Abstract
(1) Background: Skeletally anchored appliances for maxillary expansion are a less invasive alternative to surgery and are increasingly often selected as a method of treatment. The purpose of this study was to retrospectively evaluate the efficacy of maxillary expansion using distractors supported by temporary orthodontic implants (TOIs) in patients of differing sex and age. (2) Methods: The study group consisted of patients treated with distractors anchored to TOIs between July 2019 and June 2022. The group was divided into adolescent and the adult patients. Effectiveness was evaluated one and two weeks after installation of the distractors and the results were analyzed statistically. (3) Results: In total, 39 patients were treated, of which 21 were women and 18 men, ranging in age from 11 to 42. A group of 8 girls and 9 boys younger than 18 years were included. The presence of diastema at the first follow-up visit was observed in all girls and in 8 of the boys, as well as in 7 women and in none of the men. At the second visit, no diastema was found in one woman, but was noted in 3 men (p = 0.007). At the completion of distraction, diastema was observed in all the girls and in 12 women, as well as in 8 out of 9 boys and in 3 men (p = 0.015). (4) Conclusions: The effectiveness of the distraction procedure in adolescents is positive, regardless of sex. However, in adults, the procedure has limited effectiveness in males.
1. Introduction
Maxillary deficiency can manifest clinically in different ways, for example as unilateral or bilateral crossbite and crowding, as impacted canines, as ectopic eruption of the canine, or as vertical overbite of less than 1.0 mm. It has been demonstrated that a number of smaller symptoms (so-called microsyndromes) can lead to diagnosis of maxillary deficiency syndrome [1,2]. In such cases, maxillary expansion as the midpalate suture extends is a possible first stage of orthodontic treatment [3,4,5]. The study of Melsen [6] explained the transverse growth of the midpalate suture; she histologically examined the palates of boys and girls from zero to eighteen years of age and stated that suture growth occurred in three stages, in which the shape and length of the suture changed from straight and Y shaped (which appears easily separated), though more sinuous, to extremely interdigitated. She argued, in conclusion, that palatal sutures in eighteen-year-olds are so heavy that the separation of the two halves is not possible without fracturing the bone process. However, she did not describe the exact time needed for complete ossification. The histological study of Knaup et al. [7] indicates that success in palatal suture separation can probably not be reliably associated with suture ossification. They found that the ossification index was less than 5% in patients over 26 years of age, even in the most markedly ossified posterior palatal region. In Melsen’s research, maxillary expansion was performed mainly using tooth-borne appliances (such as Hyrax-type expanders) or appliances borne on the tooth and mucous membrane (e.g., Haas-type expanders); these are especially effective in children prior to the peak of skeletal growth, when the palatal suture is not very sinuous [4,8]. These methods can result in side-effects such as dentoalveolar tipping, root resorption, and gingival recession [9], generally among patients closer to eighteen years of age [2,10,11]. In patients over eighteen, treatment with these devices is ineffective and can have more serious side effects. To avoid these and to allow expansion, temporary implants have begun to be used as bone–borne anchorages. This method allows palatal expansion in patients over eighteen and helps to avoid surgically assisted palatal expansion (SARPE), which is an alternative method of treatment that requires general anesthesia; the healing process after this procedure is definitely longer and associated with more discomfort. Similarly, the removal of the distractor sometimes requires general anesthesia and is carried out in a hospital setting [2,12,13,14].
Skeletally anchored appliances for rapid maxillary expansion are a less invasive alternative to surgery. Their use is associated with surgery under local anesthesia and is performed in an outpatient setting in the dentist’s office. This approach is used increasingly often as a means of expanding the maxillary dental arch when its narrowing has resulted in unilateral or bilateral crossbite. One advantage of this treatment method, the prevention of sleep apnea, is often highlighted [5,15,16]. However, the effectiveness is not always predictable: greater predictability and certainty of success is found in younger patients, as confirmed by numerous papers [15,17,18]. For example, maxillary expanders (especially hybrid Hyrax appliances) prevents mesialization of the molars until the premolars erupt [19]. Research has pointed to inconsistent results for treatment success, especially among adults [20,21]. However, no one has unequivocally noted differences in the success of maxillary distraction in male and female patients. For this reason, we decided to investigate further.
