Exploring the Potential of Sr2+ for Improving the Post-Hardening Strength and Durability Characteristics of Cement Paste Composites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Specimens
2.1.1. Source of Strontium Cation
2.1.2. Specimen Preparation
2.2. Test Methods
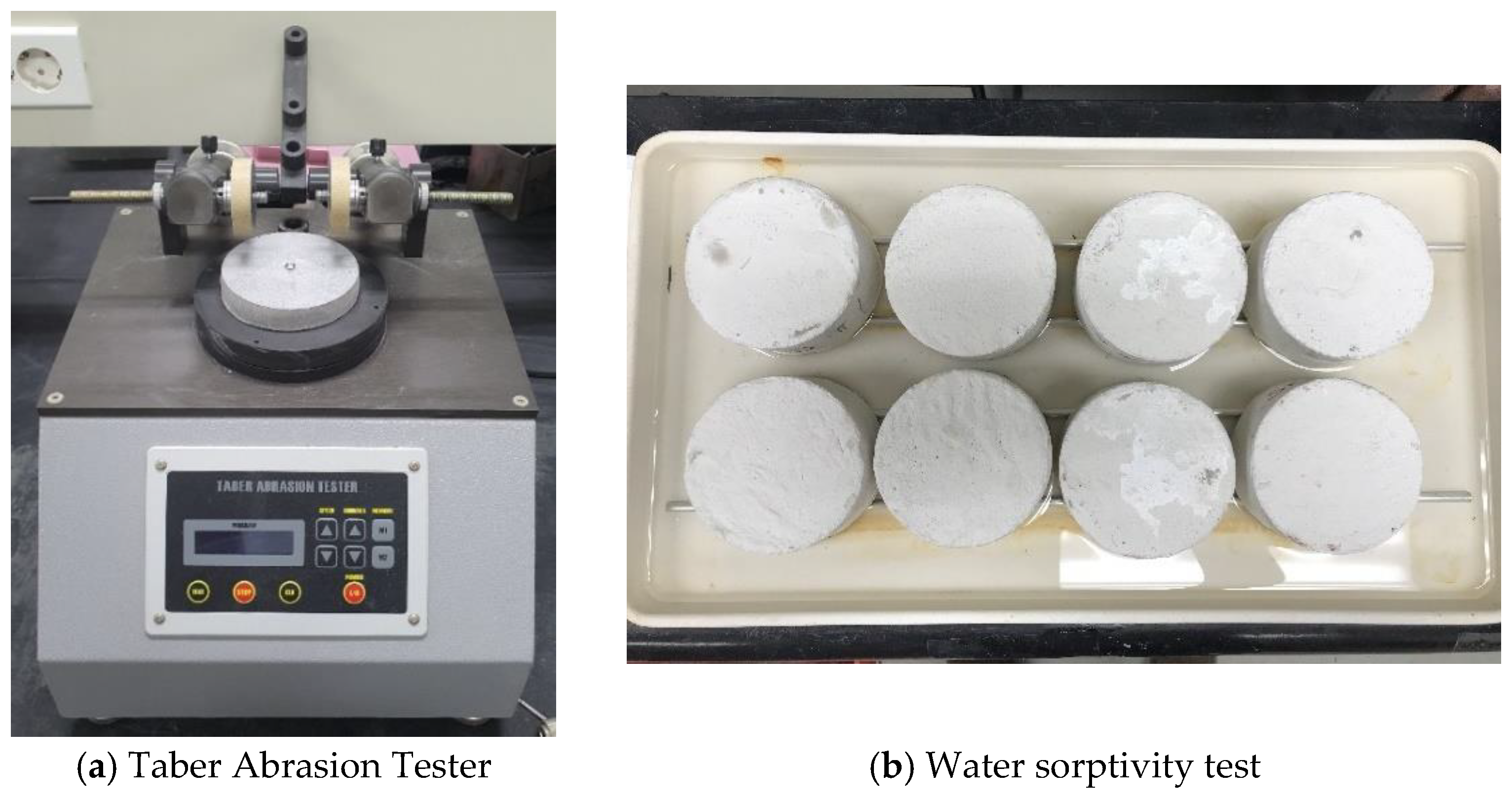
2.2.1. Mechanical Strength Tests
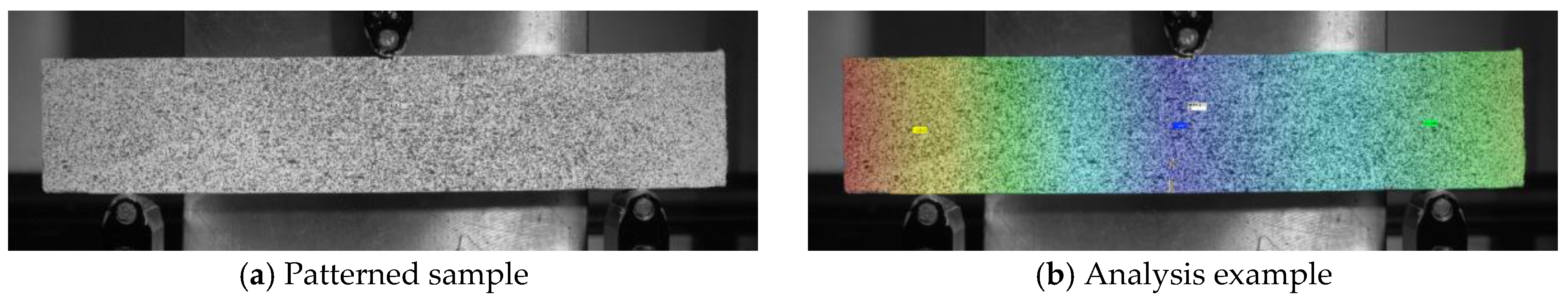
2.2.2. Digital Image Correlation (DIC)
2.2.3. Durability Performance Tests
3. Results and Discussion
3.1. Strengths Characteristics
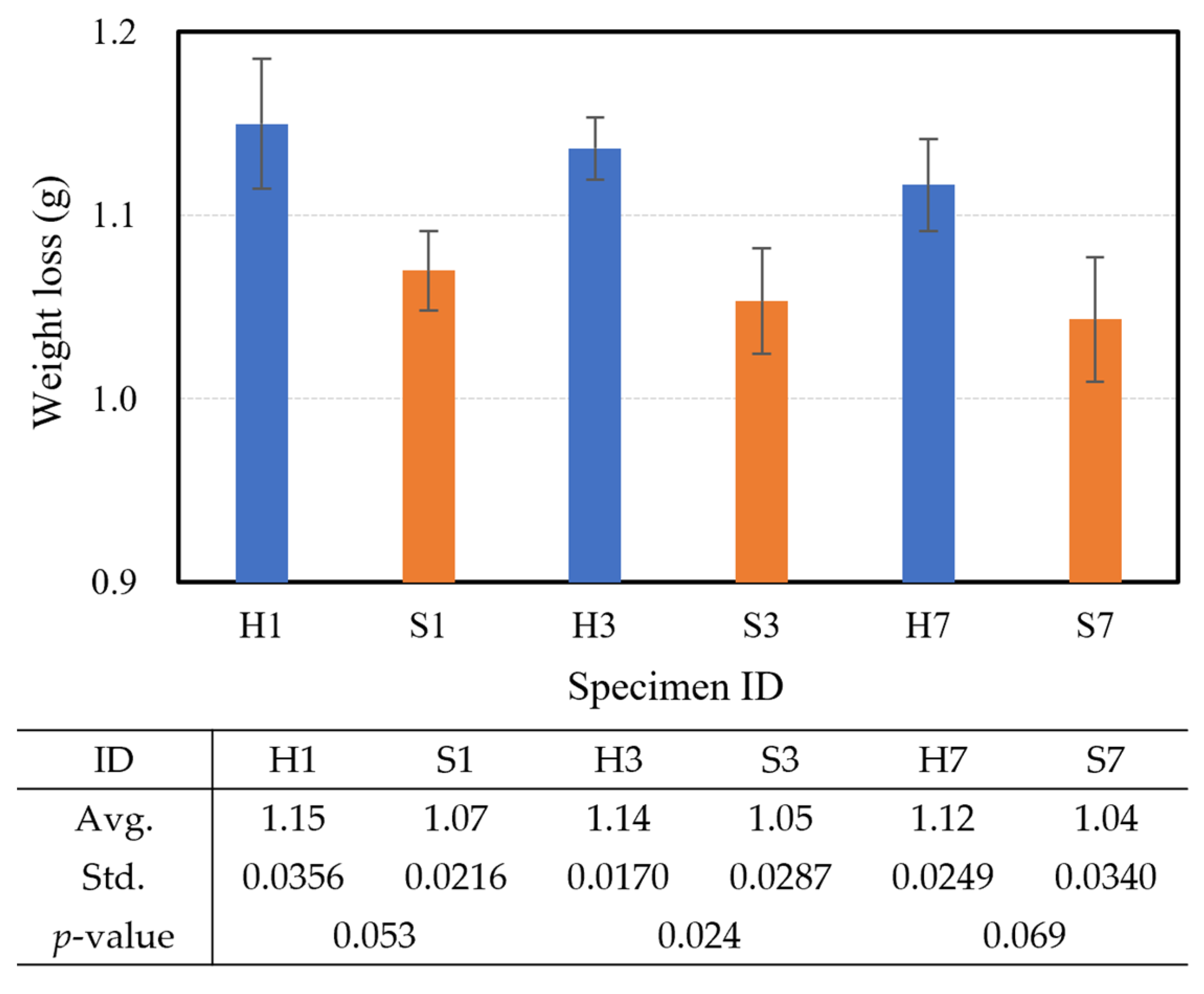
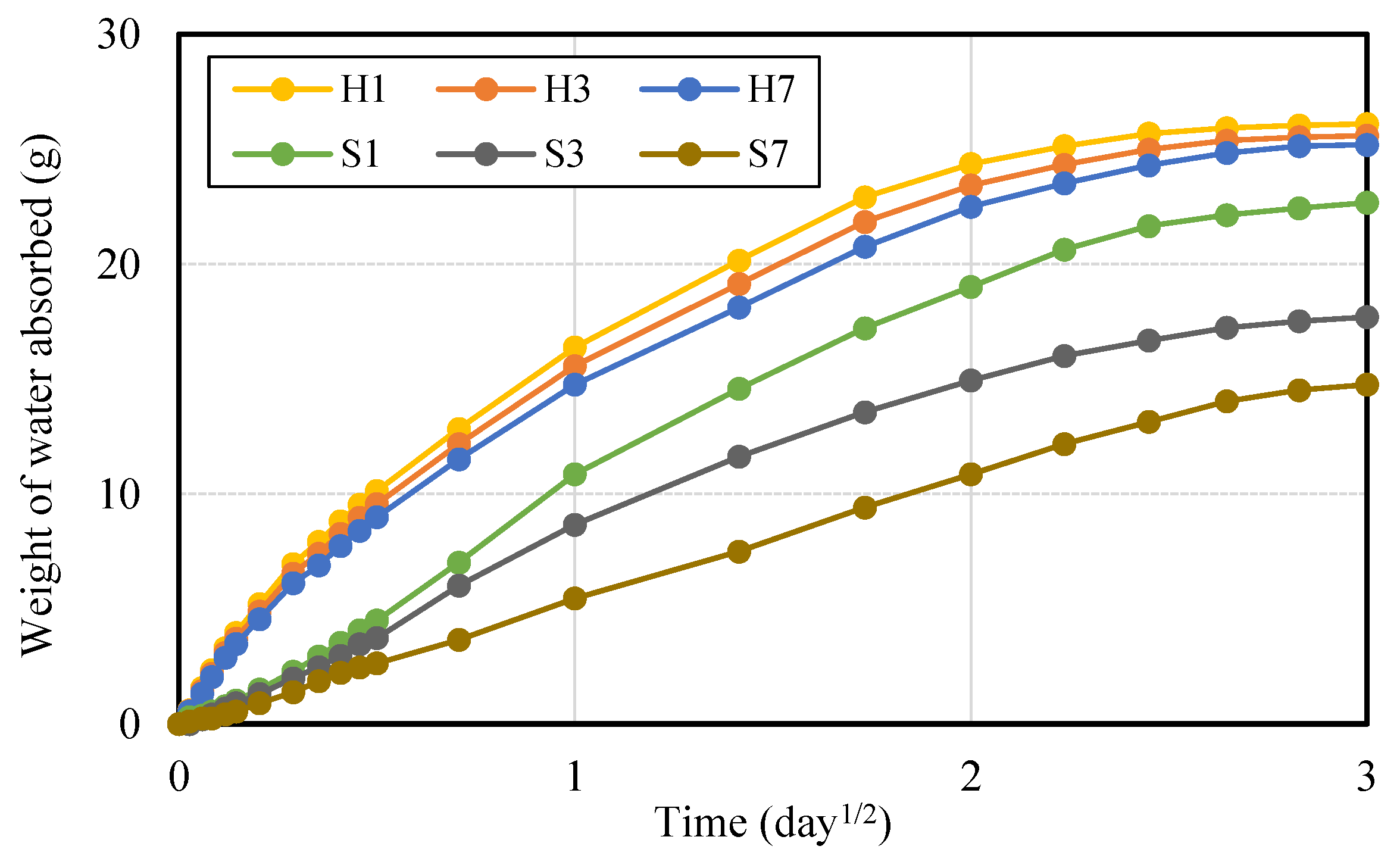
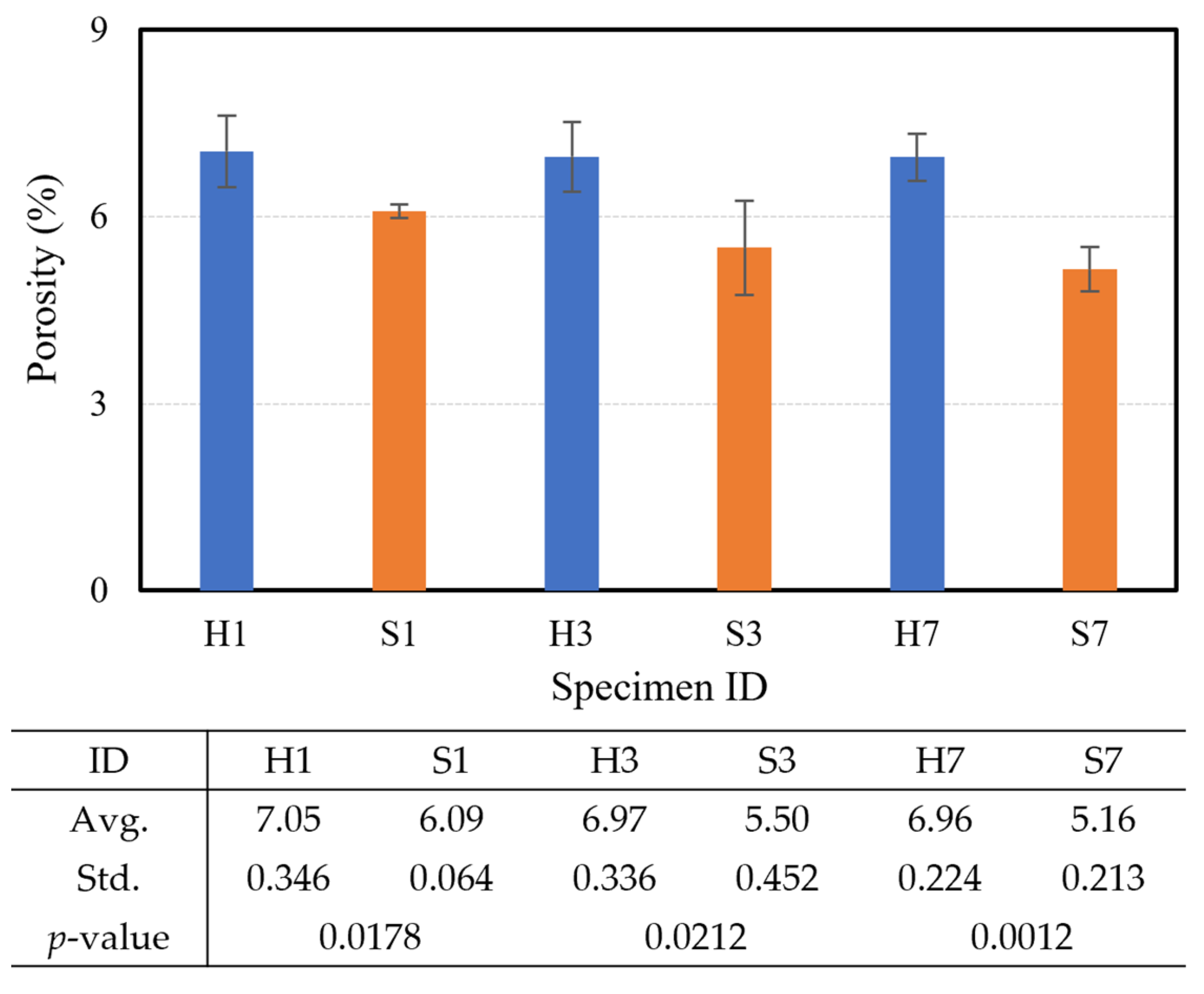
3.2. Durability Performance
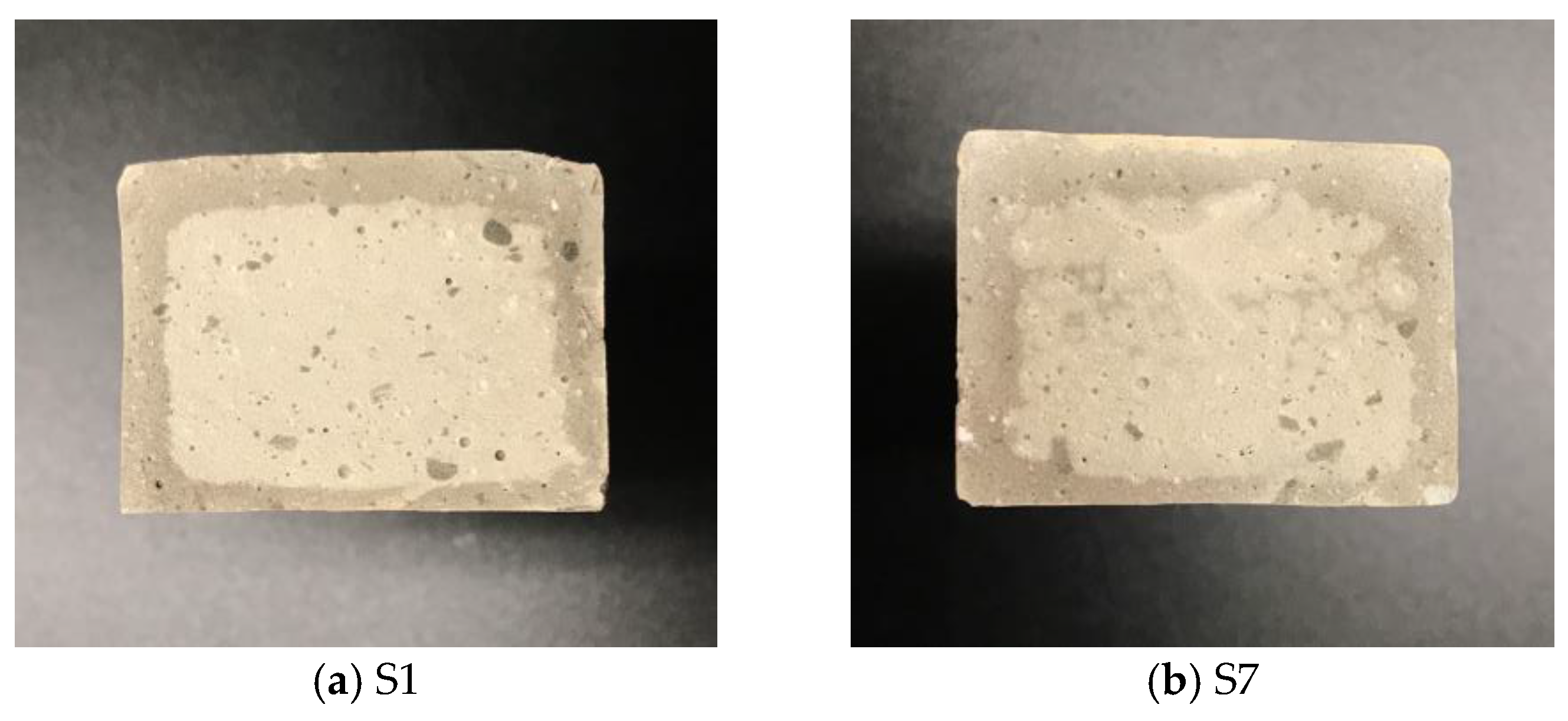
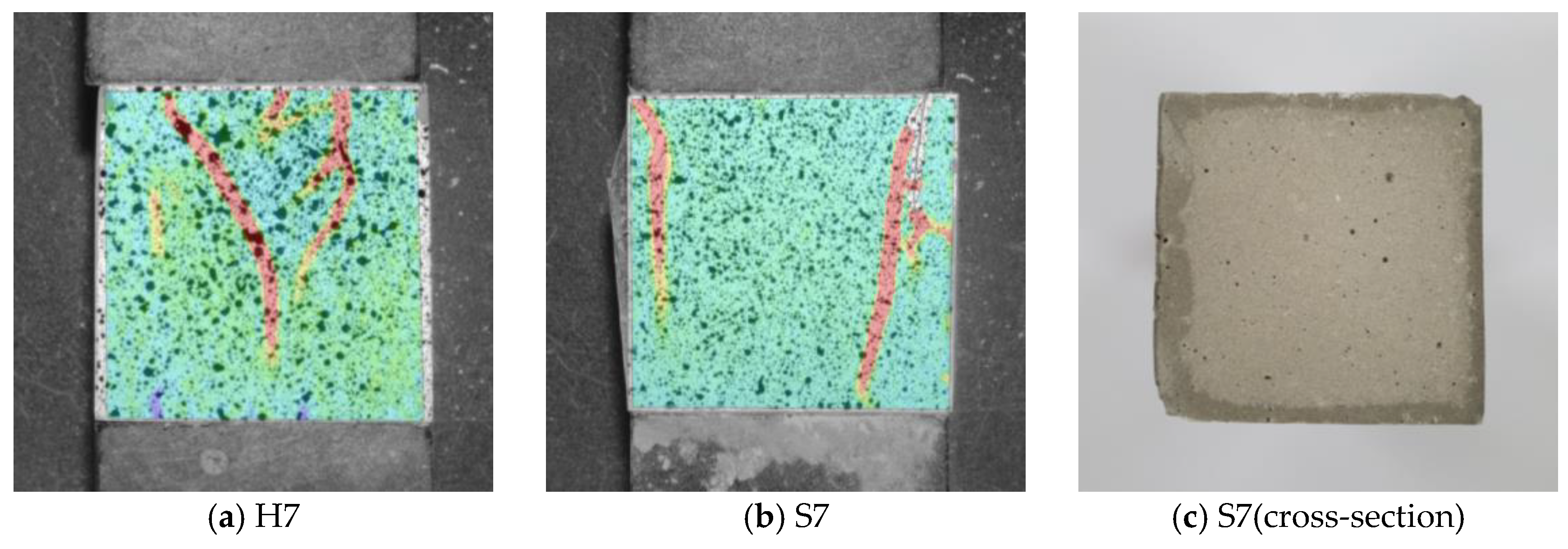
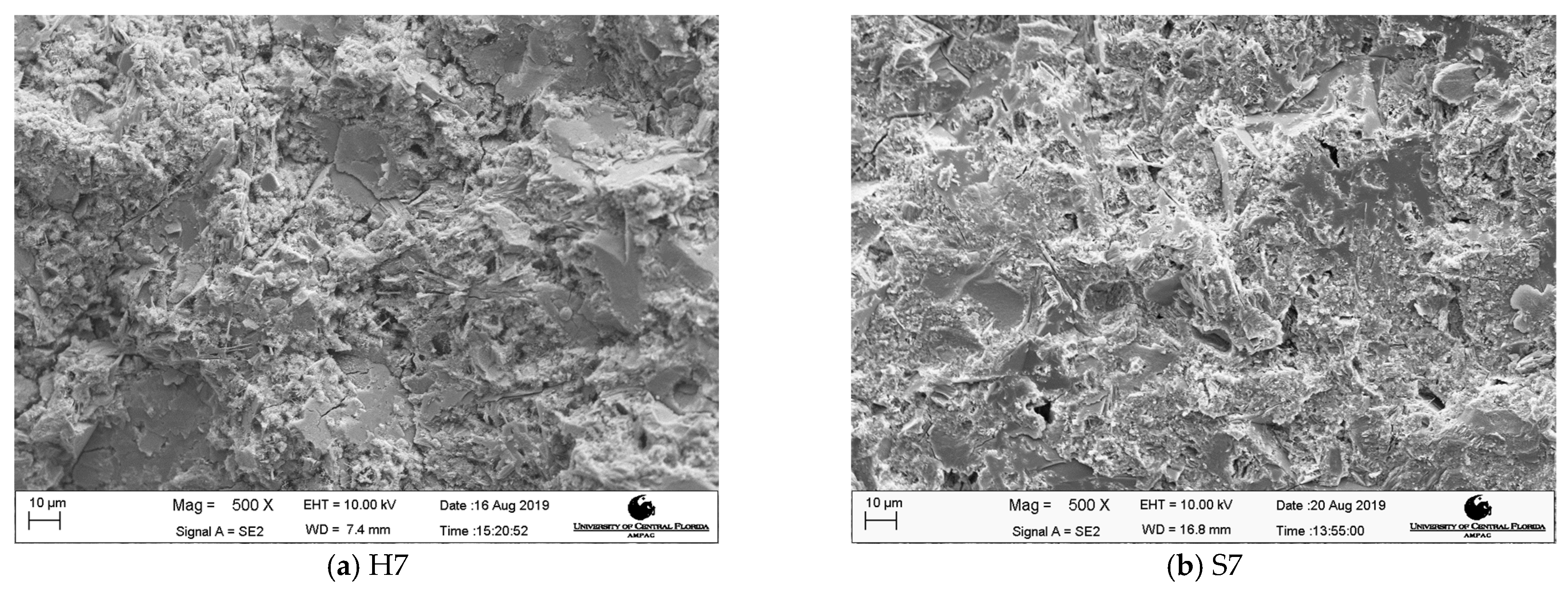
3.3. Microscopic Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alnahhal, M.F.; Alengaram, U.J.; Jumaat, M.Z.; Alsubari, B.; Alqedra, M.A.; Mo, K.H. Effect of aggressive chemicals on durability and microstructure properties of concrete containing crushed new concrete aggregate and non-traditional supplementary cementitious materials. Constr. Build. Mater. 2018, 163, 482–495. [Google Scholar] [CrossRef]
