Abstract
The immune system acts as a defense mechanism against foreign antigens. Impairment of the immune system leads to the development of chronic diseases such as respiratory infections, cancer, cardiovascular diseases, and neurodegeneration. Macrophages, natural scavengers that are part of innate immunity, are known to directly participate in scavenging foreign antigens. The functional modulation of macrophages could be an effective treatment for pathogens. Seaweeds are marine macroalgae known to exhibit multiple bioactive properties. Thus, this study evaluated the immune-enhancing properties of marine brown algae extracts of Sargassum horneri (SH), Undaria pinnatifida (UP), and Sargassum fusiforme (SF) on murine macrophage cells. The results showed that all three algal extracts stimulated cell proliferation. SH and UP outshined SF in enhancing the expression levels of IL-1β, TNF-α, and IL-6 at almost all the concentrations tested as compared to SF which showed similar effects only at 200 or 400 μg/mL. A similar trend was seen in TNF-α, NO, and PGE2 production. Additionally, only SH and SF could enhance the mRNA expression levels of IL-12, and only SH upregulated the mRNA expression level of IL-10. The algal extracts also enhanced the phagocytosis activity of macrophages at 50–400 μg/mL for SH and 100–400 μg/mL for UP and SF. In conclusion, we found that these algal extracts could be considered immunomodulators that enhance the functional activity of macrophages.
1. Introduction
The host immune system comprises complex biological processes and structures and acts as a defense system against pathogens. The alteration or dysfunction of immune system makes hosts susceptible to external stimuli causing various diseases [1]. Macrophages are the major innate immune cells responsible for removing foreign material and phagocytizing them [2,3]. Macrophages that come in direct contact with external stimuli (such as antigens or foreign particles that enter the body) are activated, resulting in the stimuli being engulfed. In addition, they recycle nutrients by digesting cellular debris, eliminate apoptotic cells, and play a significant role in tissue homeostasis [4]. When macrophages are exposed to various stimuli, they initiate a series of innate immune responses by producing various cytokines such as interleukin (IL)-1β, IL-12, tumor necrosis factor (TNF)-α, and IL-6. Although macrophages are considered the main producers of these markers, other cells such as fibroblasts and lymphocytes also produce them. Macrophages also produce prostaglandins, leukotrienes, and complement proteins.
These cytokines interact with unique cell surface receptors and produce a signaling cascade which further influences cell function by regulating various genes and transcription factors, either positively or negatively [5]. However, it is a controlled mechanism, and other cytokines (such as IL-10) are known to regulate the activities of TNF-α, IL-1β, and IL-6 [6,7].
Nitric oxide (NO), a crucial product of macrophages that is activated in the presence of microbes or cytokines, is derived from L-arginine by inducible nitric oxide synthase (iNOS, gene name: Nos2). It functions as an antimicrobial and tumoricidal molecule both in vitro and in vivo [8,9]. Cyclooxygenase-2 (COX-2, gene name: Ptgs2) catalyzes the conversion of arachidonic acid into prostaglandin E2 (PGE2) [10] and plays a crucial role in inflammation by inducing mast cells and limiting phagocytosis by macrophages [11,12,13].
The modulation of macrophage function could be considered as a therapeutic target for different diseases. Recent studies have shown the potential of natural or plant-derived bioactive compounds as immunomodulators of inflammation [14], cancer [15], and diabetes [16].
Seaweeds, also referred to as marine macroalgae, represent multiple species of multicellular and macroscopic algae [17]. Macroalgae have been reported to contain various polyphenols, proteins, polysaccharides, and vitamins [18]. Furthermore, some seaweed species are rich sources of bioactive compounds and exhibit potentially beneficial bioactivities [19,20,21,22,23]. Recent studies have reported the beneficial roles of these macroalgae in cancer [24,25,26,27,28], diabetes [29], thrombosis [30], inflammation [31,32,33], obesity [34], and cardiovascular disease [35]. Therefore, seaweed has attracted the interest of various researchers. Marine macroalgae have been also reported to be consumed as food in China and Japan.
Macroalgae such as Undaria pinnatifida, Laminaria spp., and Sargassum fusiforme, commonly called wakame, kombu, and hiziki, have been consumed by humans since at least 500 B.C., while in Europe, the consumption of these macroalgae began almost one thousand years later [36]. Their consumption is not limited to humans but also to animal species, as Europeans have been using these seaweeds in animal husbandry since the time of Ancient Romans. Iceland, Norway, and France use them in providing nutrition to domesticated animals [37]. Surprisingly, even cosmetics use marine macroalgae because of their hydrating, re-mineralizing, and brightening properties [38]. Also, various other countries use seaweeds in phycocolloids extraction or the extraction of compounds with antiviral, antibacterial, and antitumor activity [39]. Furthermore, they have been also reported to work as farming fertilizers [36].