The purpose of this study was to retrospectively evaluate the efficacy of the maxillary expansion procedure using distractors supported by temporary orthodontic implants, in patients of different sex and ages.
2. Materials and Methods
We retrospectively analyzed data from patients treated with distractors anchored to temporary orthodontic intraosseous implants between July 2019 and June 2022 at our clinic.
Consent was obtained from the Bioethical Committee of the Regional Medical Chamber to conduct the research (decision no. OIL/KBL/49/2022).
All patients treated with the orthodontic implants were included in the study in the first step, regardless of age and sex. To maintain the homogeneity of the study group, patients with the same type of implants (Dual-Top JS jet screw ø 2.5-JS-012/014/016 mm) and the same distraction screw (Power Screw, Tiger Dental, Bregenz, Austria) were included in the next step of the study. None of the patients had a prior history of orthodontic treatment with fixed appliances or surgically assisted maxillary expansion. The exclusion criterion was a cleft lip or cleft palate. Patients were divided into an adolescent group (those below eighteen years of age) and an adult group (those eighteen and over).
Application of the distractors proceeded as follows:
- Following cone beam computed tomography (CBCT) examination and analysis of the intended location of the mini-implant, the temporary orthodontic implant screws were inserted under local anesthesia. Dual-Top JS jet screw implants (Tiger Dental, Bregenz, Austria) with a diameter of 2.5 mm were used. Four implants were inserted in each patient: two mesially and two distally. Depending on the amount of bone available, implants of 12, 14, or 16 mm in length were inserted by the same operator (BWL).
- After two weeks of the healing, an alginate impression with transfers caps was taken. A master cast was produced in the in-house laboratory from Class IV plaster with four laboratory analogues. The distractor size was chosen based on the clinical situation (the available screw body sizes were 8 mm, 10 mm, 12 mm, 14 mm, and 16 mm). We refer to the appliance as the PS4 Distractor, after the Power Screw (PS) and the number of implants on which it was placed. The master cast with the selected screw and four collars for adhesive bonding was sent to the laboratory. All distractors were made in the same laboratory (Ortolab, Częstochowa, Poland).
- After a further three weeks, the distractor was cemented onto the implants using SDI Riva Self Cure HV Glass Ionomer (Melbourne, Australia) cement by the same operator (BWL). An example is presented in Figure 1.
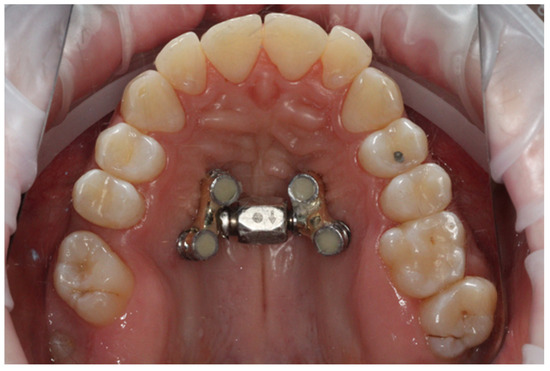 Figure 1. An example of bone–borne anchorage connected to four temporary implants before treatment.
Figure 1. An example of bone–borne anchorage connected to four temporary implants before treatment.
- 4.
- The patient (or the patient with his or her parents) was instructed on how to deal with the distractor as follows: The screw should be turned twice daily, in the morning and the evening. Each screw has six surfaces, each of which should be counted when turning. Each surface is marked with one, two, or three black dots for visual saliency. A full turn of the screw would result in an expansion of 1 mm, whereas turning the screw by one sixth of a full rotation (one surface) would give an expansion of 0.17 mm. A Power Screw open-ended spanner is used to turn the screw, with the correct direction of rotation being from the upper to the lower incisors. The patient should open the mouth as widely as possible, placing the spanner on the hexagon of the screw body so that the handle is near the upper front teeth. The spanner should then be turned until it is positioned just before the lower teeth (on the tongue). The spanner should then be detached, turned 180° around the longitudinal axis, and placed back on the upper teeth; it should then be moved downwards again, as shown in Figure 2.
