Abstract
This study aimed to assess the effect of exercise on serum electrophoretic protein pattern, C-reactive protein (CRP) and platelet aggregation in horses subjected to a jumping exercise. The possible relationship between acute-phase reactions and platelet reactivity in the context of exercise was investigated. Blood samples were collected from 10 jumper horses at rest (TREST), within 5 min from the end of exercise (TPE5), and 30 min (TPE30) and 60 min after exercise (TPE60). The serum values of total proteins; CRP; albumin; α1-, α-2, β1-, β2- and γ-globulins; and the maximum degree of aggregation and the initial velocity of aggregation (slope) were evaluated. According to one-way analysis of variance, CRP and α1-, α-2, β1- and β2-globulins increased after exercise compared with rest condition (p < 0.001), whereas albumin and platelet aggregation showed lower values after exercise than at rest (p < 0.001). CRP and α1-globulin values were negatively correlated with both platelet aggregation indices at TPE5, whereas no significant correlation among these parameters was found at TREST, TPE30 and TPE60. This study provides evidence that an acute-phase response occurred in horses after the jumping exercise and suggests a linkage between the inflammatory status and the platelet responsiveness in horses during exercise.
1. Introduction
A number of external and internal factors negatively disturb the homeostatic equilibrium of organisms, causing the so-called state of stress. Stressful events perturb an organism’s welfare status and induce energy-consuming mechanisms to counter the onset of ill effects [1]. Several stress markers that indicate that an individual is not in physiological comfort are considered and investigated in order to study the stress response of organisms and their capability to restore homeostasis [2]. Indeed, events starting from the disturbance of homeostasis initiate a series of reaction cascades involving oxidative, inflammatory and proteomic reactions [2,3,4,5,6]. These stress-induced reactions are well orchestrated and so interlinked that the generation of one compound influences the formation of another one. One example is represented by the generation of pro-inflammatory interleukins that influence the production of anti-inflammatory counterparts [4]. Among the several effects of the inflammatory reaction to stress conditions, the onset of an acute-phase response (APR) is well known. The APR is a rapid and non-specific response to any kind of injury [7] during which acute-phase proteins (APPs) are produced in the liver following the activation of receptors on various target cells by pro-inflammatory cytokines. The serum protein pattern generated by the serum electrophoresis analysis gives a good picture of the acute-phase serum protein response. Serum proteins include four fractions, namely albumin and alpha (α)-, beta (β)- and gamma (γ)-globulin fractions [8]. The α-globulin fraction includes the C-reactive protein (CRP) known as a positive APP since its concentration increases during an inflammatory response [7]. In horses, the CRP is a moderate APP and an increase in its concentration has been reported after castration, in enteritis, arthritis, laminitis and induced inflammation [9]. A reaction analogous to the APR in inflammatory conditions has also been reported after exercise in humans [10,11] and in athletic horses [12,13]. As a matter of fact, physical exercise induces various stress responses and metabolic adaptations. Depending on the duration, intensity and type of physical exercise, as well as on the training condition of the subject, equine metabolism has to adapt to nervous, cardiovascular, hemostatic, endocrine and respiratory system requirements. The exercise-induced APR has been characterized by changes in pro-inflammatory and anti-inflammatory cytokines, CRP and other acute-phase proteins, including albumin, fibrinogen and serum amyloid A [11,12]. Similarly to APPs, the platelet parameters are markers reflecting the systemic inflammatory response besides the activity/severity of many diseases [14,15]. Though many reports have been published on the effects of physical exercise on hemostasis and acute-phase proteins in horses, few studies have included in the same experimental protocol the simultaneous evaluation of CRP, albumin, globulin fractions and platelet aggregation. Specifically, data regarding the linkage between platelet activation indices and inflammatory markers are scarce and contradictory [16,17,18]. Moreover, in horses, the change in serum CRP together with electrophoretic protein pattern, including indirect markers of activation of inflammation and immunity, and their relationship with platelet activity have not been characterized in the context of exercise.
On the basis of such considerations, the aims of this study were (i) to evaluate the dynamic change in serum CRP, total proteins, albumin, globulin fractions, the maximum degree of platelet aggregation and the initial velocity of aggregation in response to exercise in well-trained jumper horses; and (ii) to assess whether the serum concentrations of CRP, albumin and globulin fractions are correlated with the platelet activity indices in order to clarify if and how platelet aggregation is modulated by inflammation status in athletic horses following physical exercise.