Brown algae, such as Undaria, Sargassum, and Laminaria species, are known to contain a high molecular weight polysaccharide called fucoidan [40]. Fucoidan are marine sulfated polysaccharides are found in the cell wall matrix of brown algae and contain large amounts of L-fucose, sulfate-fucose, and galactose, along with minor sugars [41], and are reported to have bioactive potential [42,43,44,45]. Because these are well-known brown algal species and they contain the polysaccharide fucoidan in their cell wall matrix, they were considered important for this study. In addition, there are a few different studies on Sargassum and Undaria spp.; however, to the best of our knowledge, there are no studies on the immunomodulatory and phagocytosis-enhancing properties of these extracts.
The objectives of the study were (a) to assimilate the immunomodulatory effects of different fucoidan-containing algal species [S. horneri (SH), Sargassum fusiforme (SF), and U. pinnatifida (UP)] and (b) to identify a promising immunomodulatory agent among these three through the comparative analysis of their immunomodulatory activity.
2. Materials and Methods
2.1. SH, UP, and SF Extract Preparation
Each marine algae extract was provided by BK Bio Co. (Jeju Island, Republic of Korea). SH, UP, and SF extracts were prepared using standard procedures. Briefly, each raw material was extracted in distilled water at a 1:30 (w/w) ratio for 20 h at 95 °C. The extract was concentrated to a 1:10 ratio at 45–60 °C in a vacuum of 500–700 mmHg. In comparison with the extract, three times the amount of 95% ethanol was added and precipitated for 24 h. After separating the pectin with an 18 μm mesh filter, the extract was dried at 50 °C for 24 h. The dilution of each extract was prepared in phosphate buffered saline followed by filtration and was stored at −20 °C until use.
2.2. Cell Culture and Reagents
Murine RAW 264.7 macrophage cells were supplied by the ATCC (Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle medium (DMEM, Corning, NY, USA) containing 10% fetal bovine serum and 1% antibiotic–antimycotic (Gibco, Gaithersburg, MD, USA) at 37 °C in a 5% CO2 humidified incubator.
2.3. Cell Viability Assay
The cell viability assay was performed according to previously used methods [46]. Briefly, the 2 × 104 cells/well were seeded into a 96-well culture plate and allowed to adhere for 24 h at 37 °C. After 24 h of incubation, cells were treated with different dilutions (50, 100, 200, or 400 μg/mL) of SH, UP, and SF separately for 24 h. The media were removed and replaced with fresh media containing CCK-8 and incubated again for 2 h at 37 °C. The optical density of the samples was analyzed at 450 nm using a microplate spectrophotometer (Biotek Inc., Winooski, VT, USA). The number of viable cells was counted in percentage by assuming the control as 100% viable. The experiment was repeated thrice.
2.4. NO Assay
The NO assay was performed as per previously used methods [3]. Briefly, the Griess reagent system was used to detect NO2– in various biological and experimental liquids. The principle of this assay is a chemical reaction that uses sulfanilamide and N-1-naphthylethylenediamine dihydrochloride (NED) under acidic conditions. RAW 264.7 cells were grown in a 6-well culture plate at a density of 2 × 105 cells/mL. After 24 h of incubation, cells were treated with different dilutions (0, 50, 100, 200, or 400 μg/mL) of the three algal extracts (SH, UP, and SF) separately for 24 h. NO levels were assessed using Griess reagent (Promega, Madison, WI, USA), according to the supplier’s guidelines. Briefly, 100 μL of the 100 μM nitrite solution was added to 3 wells in a 96-well plate (which were serially diluted 6-fold to 1.56 μM) and the blank (DMEM only) to generate a nitrite standard reference curve. The supernatant from the treatment group was harvested and added to the adjacent wells (50 µL/well) in triplicate and mixed with 50 µL/well of sulfanilamide for 10 min. After 10 min of incubation, the NED coloring solution was added for 10 min. The absorbance of the mixture was measured at 535 nm using an Epoch Microplate Spectrophotometer (Biotek, Inc., Winooski, VT, USA). Results were analyzed with respect to the nitrite standard curve.
2.5. RNA Isolation and Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The RNA isolation and RT-PCR were performed according to the previously used protocol [47]. Briefly, RAW 264.7 cells were grown in 6-well culture plates at a density of 2 × 105 cells/mL. After 24 h of incubation, cells were treated with different dilutions (0, 50, 100, 200, or 400 μg/mL) of three algal extracts (SH, UP, and SF) separately for 24 h. After treatment, the total RNA from the different treatment groups was extracted using an RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Republic of Korea) following the manufacturer’s instructions. The isolated RNA was quantified through Nanodrop to check the quality of the isolated RNA. A total of 50 ng of the RNA was used to form complementary DNA (cDNA) together with 4 μL of random primers and 2 μL of enzyme mix (TaKaRa Bio, Otsu, Japan). A total of 5.5 μL of synthesized cDNA was subjected to mRNA expression analysis using an ABI QuantStudio3 (Applied Biosystems, Foster City, CA, USA) and SYBR Green Master Mix (TaKaRa Bio, Otsu, Japan) using the selected gene primer pairs (forward and reverse) as shown in Table 1. PCR thermocycling consisted of three stages, namely, denaturation (94 °C, 30 s), annealing (55 °C, 30 s), and the synthesis of the complementary strand (72 °C, 1 min), and these three stages continued for 35 cycles. The target gene expression levels were adjusted to that of β-actin mRNA. For each sample, the relative abundance of the target mRNA was calculated from the −Δcycle threshold (ΔCt) values of the target and endogenous β-actin reference genes using the 2−ΔΔ Ct method.