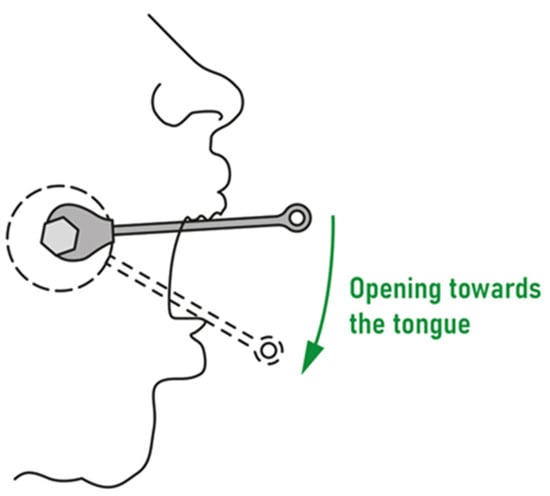 Figure 2. The direction in which the distractor screws should be turned.
Figure 2. The direction in which the distractor screws should be turned.
This usually results in rotation by one and a half surfaces. The same procedure should be repeated in the evening, resulting in a screw spread of 0.5 mm per day. The patient (and parents, where applicable) were informed that once the diastema appears, the turning should be decreased from two to one turns in the morning and one turn in the evening. The diastema should be considered to have appeared when a pin can move freely between the central incisors: this means that a gap of 1 mm has appeared.
- 5.
- One week after application, the first control visit took place.
- 6.
- Two weeks after application, the second control visit took place to evaluate the diastema.
Whether the diastema had appeared between the upper incisors after one week or after two weeks was noted in the patient’s chart. An example of a diastema produced after two weeks of expansion is presented in Figure 3.
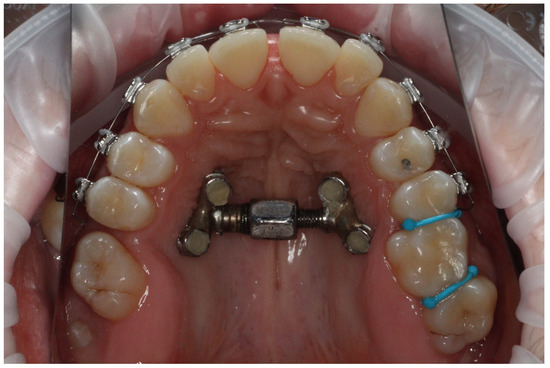
Figure 3.
An example of diastema after two weeks of expansion.
The timing of the distraction process, judged by whether a diastema had appeared between the upper incisors, was evaluated at follow-up visits one and two weeks after the distractor was applied; the timing of distraction completion was also evaluated.
A potential for bias in the test results may have resulted from a failure to follow the recommended test protocol, particularly as it relates to the frequency of screw activation.
The results were statistically analyzed using descriptive statistics by showing quantitative and qualitative features. A comparison of the qualitative variables between the groups was performed using the chi-square test (with Yates’ correction for 2 × 2 tables), or Fisher’s exact test where low expected frequencies appeared in the tables. A significance level of 0.05 was adopted in the analysis. All p values below 0.05 were interpreted as showing significant relationships. The analysis was performed in R software, version 4.2.1 [22].
3. Results
Over the three-year study period, 39 patients were treated with bone–borne distractors of the palate, of which 21 were females and 18 were males; patients ranged in age from 13 years and 1 month to 39 years and 4 months for the females and from 11 years and 10 months to 42 years and 10 months for the males. A group of 8 girls and 9 boys under 18 years of age were included in the analysis, while 13 women and 9 men were included in the adult group. A description of the qualitative characteristics of the study group is shown in Table 1.

Table 1.
Qualitative characteristics of the study group.
Table 2 shows detailed results including age (in years and months), sex, and presence of diastema for all participants. F: female; M: male; Y: presence of diastema; n: absence of diastema.

Table 2.
Sex, age, and presence of diastema in all study participants.
Diastema was observed at the first follow-up visit (one week after distraction was started) in all the girls and in eight out of nine boys; no further changes had occurred by the second follow-up visit. There was no statistically significant difference between the results.