2. Materials and Methods
Ten Italian saddle horses (geldings; 7–10 years; mean body weight 465 ± 60 kg) from the same horse training center were enrolled in this study with the owner’s informed consent. All animals enrolled in the study were healthy as assessed by the clinical examination, routine hematology and biochemistry analyses performed at rest conditions. All horses were kept in individual boxes (3.50 × 3.50 m2) and fed as described previously [12]. Horses were subjected to a standardized obstacle course preceded by a warm-up consisting of walking, trotting and galloping, including six jumps (5 verticals, 4 long jumps) of 0.80–1.20 m height and 1.15 m width. The exercise consisted of a 350 m long trail with eleven 1.25 high jumps (five vertical jumps, five long jumps and one double vertical and long jump). From each horse, blood samples were collected at rest (TREST), within 5 min from the end of exercise (TPE5), and 30 min and 60 min after exercise (TPE30 and TPE60, respectively) by jugular vein puncture into vacutainer tubes with a clot activator (Terumo Corporation Japan, Tokyo, Japan) for the assessment of serum concentrations of C-reactive protein (CRP), total proteins and globulin fractions, and into 3.6 mL tubes containing 3.8% sodium citrate (1 part citrate/9 parts blood) for platelet aggregation measurements. Blood samples collected into vacuum tubes containing clot activator were allowed to clot for 20 min at room temperature prior to centrifugation at 3000 rpm for 10 min and the obtained sera were stored at −20 °C until analysis. Through an ELISA kit specific for equine species (ab190527—CRP Horse ELISA kit, abcam, Boston, MA, USA; sensitivity 1.198 ng/mL, intra-assay coefficient of variation <10%, inter-assay coefficient of variation <10%), the serum concentration of C-reactive protein (CRP) was determined by a micro-well plate reader (Sirio, SEAC, Florence, Italy). The serum values of total protein were investigated by the Biuret method through an automated UV spectrophotometer (Slim, SEAC, Florence, Italy; commercially available kit, Biosystems S.A., Barcelona, Spain). The electrophoresis of serum protein fractions was performed through an automated system (Selvet 24, Seleo Engineering, Naples, Italy) following the procedures defined by the manufacturer as previously described [19]. Albumin and α1-, α-2, β1-, β2- and γ-globulins were the major protein fractions obtained. The determination of platelet aggregation was performed by obtaining platelet-rich plasma (PRP) and platelet-poor plasma (PPP) by centrifugation of blood samples collected into tubes containing 3.8% sodium citrate. The detailed description of the complete methodology was previously described [20,21]. Platelet aggregation response was evaluated by an analysis of the maximum degree of aggregation and the initial velocity of aggregation (slope, expressed in percentage/min) [20].
Statistical Analyses
Data were normally distributed (Shapiro–Wilk test, p > 0.05) and one-way analysis of variance for repeated measures was applied to assess significant effects of exercise on the serum concentrations of CRP, total proteins, albumin and globulins (α1-, α-2, β1-, β2-, γ-globulins) and on platelet aggregation. Pearson’s correlation coefficients were applied to assess the relationship between the investigated serum parameters (i.e., CRP and proteins fractions) and the values of platelet aggregation indices obtained in horses at each sampling time. A linear regression model (y = a + bx) was applied as well. Values of p less than 0.05 were considered statistically significant. p values <0.05 were considered statistically significant. The statistical analysis was performed using the software Prism v. 9.00 (Graphpad Software Ltd., San Diego, CA, USA, 2020).
3. Results
A significant effect of exercise (p < 0.001) on the serum concentration of CRP, albumin and α1-, α-2, β1- and β2-globulins, and on platelet aggregation, was found, while no statistical significance was observed for the serum concentration of total proteins, A/G ratio or slope (p > 0.05). Specifically, the serum values of CRP and α1-, α-2, β1- and β2-globulins increased after exercise with respect to the rest condition (p < 0.001, Figure 1); decreased values of albumin were observed after exercise compared to at rest (p < 0.001, Figure 1). Platelet aggregation exhibited lower levels after exercise than at rest (p < 0.001, Figure 2). The serum CRP and α1-globulins values showed a significant negative correlation with both platelet aggregation indices (i.e., the maximum degree of aggregation and the initial velocity of aggregation, slope) immediately after the end of exercise (TPE5), while these relationships lost statistical significance at the rest condition and after 30 and 60 min from the end of exercise. Moreover, no significant correlation among the platelet activation indices and albumin and α-2, β1- and β2-globulins was found at each time point of the experimental period. The linear regression model applied on the obtained data confirmed these results (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
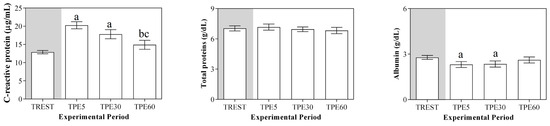
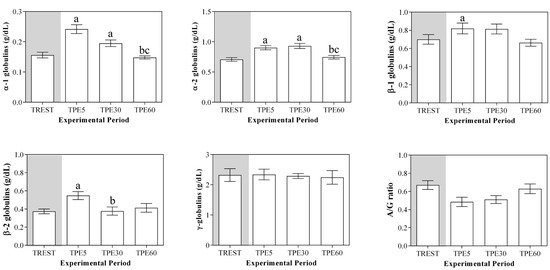
Figure 1.