Table 1.
Primer sequences of different genes of mouse origin.
2.6. Quantification of Cytokine Levels
Cells were seeded into 6-well culture plates at a density of 4–5 × 105 cells/well. After 24 h of incubation, different dilutions (0, 50, 100, 200, or 400 μg/mL) of the three algal extracts (SH, UP, and SF) were prepared in DMEM and added to the cells. All groups were compared with the control set, which was treated with DMEM only. After 24 h of treatment, the supernatant was collected. Levels of TNF-α, IL-6, and PGE2 were measured using ELISA kits (R&D Systems Inc., Minneapolis, MN, USA), following the supplier’s instructions.
2.7. Phagocytosis Assay
The phagocytosis assay was performed according to the previously published literature [7]. Briefly, 2 × 104 cells/well were seeded into a 96-well culture plate and allowed to adhere for 24 h at 37 °C. The cells were treated with different dilutions (0, 50, 100, 200, or 400 μg/mL) of the three algal extracts (SH, UP, and SF) separately for 24 h. The phagocytic properties of different algal extracts were examined using a CytoSelect 96-well phagocytosis assay kit (Cell Biolabs, Inc., San Diego, CA, USA) following the supplier’s instructions. Briefly, the cells were incubated with or without the phagocytosis inhibitor cytochalasin D (2 μM) followed by the addition of E. coli suspension. After 4 h of incubation, the external particles were blocked. The phagocytized particles were examined at 450 nm using an Epoch Microplate Spectrophotometer (Biotek Inc., Winooski, VT, USA).
2.8. Statistical Analysis
The data sets are shown as mean ± SEM. Each experiment was performed thrice. The significance among different groups were analyzed using one-way ANOVA with a post hoc (Tukey) test in GraphPad Prism 9 (Graph Pad Software Inc., San Diego, CA, USA). The data were considered significant if p < 0.05.
3. Results
3.1. The Seaweed Extracts Induce RAW 264.7 Cell Proliferation
The cytotoxicity of different algal extracts of SH, UP, and SF were observed with respect to control groups. To test this hypothesis, a CCK-8 assay was performed. None of the algal extracts induced cytotoxic effects at concentrations up to 400 µg/mL. However, there was significant cell proliferation, which was observed at high concentrations (100–400 µg/mL in SH) and even at low concentrations (50 and 100–400 µg/mL in SF; 50, 100, and 200 µg/mL in UP) when compared to the control (Figure 1a–c). Moreover, by comparing the results of all three algal extracts (at 400 µg/mL), it was observed that the cell viability of RAW 264.7 cells were significantly increased in the order SH < UP < SF (Supplementary Figure S1). All the three algal compounds were also screened in the presence of LPS by co-treating cells with 1 µg/mL of LPS and different concentrations (50, 100, 200, and 400) of SH, UP, and SF for 24 h. It was found that all the three compounds were able to reduce the LPS-induced cell viability, showing their alleviating effects on overactivation (Supplementary Figure S2a–c).
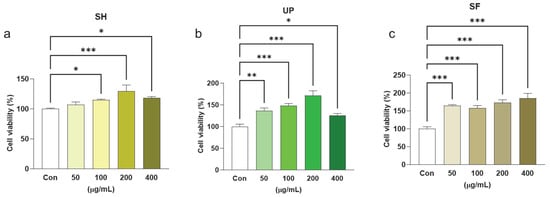
Figure 1.
Seaweed extracts induce cell proliferation in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of (a) SH, (b) UP, and (c) SF for 24 h. Cytotoxicity was observed using the CCK-8 assay. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. The Seaweed Extracts Induce Inflammation-Related Cytokine Expression in RAW 264.7 Cells
It was reported previously that macrophages are major cells of the innate immune system. When they are exposed to external stimuli such as foreign particles or pathogens, they secrete cytokines such as TNF-α, IL-1β, IL-6, and IL-12 in addition to releasing leukotrienes, chemokines, prostaglandins, and complement proteins. Together, these molecules increase vascular permeability and recruit inflammatory cells to fight infections [5]. To determine whether these seaweed extracts can induce these cytokines, RAW 264.7 cells were treated with 0, 50, 200, and 400 μg/mL of three algal extracts (SH, UP, and SF) separately for 24 h. The transcription levels of the above-mentioned cytokines were observed.
We found that all three extracts exhibit immunomodulatory properties, as all of them showed increased mRNA expression levels of TNF-α, IL-1β, IL-6, and IL-12 when compared with the control. SH significantly induced IL-1β expression in a concentration-dependent manner. UP showed higher expression of IL-1β at 100 μg/mL as compared to other concentration groups of the same extract, but SF induced IL-1β only at 200 and 400 μg/mL. In addition, SH and UP showed significant concentration-dependent effects in inducing TNF-α, but SF induced TNF-α at a concentration of 200 and 400 μg/mL. SH significantly increased IL-6 mRNA expression levels at 200 and 400 μg/mL. UP and SF showed significant induction in IL-6 mRNA expression levels at 200 and 400 μg/mL, respectively. IL-12 was also found to be significantly upregulated in the presence of different concentrations of SH (100–400 μg/mL) and SF (50, 200 and 400 μg/mL), but not in the presence of UP (Figure 2a–d).