- Roesler, J.R.; Cervantes, V.G.; Amirkhanian, A.N. Accelerated performance testing of concrete pavement with short slabs. Int. J. Pavement Eng. 2012, 13, 494–507. [Google Scholar] [CrossRef]
- Sajid, H.U.; Jalal, A.; Kiran, R.; Al-Rahim, A. A survey on the effects of deicing materials on properties of Cement-based materials. Constr. Build. Mater. 2022, 319, 126062. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Li, Z.; Jin, H.; Tang, L.J.C.; Materials, B. Influence of deicing salt on the surface properties of concrete specimens after 20 years. Constr. Build. Mater. 2021, 295, 123643. [Google Scholar] [CrossRef]
- Gan, Y.; Li, C.; Ke, W.; Deng, Q.; Yu, T. Study on pavement performance of steel slag asphalt mixture based on surface treatment. Case Stud. Constr. Mater. 2022, 16, e01131. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Lv, J.; Li, W. Performance evaluation of skid-resistant surface treatment using lithium silicate for limestone bituminous pavement. Constr. Build. Mater. 2022, 342, 127990. [Google Scholar] [CrossRef]
- Zhang, Z.; Luan, B.; Liu, X.; Zhang, M. Effects of surface texture on tire-pavement noise and skid resistance in long freeway tunnels: From field investigation to technical practice. Appl. Acoust. 2020, 160, 107120. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, J.; Sohn, D.; Bae, S. Optimal longitudinal texture on concrete pavement to reduce lateral vibration of vehicles. Appl. Sci. 2022, 12, 9661. [Google Scholar] [CrossRef]
- Zhao, Z.; Qu, X.; Li, J. Application of polymer modified cementitious coatings (PCCs) for impermeability enhancement of concrete. Constr. Build. Mater. 2020, 249, 118769. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.; Lv, Y.; Gao, Z.; Dai, S. Effect of polymer coatings on the permeability and chloride ion penetration resistances of nano-particles and fibers-modified cementitious composites. Polymers 2022, 14, 3258. [Google Scholar] [CrossRef]
- Li, J.; Yi, Z.; Xie, Y. Progress of silane impregnating surface treatment technology of concrete structure. Mater. Rev. 2012, 26, 120–125. [Google Scholar]