Mean values serum total proteins, C-reactive protein, albumin, globulin fractions and albumin/globulin ratio (A/G) (±SEM) obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60). Significant effect of exercise (p < 0.001): a vs. TREST; b vs. TPE5; c vs. TPE30.
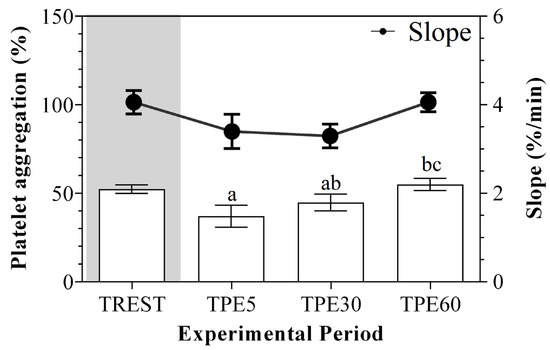
Figure 2.
Mean values ± standard error of the mean (±SEM) of platelet aggregation and slope values obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60). Significant effect of exercise (p < 0.001): a vs. TREST; b vs. TPE5; c vs. TPE30.
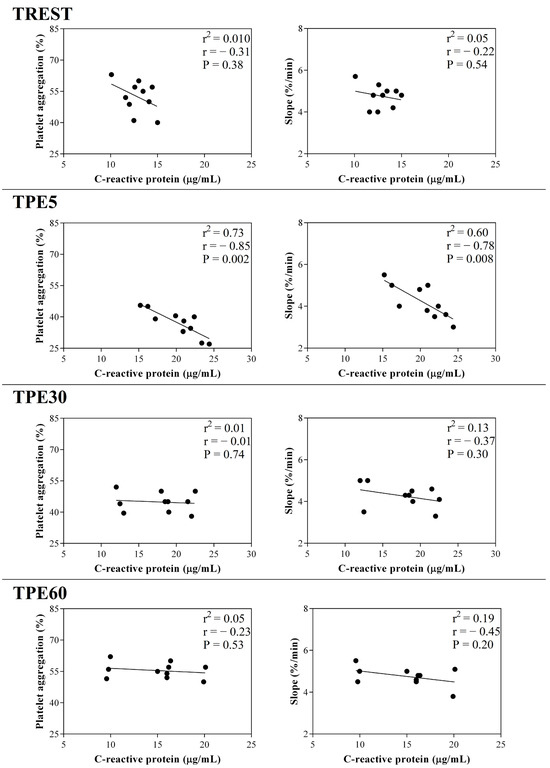
Figure 3.
Linear regression results found between the serum concentration of C-reactive protein and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
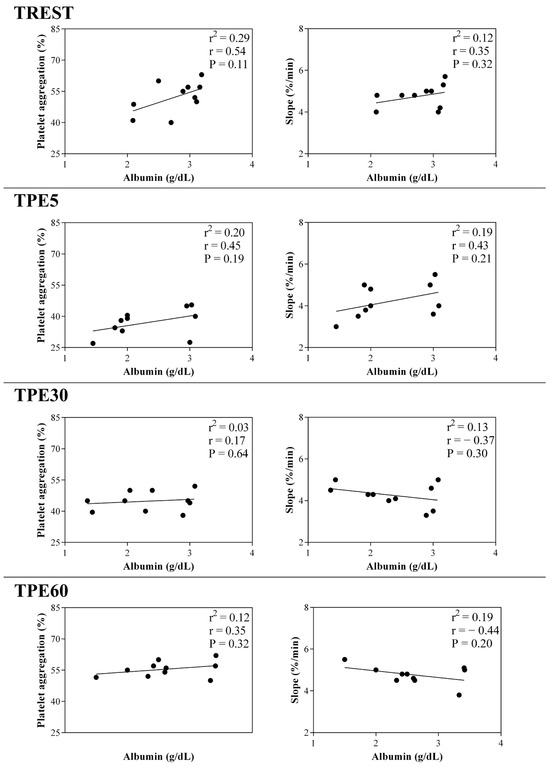
Figure 4.