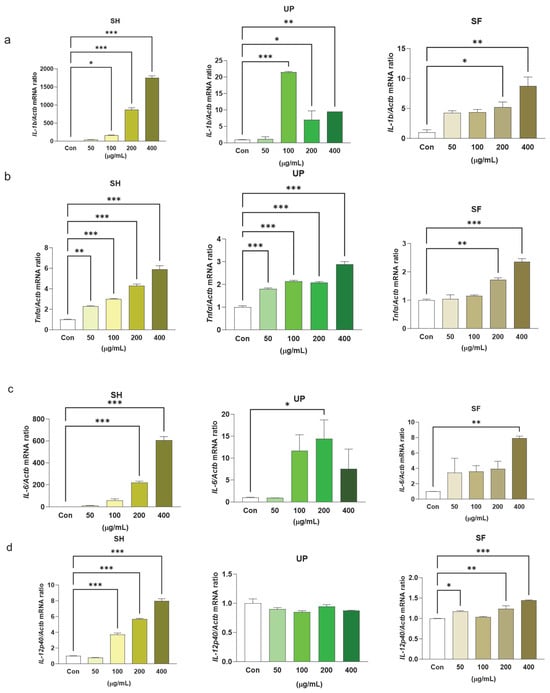
Figure 2.
Seaweed extracts upregulate transcriptional levels of pro-inflammatory markers in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of SH, UP, and SF for 24 h. The transcription expression levels of (a) IL-1β, (b) TNF-α, (c) IL-6, and (d) IL-12 were measured by RT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001.
The inflammatory response could be beneficial for the host only when the cytokines are generated in optimum amounts. The deregulation of these cytokines can also cause serious toxicity. IL-10 is another cytokine produced by macrophages that regulates inflammation [48]. Its main function is to suppress excessive macrophage activation by suppressing various other cytokines, namely IL-1β, IL-12, TNF-α, IL-8, and IL-6 [6]. In our study, we found that SH significantly induced the expression levels of IL-10 at different concentrations (100–400 μg/mL), but similar effects were not observed with UP and SF (Figure 3a–c).
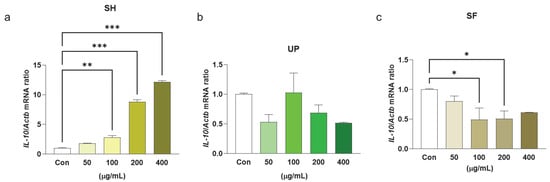
Figure 3.
Seaweed extracts modulate transcriptional levels of anti-inflammatory cytokine IL-10 in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of (a) SH, (b) UP, and (c) SF for 24 h. The transcriptional levels level of IL-10 was determined using RT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001.
ELISA was performed to observe the protein expression of the two major pro-inflammatory cytokines, TNF-α and IL-6. It was found that, compared to the control, SH was able to induce TNF-α cytokine production significantly at 100, 200, and 400 μg/mL. However, UP showed similar significant effects even at lower concentrations of 50 μg/mL with respect to the control. SF significantly upregulated TNF-α cytokine production at 200 and 400 μg/mL compared to the control. It was found that all three algal extracts induced significant IL-6 production at 200 and 400 μg/mL (Figure 4a,b).
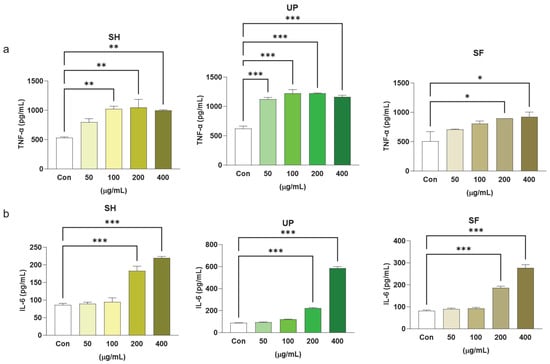
Figure 4.
Seaweed extracts upregulate protein expression levels of pro-inflammatory markers in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of SH, UP, and SF for 24 h. The protein expression levels of (a) TNF-α and (b) IL-6 were determined using RT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. The Seaweed Extracts Induce NO, Ptgs2, and PGE2 Production in RAW 264.7 Cells
As previously mentioned, NO, Ptgs2, and PGE2 are crucial factors in immune response. To determine whether these algal extracts have any significant immunomodulatory effects, RAW 264.7 cells were treated with 0, 50, 100, 200, and 400 µg/mL of SH, UP, and SF separately for 24 h. It was found that all three algal extracts induced mRNA expression levels of Nos2 with respect to the control. However, only SH showed significantly upregulated expression of Nos2 at 50 µg/mL, whereas UP and SF showed upregulation only at concentrations at or above 100 µg/mL. Only SH and UP showed promising results in inducing Ptgs2 expression levels significantly at 200–400 µg/mL, but SF showed a significant upregulation of Ptgs2 at the 400 µg/mL concentration (Figure 5a,b).