- Xiong, B.B.; Gao, L.; Chen, J.G.; Lu, X.C.; Tian, B.; Chen, B.F.; Li, Y.B. Action mechanism for improving water impermeability of concrete surface based on deep penetrating sealer. Constr. Build. Mater. 2022, 322, 126424. [Google Scholar] [CrossRef]
- Dong, Q.; Cao, X.; Wang, S.; Chen, X.; Yang, B.; Xie, S.; Cheng, Z. Design and Evaluation of an Innovative Composite Silicate-based Surface Treatment Agent of Concrete. Case Stud. Constr. Mater. 2023, 18, e02207. [Google Scholar] [CrossRef]
- Herath, C.; Gunasekara, C.; Law, D.W.; Setunge, S. Performance of high volume fly ash concrete incorporating additives: A systematic literature review. Constr. Build. Mater. 2020, 258, 120606. [Google Scholar] [CrossRef]
- Villain, G.; Platret, G. Two experimental methods to determine carbonation profiles in concrete. ACI Mater. J. 2006, 103, 265. [Google Scholar]
- Atkins, M.; Glasser, F.P. Application of Portland cement-based materials to radioactive waste immobilization. Waste Manag. 1992, 12, 105–131. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Z.; Deng, Y.; Shi, C.; Zhang, Z. Advances in immobilization of radionuclide wastes by alkali activated cement and related materials. Cem. Concr. Compos. 2022, 126, 104377. [Google Scholar] [CrossRef]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental composites with calcium/strontium phosphates and polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Seeman, E.; De Vernejoul, M.; Adami, S.; Compston, J.; Phenekos, C.; Devogelaer, J.-P.; Curiel, M.D.; Sawicki, A.; Goemaere, S. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J. Clin. Endocrinol. Metab. 2005, 90, 2816–2822. [Google Scholar] [CrossRef]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium (II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Shiner, M.E.; Klein-BenDavid, O.; L’Hôpital, E.; Dauzères, A.; Neji, M.; Teutsch, N.; Bar-Nes, G. Retention of strontium in high-& low-pH cementitious matrices–OPC vs. model systems. Cem. Concr. Res. 2022, 152, 106659. [Google Scholar]