Linear regression results found between the serum concentration of albumin and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
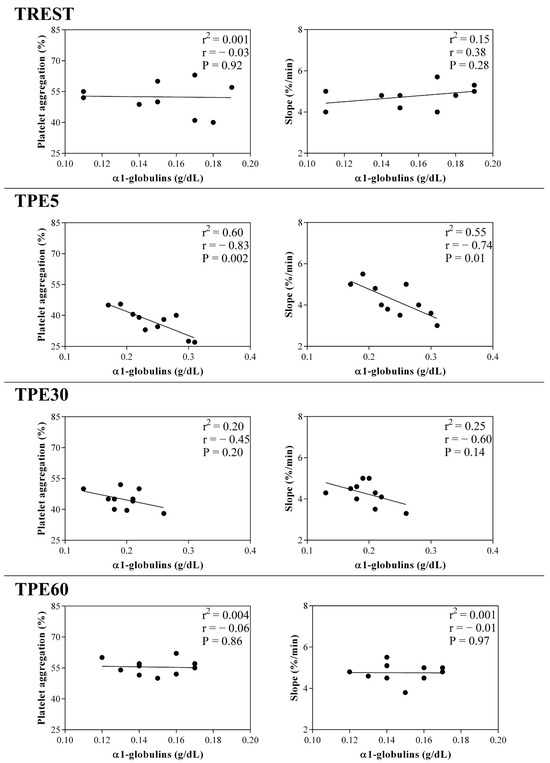
Figure 5.
Linear regression results found between the serum concentration of α1-globulin fraction and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
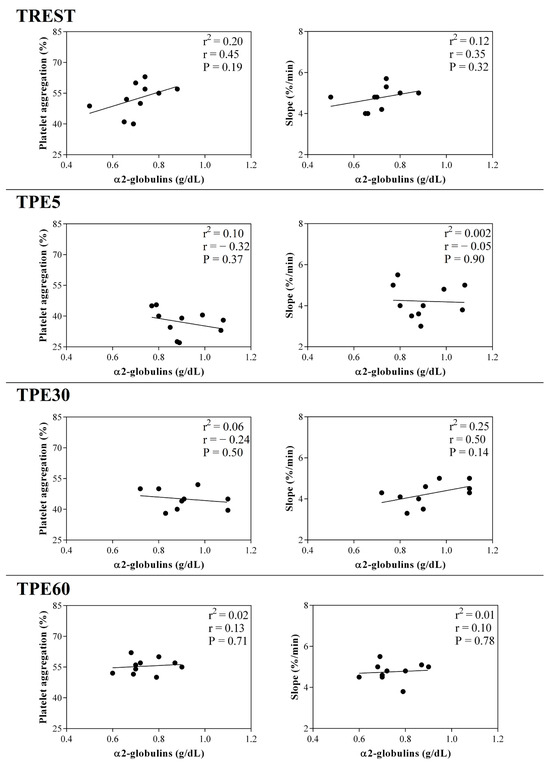
Figure 6.
Linear regression results found between the serum concentration of α2-globulin fraction and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
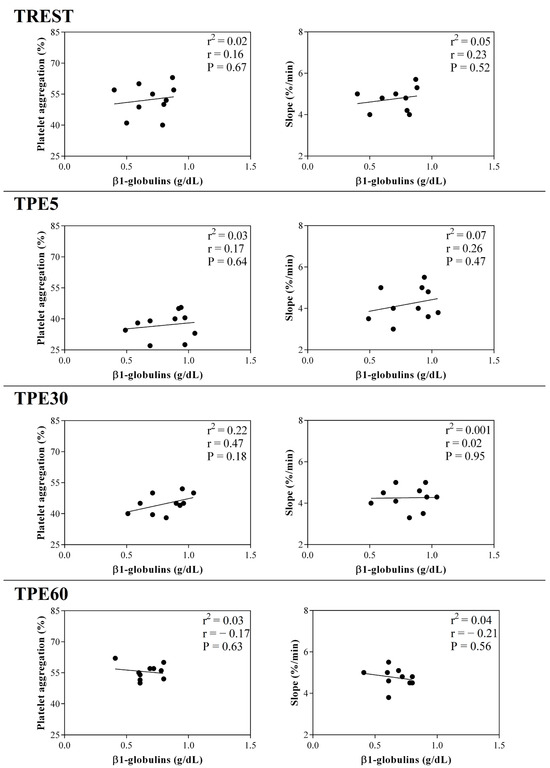
Figure 7.