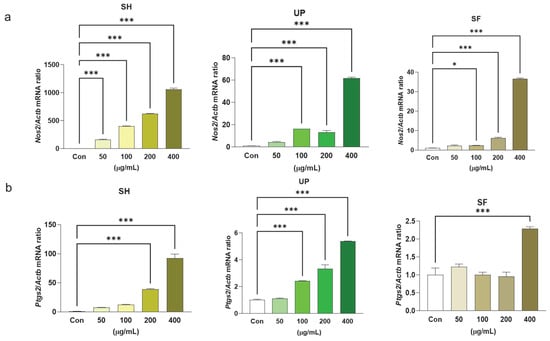
Figure 5.
Seaweed extracts induce Nos2 and Ptgs2 mRNA expression levels in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of SH, UP, and SF for 24 h. The mRNA expression levels of (a) Nos2 and (b) Ptgs2 were determined by RT-PCR. * p < 0.05, *** p < 0.001.
Similar treatments were administered and NO and PGE2 production were measured using the Griess reagent assay and ELISA kits. It was found that SH and UP induced NO production significantly within the concentration range of 100–400 µg/mL. However, SF significantly induced NO only at 200 and 400 µg/mL. When compared with PGE2 production levels, SH significantly upregulated PGE2 production at 100, 200, and 400 µg/mL, while UP did so at 50, 100, 200, and 400 µg/mL. SF was found to be significantly effective only at 400 µg/mL (Figure 6a,b). By comparing the effects of all three algal extracts (at 400 µg/mL) on NO production, a significant increase in NO production was observed in the sequence UP > SH > SF (Supplementary Figure S3). This indicates the high immunomodulatory effects of UP compared to SH and SF. In addition, the anti-inflammatory properties of these algal compounds in ameliorating NO production were also analyzed in the presence of LPS. Cells were co-treated with 1 µg/mL of LPS and different concentrations (50, 100, 200, and 400 µg/mL) of SH, UP, and SF for 24 h. It was found that all the three compounds were able to downregulate LPS-induced NO levels at the concentration range of 100–400 µg/mL (Supplementary Figure S4a–c).
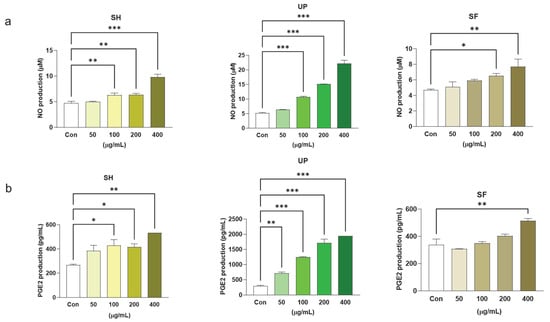
Figure 6.
Seaweed extracts induce NO and PGE2 production in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of SH, UP, and SF for 24 h. The production of (a) NO and (b) PGE2 was observed using Griess reagent assay and ELISA. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.4. The Seaweed Extracts Influence the Phagocytosis Activity of Macrophages
Phagocytosis is a non-specific host defense mechanism operated by macrophages as part of the innate immune response. It occurs when macrophages are exposed to external stimuli or pathogens and is marked as an important indicator of immune function [49,50]. RAW 264.7 cells were treated with different concentrations (0–400 μg/mL) of SH, UP, and SF and tested for phagocytosis activity against the control. All three extracts were found to exhibit phagocytic activity. SH showed a significant enhancement of phagocytic activity at all concentrations, UP showed significant enhancement at 100 and 200 μg/mL, and SF showed significant enhancement at 100, 200, and 400 μg/mL (Figure 7a–c).
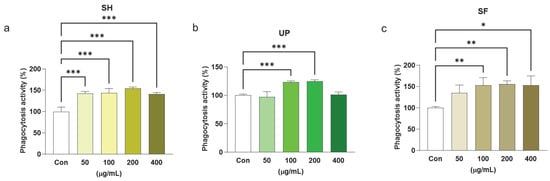
Figure 7.
Seaweed extracts enhance phagocytosis activity in RAW 264.7 cells. RAW 264.7 cells were exposed to 50, 100, 200, and 400 μg/mL of (a) SH, (b) UP, and (c) SF for 24 h. Phagocytosis was observed using CytoSelect 96-well phagocytosis assay. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
We are exposed to a variety of pathogens during our lifespan. Of those, a small number of pathogens manage to cause disease by impairing the immune system. The innate immune system provides the first line of defense against these pathogens [51,52]. Different innate immune cells such as monocytes, macrophages, and dendritic cells come into direct contact with these pathogens and trigger a series of reactions to fight against them. Macrophages scavenge and dispose of pathogens. They implement multiple and complex signaling pathways to deal with infectious pathogens by expressing pattern recognition receptors [53], which recognize unique conserved molecular patterns of pathogen surfaces and produce various cytokines such as TNF-α, IL-1β, IL-6, and IL-12. This initiates inflammation and the phagocytosis of the pathogen [54].