- Wieland, E.; Tits, J.; Kunz, D.; Dähn, R. Strontium uptake by cementitious materials. Environ. Sci. Technol. 2008, 42, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Tits, J.; Wieland, E.; Müller, C.J.; Landesman, C.; Bradbury, M.H. Strontium binding by calcium silicate hydrates. J. Colloid Interface Sci. 2006, 300, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Haga, K.; Hosokawa, Y.; Yamada, K.; Igarashi, G.; Maruyama, I. Modeling of the adsorption behavior of Cs and Sr on calcium silicate hydrates. J. Adv. Concr. Technol. 2021, 19, 1061–1074. [Google Scholar] [CrossRef]
- Iwaida, T.; Nagasaki, S.; Tanaka, S. Sorption study of strontium onto hydrated cement phases using a sequential desorption method. Radiochim. Acta 2000, 88, 483–487. [Google Scholar] [CrossRef]
- Cho, B.H.; Nam, B.H.; Santra, S.; Barry, M.; Novak, S. Enhanced surface hardening of hydrated concrete composite by strontium nitrate (Sr(NO3)2) aqueous solution. J. Build. Eng. 2021, 40, 102696. [Google Scholar] [CrossRef]
- Winslow, D.N.; Dolch, W.L. Cement paste hydrated in Sr(OH)2. Cem. Concr. Res. 1976, 6, 309–319. [Google Scholar] [CrossRef]