Linear regression results found between the serum concentration of β1-globulin fraction and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
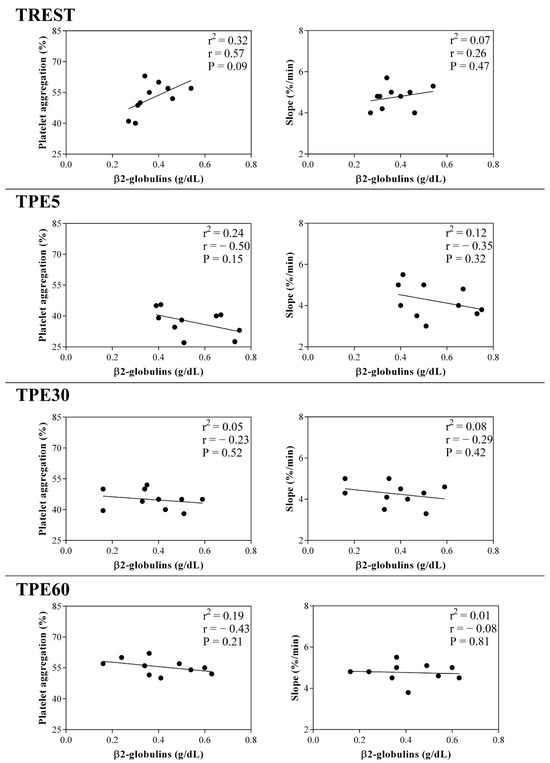
Figure 8.
Linear regression results found between the serum concentration of β2-globulin fraction and the values of platelet aggregation and slope obtained from horses at rest condition (TREST), within 5 min from the end of exercise (TPE5) and 30 min (TPE30) and 60 min after exercise (TPE60).
4. Discussion
The innate immune system elicits certain key and prompt responses to defend the body against infection, inflammation, tissue injury and stress from exercise [13]. These systemically activated and complex early defensive responses, termed acute-phase responses, are promoted by APPs, which are serum components, produced primarily by hepatocytes [22,23]. The serum concentration of positive APPs, mainly included in the α-globulin fraction, markedly increases following inflammation [22], whereas the serum values of negative APPs, such as albumin, decrease in response to inflammation as well as stress [23]. Many researchers revealed the influence of these proteins on human and animal physiology, resulting in them being termed “molecular thermometers” [22,23,24,25]. Recent studies carried out on animals pointed out the sensitivity of different APPs, suggesting them as strong markers of herd health in large animals and proposing them as optimum markers of stress conditions [25,26,27]. The results gathered in the current study showed a significant effect of exercise on all investigated parameters with the exception of serum total proteins, γ-globulins, A/G ratio and slope values. The concentrations of all the investigated parameters fall within the physiological range proposed for the equine species and are in agreement with the findings reported in a previous investigation carried out on athletic horses [19]. The analysis of protein electrophoretograms obtained from serum samples of horses after exercise showed a remodeling of the different factions within the protein pool. As a matter of fact, though the concentration of total proteins did not undergo a significant variation during the experimental period, albumin and α1-, α2-, β1- and β2-globulin fractions increased after exercise while albumin showed an opposite trend. Albumin is known as a negative APP, whereas the α- and β-globulin fractions are known to include both positive and negative APPs including haptoglobin, C-reactive protein, serum amyloid A, ceruloplasmin, fibrinogen, alpha 1-acid glycoprotein, complement proteins and transferrin. According to the results gathered in the current study, the CRP also showed increased serum values after exercise. In the context of exercise, increases in CRP concentrations have been shown in sled dogs [28,29] and in humans after exhausting exercise, but not in typical training [30,31], whereas any change in CRP values has been found after limited- and long-distance endurance rides in horse [32]. In agreement with previous findings [12], the results gathered in the present study suggest that jumping exercise induces an inflammation-like state, reinforcing the supposition that this kind of exercise reflects a classical APR in athletic horse. It is well known that the APR, in addition to its important functions to fight bacterial and viral infections, also stimulates clearance of injured tissue, setting the stage for tissue restoration and growth [33]. These latter activities might theologically explain why an APR is initiated by exercise. Regarding the mechanism orchestrating the activation of APR following exercise, it is widely accepted that intense physical activity is able to induce this response through the induction of interleukin-6 [3,34]. Together with the increase in pro-inflammatory interleukins, exercise also leads to an augmentation of several anti-inflammatory mediators, such as Il-1 receptor antagonist [13,35]. Thus, during physical exercise, it seems like a parallel “protective” anti-inflammatory counter-regulation occurs within the APR. It has been proposed that exercise-induced muscle injury was the primary stimulus for the release of IL-6 and the cascade reactions that this interleukin entails. It has been assumed that the signals generated by muscles during exercise lead to IL-6 release regardless of the presence of muscle damage [36]. Subsequently, muscle damage per se elicits a repair response, including macrophage entry into the muscle, causing further IL-6 production [36]. The exercise-activated muscle has been also suggested to modulate platelets’ reactivity. According to the results obtained herein, decreased platelet aggregation values were found after exercise compared with the rest condition, whereas no significant changes in slope were observed. These results complied with the findings obtained in previous studies carried out on athletic horses [21,30].