Recent studies have reported various immunomodulators that regulate macrophage function and target various chronic diseases. Natural or plant-derived immunomodulators are considered a promising approach in modulating macrophage function while dealing with different pathogens or chronic diseases, because they contain bioactive compounds [14,15,16]. Brown algae contain a common sulfated polysaccharide, fucoidan, which has been studied in various diseases for its potentially bioactive properties, such as antiviral, anticancer, and anti-inflammatory properties attributed to molecular weights and sulfate contents [42,43,44,45]. Sargassum horneri has also been recognized as an environmental pollutant, particularly on Jeju Island, Korea [55]. Fish farms were found to be polluted with it, and an unpleasant odor on the beaches could be observed owing to its presence [56], which is regarded as an economic burden [57]. Thus, the Korean government and researchers are constantly contributing to efforts to use these materials as beneficial materials [58,59]. Previous studies have supported the beneficial role of Sargassum sp. as anti-inflammatory [60,61], anticancer [62], antioxidant [63], and immunoenhancing agents [64]. On the other hand, Undaria pinnatifida is found in Asian countries (such as Korea, Japan, and China), as well as in Australia and European countries. They are considered a popular seaweed that has been used as food in Asian regions for a longer period [65]. Sporophyll, a byproduct of U. pinnatifida, contains more polysaccharides than its edible portion. U. pinnatifida possesses various bioactivities, including anti-inflammatory [66], antioxidant [67], and antitumor [68] properties.
In our study, we focused on evaluating the immunomodulatory roles of the extracts of brown algae, such as S. horneri, S. fusiforme, and U. pinnatifida. We found that none of these extracts were harmful or caused any toxicity to RAW 264.7 cells when exposed for 24 h.
Cytokines and chemokines released by macrophages as a host defense mechanism play crucial roles in immune impairment. The IL-1β, IL-6, TNF-α, and IL-12 have been reported to be involved in providing innate and adaptive immunity [69]. Kim et al. described the immune-enhancing effects of SH by increasing IL-1β, IL-6, and TNF-α expression in immunosuppressed mice and primary cultured splenocytes [70]. Additionally, Yu et al. analyzed the effect of a novel polysaccharide, UPP-2, from UP and observed its immune-enhancing effects on RAW264.7 cells [71]. Also, the immune-enhancing effects of SH on immunosuppressed BALB/c mice and cultured primary splenocytes were reported [70]. Our study followed in the footsteps of the previous findings, and we found that all three algal extracts were able to affect the mRNA expression levels of IL-1β, TNF-α, and IL-6 and the protein expression levels of TNF-α and IL-6, as shown in a previous study on fucoidan extracted from Laminaria in THP-1 cells [72]. In addition, SH and SF showed upregulation in IL-12 mRNA expression. Only SH was able to upregulate the mRNA expression level of IL-10. Toll-like receptors (TLRs) are the pattern recognition receptors present on the cell surface of innate immune cells including macrophages and are involved in macrophage-mediated inflammation in various diseases [73,74]. Recent studies showed the effects of natural plant compounds in mitigating TLR/nuclear factor kappa-light-chain-enhancer of activated B cells (TLR/NF-κB) signaling to ameliorate inflammation in various disease models [75,76]. Future studies could be designed to observe the effects of these algal extracts in targeting TLR/NF-κB signaling. Macrophages serve as a double-edged sword depending upon their external environment, where some are known as classical or inflammatory (M1 phenotype) and others are known as alternative or anti-inflammatory (M2 phenotype) macrophages [77]. Recent studies have focused on using natural compounds to polarize macrophages toward an M2 phenotype to fight against various diseases. Based on the modulatory properties of these algal extracts, they could be studied for their potential to polarize macrophages in various disease models.
NO functions as a critical cellular messenger known to be involved in both immune and inflammatory responses. It is involved in both regulatory (immunosuppressive) functions (e.g., the modulation of cytokine response and the inhibition of lymphocyte proliferation) and effector functions, such as tissue destruction (immunopathological) and the killing of microbial pathogens (immunoprotective) [78]. Moreover, Takeda et al. demonstrated the antitumor effects of the supernatant derived from fucoidan-stimulated RAW 264.7 and reported the role of NO in imparting antitumor properties in S-180 cells [79]. We also found that SH, UP, and SF extracts were able to induce the activity of both iNOS and NO, which indicated their immune-enhancing properties. Ptgs2 is involved in catalyzing the conversion of arachidonic acid to prostaglandin E2 (PGE2) [10], which plays an essential role in inflammation by activating mast cells and limiting phagocytosis and pathogen killing by macrophages [11,12,13]. Laminaria japonica extract has been shown to exert anti-inflammatory effects against UVB-induced inflammation by reducing Ptgs2 and PGE2. In contrast, our findings showed that all three algal extracts could upregulate mRNA expression levels of Ptgs2 and PGE2 protein production.This suggests that brown seaweeds could be used as anti-inflammatory or immune-enhancing agents in different diseases.
Macrophage phagocytosis is a crucial step in the treatment of foreign antigens or pathogens. Various studies have highlighted that impaired phagocytosis by macrophages is a critical event that occurs in chronic diseases. Fajriah et al. demonstrated the effects of edible green seaweed extracts (such as Caulerpa lentillifera) on the phagocytic properties of RAW 264.7 cells [80] and found that beta-glucan, a bioactive component of seaweed, increased the phagocytic activity of RAW 264.7 cells. Like previous findings, our study also found that SH, UP, and SF induce the phagocytic properties of RAW 264.7 cells.