Among the adults, diastema was present in seven out of the thirteen women and in none of the men after the first week; that difference was statistically significant (p = 0.017 in the chi-square test). At the second follow-up visit, diastema was lacking in one woman and was present in three of the nine males; this difference was also statistically significant (p = 0.007). The results are shown in Table 3.

Table 3.
Distraction results in the adult patients.
Upon completion of distraction, diastema had appeared in all the girls, in twelve of the thirteen women, in eight of the nine boys, and in three of the nine men. Detailed results for all groups by sex are presented in Table 4.

Table 4.
Distraction results in all patient groups.
4. Discussion
We have presented here results on the use of bone–borne distractors for maxillary expansion in two groups of patients. Among the adolescent patients, we obtained a success rate of 100% in the girls and of 89% in the boys (corresponding to 8 out of 9 boys) after one week of treatment, this did not change in the second week of observation. The results in the younger group of patients were similar to those obtained by Jeon et al. [21], even though the procedure they employed was different: they performed one turn of the screw per day (0.2 mm per turn), whereas our procedure used 0.5 mm expansion a day. The success rate reported in Jia et al. [12] was 100% in both sexes in their two younger age groups (from 11 to 19 years). They used an expansion procedure similar to ours, but with Hyrax-type expanders. This may suggest that the speed of expansion does not play a significant role in the process of maxillary suture opening in younger patients.
In our adult patients, expansion for one week produced a diastema in 54% of the women and in none of the men; this was a significant difference. At the next appointment one week later, diastema had occurred in 92% of women and in 33% of men; this result was also statistically significantly different. The overall success rate in the adult group of thirteen females and nine males was 68%. This differs from the results of Winsauer et al. [20], who had a success rate of 84% in a group of nineteen female adults and eight male adults, and where the difference between the sexes was not statistically significant. On the other hand, they had an 86% success rate among their female adults, whereas we saw a 92% success rate among female adults. Our research and theirs followed different protocols: they waited twelve weeks after the implants were installed; a distraction of 0.34 mm per day was then recommended for the first week (0.5 mm in our protocol). They also measured the forces with a spring scale, ensuring they did not exceed 500 cN. This was not measured in our protocol. However, our patients were verbally instructed not to activate the distractor with too much force. If a patient had difficulty activating it, then he or she was to abandon it or carry it out at intervals. So, if patients attempt to activate the device in the evening but feel either too much resistance from the screw or too much tension in the facial area, then they are to abandon activation. They might take a one-hour break before attempting activation again. Patient medical history data show that this type of activation in stages yields positive results.
Similar results on distraction in patients fifteen years and older were obtained by Jeon et al. [21], despite their different expansion protocol. This group saw success in 93% of females and in 54% of males, which is similar to our results for females. They also pointed out that there were no successful male cases older than thirty. Our oldest male patient was 26 years old, while our oldest female was 39. This might suggest that interdigitation is not the only factor that can make the palatal suture inseparable: the success of palatal distraction may also be associated with age and sex. A prospective study by Kapetanovic et al. [23] found a success rate of 94% in a group of 34 patients who ranged in age from 16 to 34 and, 26 of whom were women and 8 of whom were men. However, they did employ a longer observation time of 23 to 39 days. Our present research agrees with the positive results obtained for distraction periods longer than two weeks. The research of Alkhani et al. [24] on palatal distraction in rats explained the histological process as a biological reaction in which inflammation activates osteoclasts (as a catabolic reaction to tensile transverse forces) in the first period of distraction, which takes a month before the process of bone separation begins. Osteoblasts are then activated in response to the number of osteoclasts. In the next step, new bone is produced. The contribution of the inflammation process was confirmed in the control group of rats, which were medicated with anti-inflammatories; a decrease in osteoblast activity was observed in them. This confirmed the result of a previous study by Sims et al. [25]. These studies show that there is a need to pay attention to the painkillers patients are taking in order to ensure they have no anti-inflammatory component. Alkhani et al. [24] also considered previous research conducted on dry skulls and finite elements [26,27], where the palatal suture bone distraction was described in terms of transversal tensile forces, without taking into account the biological reaction. On the other hand, they noted that the smaller initial sutural width in adult patients means that the osteoclast activation time may be longer; this suggests that strong forces over short periods are contraindicated in that group of patients. Based on this research, Jeon et al. [21] suggested that, even if distraction fails in the initial stage of expansion, activation of the catabolic phase after a resting period of approximately one month can allow the suture to be separated after the resumption of expansion. Similarly, the unexplored biological reaction could be associated with sex hormones, which can play an important differentiating role in sex-specific reactions to distraction forces in suture soft tissue. The research of Alkhani et al. [24] used only female rats, so sex differences were not examined. It is also worth noting that, in our study group of men who did not develop maxillary distraction, we recommended stopping the daily activation by changing the maintenance protocol to activation once a week and setting the next visit for a month later. All patients in whom this type of emergency protocol was implemented returned to us after a month with visible diastema. They reported that they went to bed in the evening with noticeable tension, that in the morning they no longer felt tension, and that they noticed the diastema in the mirror when they first visited the bathroom.