The blood hemoconcentration occurring during physical exercise leads to an increased concentration of sodium citrate, resulting in a lowered ionized calcium level and, consequently, decreased platelet aggregation [37]. Moreover, the catecholamine increase known to occur during exercise could contribute to the decrease in platelet aggregation after physical activity [38,39,40]. As a matter of fact, increased adrenaline levels result in decreased platelet aggregation, probably due to adrenergic receptor downregulation [38]. Noradrenaline is capable of exciting endothelial cells to release two strong potent inhibitors of platelet aggregation (i.e., nitric oxide and prostacyclin) [39]. Effectively, the increased prostacyclin production and plasma nitric oxide metabolites may suppress platelet reactivity [40]. It is likely that the changes observed in platelet reactivity represent a protective endothelial mechanism of the organism through the production of nitric oxide in response to physical exercise. Noteworthy in this survey is that platelet aggregation and slope showed a strong negative correlation with the serum values of CRP and α1-globulins after exercise, suggesting a link between inflammatory status and platelet responsiveness during exercise. These relationships immediately after exercise (within 5 min from the end of exercise) could be due to the high adaptation response of the body systems of horses, including the inflammatory and hemostatic systems, because of the recent stressful event (i.e., physical activity). As a matter of fact, the significant relationship among the investigated parameters was lost in the other conditions (at rest) and after 30 and 60 min from the end of the exercise, when the horse’s homeostasis is recovering. It must be taken into account that the horses enrolled in the present study were regularly trained with a standard training program; thus, the biochemical adaptation to the stress induced by physical exercise may have been optimized by training.
A possible inflammatory–thrombotic link has been proposed in a study on human blood ex vivo and in murine blood in vivo in which a monocyte–platelet aggregation promotion by CRP has been demonstrated [17]. An in vitro study investigating the role of CRP in thrombus formation on different vascular substrates under flow conditions suggested that the CRP has contrasting effects on thrombus formation according to its conformation. Whereas native CRP had no effect, the monomeric forms of CRP strengthened the activation and adhesion of platelet as well as thrombus growth under arterial flow conditions [41]. It has been demonstrated that circulating blood native CRP could be transformed into prothrombotic monomeric CRP under flow conditions on the surface of platelets attached to collagen [42]. In addition to collagen, other substrates that trigger platelet adhesion and aggregation also support CRP dissociation [18]. It has been suggested that a vascular injury with mural platelet adhesion can induce a dissociation of native CRP into monomeric CRP, highlighting that this dissociation is dependent on deposited activated platelets [18]. Contrariwise, dated investigations showed that the effect of CRP on platelet aggregation is not consistent with enhanced thrombosis as, when applied to platelets, CRP suppresses aggregation [43,44]. The results gathered in another in vitro study indicated that CRP inhibits platelet-activating factor-induced platelet aggregation and lysophosphatidylcholine-induced platelet lysis [16]. It has been hypothesized that this APP contrasts the tissue damage by binding to membrane phospholipids, thus stabilizing cellular membranes against the detergent-like effect of lysolipids.
Though further investigations enrolling a larger study population are advocated to better highlight the associations found herein, the results gathered in the current study seem to denote a normal response with no pathological significance in the athletic horses.
5. Conclusions
The present study provides evidence that APR occurred in horses after jumping exercise. This exercise-induced APR should be taken into consideration to maintain the welfare state of the horse, especially in light of the significant negative correlation found between the platelet aggregation indices and the serum concentration of CRP and α1-globulin after exercise. These correlation results suggest a linkage between the inflammatory status and the platelet responsiveness during exercise in horses. As the α1-globulin fraction includes many other APPs in addition to CRP, the negative correlation found between this globulin fraction and platelet activity advocates further studies in order to assess if and which other APPs could interact with hemostatic activity during exercise. Moreover, considering that the nature and the extent of the modifications in APR and platelet aggregation depend on the exercise type and intensity, further investigations are needed to better delineate the mechanisms by which physical activity affects the inflammatory process and to yield a full understanding of the correlation between APR and platelet reactivity in athletic horses subjected to different workloads.