In addition to exhibiting immunomodulation properties by inducing pro-inflammatory and anti-inflammatory cytokines expression, these algal extracts also proved their potential in downregulating NO production in the presence of LPS and in reducing LPS-induced cell viability (data shown in Supplementary Materials). These findings indicated the futuristic potential of these compounds as a therapeutic agent in various immunocompromised or inflammatory diseases.
Although this study reported the immunomodulatory potential of algal extracts, their effects could also be studied in the future in the presence of disease-causing agents such as LPS, Aβ, or H2O2, which could give a clear idea about the mode of action of these algal extracts under different disease conditions. Also, in depth studies could be designed to fully explore the bioactive potential of these algal extracts, which might include in vitro and in vivo disease models studying various inflammation-related pathways such as TLR/NF-κB signaling, extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling, apoptosis, macrophage polarization, etc.
5. Conclusions
This study demonstrated that S. horneri, U. pinnatifida, and S. fusiforme are effective in enhancing macrophage activation and phagocytosis. Notably, S. horneri and U. pinnatifida outshined S. fusiforme in enhancing the expression levels IL-1β, TNF-α, and IL-6 at almost all the concentrations tested as compared to S. fusiforme, which showed similar effects only at 200 and 400 μg/mL. A similar trend was seen in TNF-α, NO, and PGE2 production. Additionally, only SH and SF could enhance the mRNA expression levels of IL-12. This study suggests that these macroalgae could be used as potential therapeutic agents for immune-impaired disorders. However, further studies are still required to fully exploit the maximum benefit derived from these seaweeds by assessing their immunomodulatory effects in various immunocompromised disorders such as respiratory infections, cancer, cardiovascular diseases, and neurodegeneration. Additionally, there is the need to identify novel approaches to inherit these seaweeds in functional foods or therapeutics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14051794/s1. Figure S1. Seaweed extracts induce cell proliferation in RAW 264.7 cells. RAW 264.7 cells were treated with 400 μg/mL of SH, UP, and SF for 24 h. Cytotoxicity was observed using the CCK-8 assay. * p < 0.05, ** p < 0.01, *** p < 0.001. Figure S2. Seaweed extracts reduce LPS-induced cell proliferation in RAW 264.7 cells. RAW 264.7 cells were treated with 1 µg/mL of LPS alone and co-treated with different concentrations (50, 100, 200, and 400 μg/mL) of SH, UP, and SF for 24 h. Cytotoxicity was observed using the CCK-8 assay. * p < 0.05, ** p < 0.01, *** p < 0.001. Figure S3. Seaweed extracts induce NO production in RAW 264.7 cells. RAW 264.7 cells were treated with 400 μg/mL of SH, UP, and SF for 24 h. The production of NO was observed using a Griess reagent assay. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Figure S4. Seaweed extracts reduce LPS-induced NO in RAW 264.7 cells. RAW 264.7 cells were treated with 1 µg/mL of LPS alone and co-treated with different concentrations (50, 100, 200, and 400 μg/mL) of SH, UP, and SF for 24 h. The production of NO was observed using a Griess reagent assay. * p < 0.05, ** p < 0.01, *** p < 0.001.
Author Contributions
H.-J.L. and E.-J.P. conceived the idea. H.-J.L., E.-J.P. and S. designed the experiments. E.-J.P. and S. performed the laboratory experiments, analyzed the data, prepared the figures, and wrote the original draft. H.-J.L. revised the manuscript and supervised the study. H.-J.L., E.-J.P., S. and N.Y.Y. contributed to data curation and reviewed/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Development of technology for biomaterialization of marine fisheries by-products of Korea institute of Marine Science & Technology Promotion (KIMST), funded by the Ministry of Oceans and Fisheries (KIMST-20220128) and by a grant from the National Institute of Fisheries Science in Korea (R2024058).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Rachit Sood, Sung-Min Kim, and Hae-Sun Park for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frisch, M.; Biggar, R.J.; Engels, E.A.; Goedert, J.J.; AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001, 285, 1736–1745. [Google Scholar] [CrossRef]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, H.-J. Immunomodulatory effects of fermented Platycodon grandiflorum extract through NF-κB signaling in RAW 264.7 cells. Nutr. Res. Pract. 2020, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sieweke, M.H.; Allen, J.E. Beyond stem cells: Self-renewal of differentiated macrophages. Science 2013, 342, 1242974. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Lee, Y.-S.; Kim, S.M.; Jung, A.J.; Yoo, J.-H.; Lee, S.-H.; Jeong, H.C.; Lee, H.-J. Immune-enhancing effects of red Platycodon grandiflorus root extract via p38 MAPK-mediated NF-κB activation. Appl. Sci. 2020, 10, 5457. [Google Scholar] [CrossRef]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Asano, K.; Seki, H.; Shimamura, T. An essential role of prostaglandin E on mouse mast cell induction. J. Immunol. 1995, 155, 2134–2142. [Google Scholar] [CrossRef]