Our retrospective research has shown that there are correlations between sex and age on one hand and the success rate of palatal expansion on the other, but future prospective studies are necessary to optimize the frequency of activation, given need to limit the induced force.
The effectiveness of the distraction procedure in adolescents is positive, regardless of sex. However, in adults, the procedure has limited effectiveness in males. This is also important for predictions of orthodontic treatment plans.
5. Conclusions
The distraction procedure is effective in adolescents, regardless of sex, but has limited effectiveness in adult males. The mechanism behind this phenomenon requires further research.
Author Contributions
Conceptualization: B.W.L. and J.E.L.; methodology: B.W.L.; formal analysis: B.W.L.; investigation: B.W.L. and Z.J.; writing—original draft preparation: J.E.L. and Z.J.; writing—review and editing: B.W.L. and Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethical Committee of the Regional Medical Chamber to conduct the research (approval no. OIL/KBL/49/2022, 12 August 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the authors of the article upon individual request at the following e-mail address: bwloster@gmail.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Williams, S.; Loster, J.E.; Loster, B.W. The relationship between maxillary dental and occlusal anomalies: Evidence of a ‘Maxillary Deficiency Syndrome’. Australas. Orthod. J. 2018, 34, 212–224. [Google Scholar] [CrossRef]
- Lin, L.; Ahn, H.W.; Kim, S.J.; Moon, S.C.; Kim, S.H.; Nelson, G. Tooth-borne vs bone-borne rapid maxillary expanders in late adolescence. Angle Orthod. 2015, 85, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lagravere, M.O.; Major, P.W.; Flores-Mir, C. Long-term skeletal changes with rapid maxillary expansion: A systematic review. Angle Orthod. 2005, 75, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Podesser, B.; Williams, S.; Crismani, A.G.; Bantleon, H.P. Evaluation of the effects of rapid maxillary expansion in growing children using computer tomography scanning: A pilot study. Eur. J. Orthod. 2007, 29, 37–44. [Google Scholar] [CrossRef]
- Lagravere, M.O.; Ling, C.P.; Woo, J.; Harzer, W.; Major, P.W.; Carey, J.P. Transverse, vertical, and anterior-posterior changes between tooth-anchored versus Dresden bone-anchored rapid maxillary expansion 6 months post-expansion: A CBCT randomized controlled clinical trial. Int. Orthod. 2020, 18, 308–316. [Google Scholar] [CrossRef]
- Melsen, B. Palatal growth studied on human autopsy material. A histologic microradiographic study. Am. J. Orthod. 1975, 68, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Knaup, B.; Yildizhan, F.; Wehrbein, H. Age-related changes in the midpalatal suture. A histomorphometric study. J. Orofac. Orthop. 2004, 65, 467–474. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; Cameron, C.G.; McNamara, J.A., Jr. Treatment timing for rapid maxillary expansion. Angle Orthod. 2001, 71, 343–350. [Google Scholar] [CrossRef]
- Weissheimer, A.; de Menezes, L.M.; Mezomo, M.; Dias, D.M.; de Lima, E.M.; Rizzatto, S.M. Immediate effects of rapid maxillary expansion with Haas-type and hyrax-type expanders: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 366–376. [Google Scholar] [CrossRef]
- Winsauer, H.; Vlachojannis, J.; Winsauer, C.; Ludwig, B.; Walter, A. A bone-borne appliance for rapid maxillary expansion. J. Clin. Orthod. 2013, 47, 375–381; quiz 388. [Google Scholar]