- ASTM-C150; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM-C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM-C293; Standard Test Method for Flexural Strength of Concrete (Using Simple Beam with Center-Point Loading). ASTM International: West Conshohocken, PA, USA, 2016.
- Sutton, M.A.; Wolters, W.J.; Peters, W.H.; Ranson, W.F.; McNeill, S.R. Determination of displacements using an improved digital correlation method. Image Vis. Comput. 1983, 1, 133–139. [Google Scholar] [CrossRef]
- ASTM-C1585; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM-C1754; Standard Test Method for Density and Void Content of Hardened Pervious Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- Faure, G.; Powell, J.L. Strontium Isotope Geology; Springer Science & Business Media: Berlin, Germany, 2012; Volume 5. [Google Scholar]
- ASTM-C295/C295M-18; Standard Guide for Petrographic Examination of Aggregates for Concrete. ASTM International: West Conshohocken, PA, USA, 2018.






| Component | SiO2 | CaO | Al2O3 | SO3 | Fe2O3 | IR (1) | LOI (2) |
|---|---|---|---|---|---|---|---|
| Percentage (%) | 21.5 | 64.9 | 4.2 | 0.7 | 3.5 | 1.1 | - |
| Solutions for Additional Curing | Period of Additional Curing (Day) | ||
|---|---|---|---|
| 1 | 3 | 7 | |
| In 30% Sr(NO3)2 | S1 | S3 | S7 |
| In deionized water | H1 | H3 | H7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.H. Exploring the Potential of Sr2+ for Improving the Post-Hardening Strength and Durability Characteristics of Cement Paste Composites. Appl. Sci. 2024, 14, 1841. https://doi.org/10.3390/app14051841
Cho BH. Exploring the Potential of Sr2+ for Improving the Post-Hardening Strength and Durability Characteristics of Cement Paste Composites. Applied Sciences. 2024; 14(5):1841. https://doi.org/10.3390/app14051841
Chicago/Turabian StyleCho, Byoung Hooi. 2024. "Exploring the Potential of Sr2+ for Improving the Post-Hardening Strength and Durability Characteristics of Cement Paste Composites" Applied Sciences 14, no. 5: 1841. https://doi.org/10.3390/app14051841
APA StyleCho, B. H. (2024). Exploring the Potential of Sr2+ for Improving the Post-Hardening Strength and Durability Characteristics of Cement Paste Composites. Applied Sciences, 14(5), 1841. https://doi.org/10.3390/app14051841







