. Applied Sciences, 14(7), 2723. https://doi.org/10.3390/app14072723