Author Contributions
Conceptualization, F.A. (Francesca Arfuso) and G.P.; methodology, F.A. (Federica Arrigo), C.F., E.G. and M.R.; software, M.R.; validation, F.A. (Francesca Arfuso), G.P. and C.G.; formal analysis, F.A.; investigation, F.A. (Francesca Arfuso); writing—original draft preparation, F.A. (Francesca Arfuso); writing—review and editing, F.A. (Francesca Arfuso) and C.G.; visualization, C.G. and E.G.; supervision, C.G.; project administration, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All treatments, housing, and animal care were carried out in accordance with the standards recommended by the European Directive 2010/63/EU for animal experiments.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reason.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Marco-Ramell, A.; De Almeida, A.M.; Cristobal, S.; Rodrigues, P.; Roncada, P.; Bassols, A. Proteomics and the Search for Welfare and Stress Biomarkers in Animal Production in the One-Health Context. Mol. Biosyst. 2016, 12, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Fazio, F.; Giannetto, C.; Giudice, E.; Piccione, G. Influence of Transportation on Serum Concentrations of Acute Phase Proteins in Horse. Res. Vet. Sci. 2012, 93, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Marycz, K. The Effect of Chronic Inflammation and Oxidative and Endoplasmic Reticulum Stress in the Course of Metabolic Syndrome and Its Therapy. Stem Cells Int. 2018, 2018, 4274361. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, V.; Sessa, F.; Valenzano, A.; Salerno, M.; Bitetti, I.; Precenzano, F.; Marotta, R.; Lavano, F.; Lavano, S.M.; et al. Sympathetic, Metabolic Adaptations, and Oxidative Stress in Autism Spectrum Disorders: How Far from Physiology? Front. Physiol. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Van Der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-Wide Analysis of Cysteine Oxidation Reveals Metabolic Sensitivity to Redox Stress. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M.H. Application of Acute Phase Protein Measurements in Veterinary Clinical Chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Academic Press Inc.: San Diego, CA, USA, 1997. [Google Scholar]
- Takiguchi, M.; Fujinaga, T.; Naiki, M.; Mizuno, S.; Otomo, K. Isolation, characterization and quantitative analysis of C—Reactive protein from horses. Am. J. Vet. Res. 1990, 51, 1215–1220. [Google Scholar] [CrossRef]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in Markers of Muscle Damage, Inflammation and HSP70 after an Ironman Triathlon Race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef]
- Peeling, P.; Brian, A.E.; Ae, D.; Goodman, C.; Grant, A.E.; Ae, L.; Trinder, D. Athletic Induced Iron Deficiency: New Insights into the Role of Inflammation, Cytokines and Hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Arfuso, F.; Giannetto, C.; Fazio, F.; Panzera, F.; Piccione, G. Training Program Intensity Induces an Acute Phase Response in Clinically Healthy Horses. J. Equine Vet. Sci. 2020, 88, 102986. [Google Scholar] [CrossRef] [PubMed]
- Mihelić, K.; Vrbanac, Z.; Bojanić, K.; Kostanjšak, T.; Ljubić, B.B.; Gotić, J.; Vnuk, D.; Bottegaro, N.B. Changes in Acute Phase Response Biomarkers in Racing Endurance Horses. Animals 2022, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zaja̧c, A.; Grzanka, A.; Jarzab, J.; Misiołek, M.; Wyszyńska-Chłap, M.; Kasperski, J.; Machura, E. The Association between Platelet Count and Acute Phase Response in Chronic Spontaneous Urticaria. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yuri Gasparyan, A.; Ayvazyan, L.; Mikhailidis, D.P.; Kitas, G.D. Mean Platelet Volume: A Link between Thrombosis and Inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Vigo, C. Effect of C-Reactive Protein on Platelet-Activating Factor-Induced Platelet Aggregation and Membrane Stabilization. J. Biol. Chem. 1985, 260, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Danenberg, H.D.; Kantak, N.; Grad, E.; Swaminathan, R.V.; Lotan, C.; Edelman, E.R. C-Reactive Protein Promotes Monocyte-Platelet Aggregation: An Additional Link to the Inflammatory-Thrombotic Intricacy. Eur. J. Haematol. 2007, 78, 246–252. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, R.; Peña, E.; Vilahur, G.; Slevin, M.; Badimon, L. Monomerization of C-Reactive Protein Requires Glycoprotein IIb-IIIa Activation: Pentraxins and Platelet Deposition. J. Thromb. Haemost. 2013, 11, 2048–2058. [Google Scholar] [CrossRef]
- Piccione, G.; Arfuso, F.; Marafioti, S.; Giannetto, C.; Giudice, E.; Fazio, F. Different training schedules influence serum electrophoretic protein profile in the athletic horse. J. Equine Vet. Sci. 2015, 35, 856–859. [Google Scholar] [CrossRef]