- Wilmes, B.; Nienkemper, M.; Drescher, D. Application and effectiveness of a mini-implant- and tooth-borne rapid palatal expansion device: The hybrid hyrax. World J. Orthod. 2010, 11, 323–330. [Google Scholar] [PubMed]
- Jia, H.; Zhuang, L.; Zhang, N.; Bian, Y.; Li, S. Age-dependent effects of transverse maxillary deficiency treated by microimplant-assisted rapid palatal expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Cevidanes, L.H.; Franchi, L.; Goncalves, J.R.; Benavides, E.; McNamara, J.A., Jr. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.; McNamara, J.A., Jr. Diagnostic performance of skeletal maturity for the assessment of midpalatal suture maturation. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Montigny, M. Mini-implant assisted rapid palatal expansion: New perspectives. J. Dentofac. Anom. Orthod. 2017, 20, 18. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Palmisano, R.G.; Poole, M.D. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 1998, 21, 831–835. [Google Scholar] [CrossRef]
- Choi, S.H.; Shi, K.K.; Cha, J.Y.; Park, Y.C.; Lee, K.J. Nonsurgical miniscrew-assisted rapid maxillary expansion results in acceptable stability in young adults. Angle Orthod. 2016, 86, 713–720. [Google Scholar] [CrossRef]
- Chane-Fane, C.; Darque, F. Rapid maxillary expansion assisted by palatal mini-implants in adolescents—Preliminary study. Int. Orthod. 2015, 13, 96–111. [Google Scholar] [CrossRef]
- Walter, A.; Wendl, B.; Ploder, O.; Mojal, S.; Puigdollers, A. Stability determinants of bone-borne force-transmitting components in three RME hybrid expanders-an in vitro study. Eur. J. Orthod. 2017, 39, 76–84. [Google Scholar] [CrossRef]
- Winsauer, H.; Walter, A.; Katsaros, C.; Ploder, O. Success and complication rate of miniscrew assisted non-surgical palatal expansion in adults—A consecutive study using a novel force-controlled polycyclic activation protocol. Head Face Med. 2021, 17, 50. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Choi, S.H.; Chung, C.J.; Lee, K.J. The success and effectiveness of miniscrew-assisted rapid palatal expansion are age- and sex-dependent. Clin. Oral Investig. 2022, 26, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 12 August 2022).
- Kapetanovic, A.; Odrosslij, B.; Baan, F.; Bergé, S.J.; Noverraz, R.R.M.; Schols, J.G.J.H.; Xi, T. Efficacy of Miniscrew-Assisted Rapid Palatal Expansion (MARPE) in late adolescents and adults with the Dutch Maxillary Expansion Device: A prospective clinical cohort study. Clin. Oral Investig. 2022, 26, 6253–6263. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Alansari, S.; Al Jearah, M.; Gadhavi, N.; Hamidaddin, M.A.; Shembesh, F.A.; Sangsuwon, C.; Nervina, J.M.; Teixeira, C.C. Biphasic sutural response is key to palatal expansion. J. World Fed. Orthod. 2019, 8, 9–17. [Google Scholar] [CrossRef]
- Sims, N.A.; Vrahnas, C. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch. Biochem. Biophys. 2014, 561, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Boryor, A.; Hohmann, A.; Wunderlich, A.; Geiger, M.; Kilic, F.; Kim, K.B.; Sander, M.; Böckers, T.; Sander, C. Use of a modified expander during rapid maxillary expansion in adults: An in vitro and finite element study. Int. J. Oral Maxillofac. Implant. 2013, 28, e11–e16. [Google Scholar] [CrossRef][Green Version]
- Gautam, P.; Valiathan, A.; Adhikari, R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: A finite element method study. Am. J. Orthod. Dentofacial. Orthop. 2007, 132, e1–e11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).