- Arfuso, F.; Giannetto, C.; Giudice, E.; Fazio, F.; Piccione, G. Dynamic Modulation of Platelet Aggregation, Albumin and Nonesterified Fatty Acids during Physical Exercise in Thoroughbred Horses. Res. Vet. Sci. 2016, 104, 86–91. [Google Scholar] [CrossRef]
- Fontenot, R.L.; Sink, C.A.; Werre, S.R.; Weinstein, N.M.; Dahlgren, L.A. Simple Tube Centrifugation for Processing Platelet-Rich Plasma in the Horse. Can. Vet. J. 2012, 53, 1266. [Google Scholar]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, N.S.; Stefanovski, D.; Johnson, A.L. Utility of C-reactive Protein and Serum Amyloid A in the Diagnosis of Equine Protozoal Myeloencephalitis. J. Vet. Intern. Med. 2018, 32, 1726. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517. [Google Scholar] [PubMed]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute Phase Proteins in Ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Gånheim, C.; Alenius, S.; Persson Waller, K. Acute Phase Proteins as Indicators of Calf Herd Health. Vet. J. 2007, 173, 645. [Google Scholar] [CrossRef] [PubMed]
- Wakshlag, J.J.; Balkman, C.A.; Morgan, S.K.; McEntee, M.C. Evaluation of the Protective Effects of All-Trans-Astaxanthin on Canine Osteosarcoma Cell Lines. Am. J. Vet. Res. 2010, 71, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wakshlag, J.J.; Stokol, T.; Geske, S.M.; Greger, C.E.; Angle, C.T.; Gillette, R.L. Evaluation of Exercise-Induced Changes in Concentrations of C-Reactive Protein and Serum Biochemical Values in Sled Dogs Completing a Long-Distance Endurance Race. Am. J. Vet. Res. 2010, 71, 1207–1213. [Google Scholar] [CrossRef]
- Scharhag, J.; Meyer, T.; Gabriel, H.H.W.; Schlick, B.; Faude, O.; Kindermann, W. Does Prolonged Cycling of Moderate Intensity Affect Immune Cell Function? Br. J. Sports Med. 2005, 39, 171–177. [Google Scholar] [CrossRef]
- Neubauer, O.; König, D.; Wagner, K.H. Recovery after an Ironman Triathlon: Sustained Inflammatory Responses and Muscular Stress. Eur. J. Appl. Physiol. 2008, 104, 417–426. [Google Scholar] [CrossRef]
- Cywińska, A.; Szarska, E.; Górecka, R.; Witkowski, L.; Hecold, M.; Bereznowski, A.; Schollenberger, A.; Winnicka, A. Acute Phase Protein Concentrations after Limited Distance and Long Distance Endurance Rides in Horses. Res. Vet. Sci. 2012, 93, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Cannon, J.G. The Metabolic Effects of Exercise-Induced Muscle Damage. Exerc. Sport. Sci. Rev. 1991, 19, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Nukina, H.; Sudo, N.; Aiba, Y.; Oyama, N.; Koga, Y.; Kubo, C. Restraint Stress Elevates the Plasma Interleukin-6 Levels in Germ-Free Mice. J. Neuroimmunol. 2001, 115, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-Derived Interleukin-6: Possible Biological Effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, K.W.; Kaneps, A.J. Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete; Saunders Company: Edinburgh, UK; London, UK, 2004. [Google Scholar]
- Kjeldsen, S.E.; Weder, A.B.; Egan, B.; Neubig, R.; Zweifler, A.J.; Julius, S. Effect of Circulating Epinephrine on Platelet Function and Hematocrit. Hypertension 1995, 25, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.H.; DeFily, D.V.; Patterson, J.L.; Chilian, W.M. Endothelium-Dependent Relaxation Competes with Alpha 1- and Alpha 2-Adrenergic Constriction in the Canine Epicardial Coronary Microcirculation. Circulation 1993, 87, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, J.C.; Banga, J.D.; Moncada, S.; Palmer, R.M.J.; De Groot, P.G.; Sixma, J.J. Nitric Oxide Functions as an Inhibitor of Platelet Adhesion under Flow Conditions. Circulation 1992, 85, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Molins, B.; Peña, E.; Vilahur, G.; Mendieta, C.; Slevin, M.; Badimon, L. C-Reactive Protein Isoforms Differ in Their Effects on Thrombus Growth. Arter. Thromb. Vasc. Biol. 2008, 28, 2239–2246. [Google Scholar] [CrossRef]
- Molins, B.; Pea, E.; De La Torre, R.; Badimon, L. Monomeric C-Reactive Protein Is Prothrombotic and Dissociates from Circulating Pentameric C-Reactive Protein on Adhered Activated Platelets under Flow. Cardiovasc. Res. 2011, 92, 328–337. [Google Scholar] [CrossRef]
- Fiedel, B.A.; Gewurz, H. Effects of C-Reactive Protein on Platelet Function. I. Inhibition of Platelet Aggregation and Release Reactions. J. Immunol. 1976, 116, 1289–1294. [Google Scholar] [CrossRef]
- Fiedel, B.A.; Gewurz, H. Effects of C-Reactive Protein on Platelet Function. II. Inhibition by CRP of Platelet Reactivities Stimulated by Poly-L-Lysine, ADP, Epinephrine, and Collagen. J. Immunol. 1976, 117, 1073–1078. [Google Scholar] [CrossRef]
- Fiedel, B.A.; Simpson, R.M.; Gewurz, H. Effects of C-reactive protein (CRP) on platelet function. Ann. N. Y. Acad. Sci. 1982, 389, 263–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).