- Aronoff, D.M.; Canetti, C.; Peters-Golden, M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J. Immunol. 2004, 173, 559–565. [Google Scholar] [CrossRef]
- Serezani, C.H.; Chung, J.; Ballinger, M.N.; Moore, B.B.; Aronoff, D.M.; Peters-Golden, M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am. J. Respir. Cell Mol. Biol. 2007, 37, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-inflammatory activity of bioactive compounds from microalgae and cyanobacteria by focusing on the mechanisms of action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef]
- Dimova, V.; Sencheva-Petrevska, M.; Jankulovska-Petkovska, M. Vitamin C equivalent antioxidant capacity prediction for set of flavones which influence food quality. J. Agric. Food Environ. Sci. JAFES 2022, 76, 28–35. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef]
- Qiu, S.-M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Bioactive polysaccharides from red seaweed as potent food supplements: A systematic review of their extraction, purification, and biological activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.; Yang, R.; Zhao, W. Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive compounds from marine organisms: Potential for bone growth and healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader-Ul-Ain, H.; Suleria, H.A.R. Therapeutic Potential of Seaweed Bioactive Compounds; IntechOpen: London, UK, 2018. [Google Scholar]
- Rai, S.K.; Smriti, B.; Gunaseelan, S.; Ashokkumar, B.; Varalakshmi, P. Polyphenolic Compound from Brown Macroalga padina tetrastromatica Imparts Oxidative Stress Tolerance in SH-SY5Y, RAW 264.7, HeLa Cell Lines and in Caenorhabditis elegans. ChemistrySelect 2019, 4, 6342–6347. [Google Scholar] [CrossRef]
- Ruan, B.-F.; Ge, W.-W.; Lin, M.-X.; Li, Q.-S. A review of the components of seaweeds as potential candidates in cancer therapy. Anti-Cancer Agents Med. Chem. 2018, 18, 354–366. [Google Scholar] [CrossRef]
- Ghannam, A.; Murad, H.; Jazzara, M.; Odeh, A.; Allaf, A.W. Isolation, Structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. Int. J. Biol. Macromol. 2018, 108, 916–926. [Google Scholar] [CrossRef]
- Desamero, M.J.; Kakuta, S.; Chambers, J.K.; Uchida, K.; Hachimura, S.; Takamoto, M.; Nakayama, J.; Nakayama, H.; Kyuwa, S. Orally administered brown seaweed-derived β-glucan effectively restrained development of gastric dysplasia in A4gnt KO mice that spontaneously develop gastric adenocarcinoma. Int. Immunopharmacol. 2018, 60, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Nedel, F.; Guimarães, V.B.; Da Silva, A.F.; Colepicolo, P.; De Pereira, C.M.; Lund, R.G. Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front. Microbiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Rocha, D.H.; Seca, A.M.; Pinto, D.C. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Sharifuddin, Y.; Chin, Y.-X.; Lim, P.-E.; Phang, S.-M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Sapkota, K.; Choi, J.-H.; Kim, Y.-S.; Kim, S.; Kim, S.-J. Direct acting anti-thrombotic serine protease from brown seaweed Costaria costata. Process Biochem. 2013, 48, 340–350. [Google Scholar] [CrossRef]
- Kellogg, J.; Esposito, D.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan seaweeds lower inflammation in RAW 264.7 macrophages and decrease lipid accumulation in 3T3-L1 adipocytes. J. Funct. Foods 2015, 15, 396–407. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Q.; Luo, H.; Lei, D.; Tang, Z.; Lei, S.; Xiao, H. Inhibitory effects of polyphenols-rich components from three edible seaweeds on inflammation and colon cancer in vitro. Front. Nutr. 2022, 9, 856273. [Google Scholar] [CrossRef]
- Olsthoorn, S.E.; Wang, X.; Tillema, B.; Vanmierlo, T.; Kraan, S.; Leenen, P.J.; Mulder, M.T. Brown seaweed food supplementation: Effects on allergy and inflammation and its consequences. Nutrients 2021, 13, 2613. [Google Scholar] [CrossRef]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J. Fucoidan extracted from the New Zealand Undaria pinnatifida—Physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Decharneux, T.; Dubois, F.; Beauloye, C.; De Coninck, S.W.; Wattiaux, R. Effect of various flavonoids on lysosomes subjected to an oxidative or an osmotic stress. Biochem. Pharmacol. 1992, 44, 1243–1248. [Google Scholar] [CrossRef]
- Sharma, A.; Jaiswal, V.; Park, M.; Lee, H.-J. Biogenic silver NPs alleviate LPS-induced neuroinflammation in a human fetal brain-derived cell line: Molecular switch to the M2 phenotype, modulation of TLR4/MyD88 and Nrf2/HO-1 signaling pathways, and molecular docking analysis. Biomater. Adv. 2023, 148, 213363. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Sharma, A.; Lee, H.-J. Honeyberry-derived carbon quantum dots ameliorate LPS-induced neuroinflammation and oxidative stress through Nrf2/HO-1 signalling in HMC3 cells. Artif. Cells Nanomed. Biotechnol. 2023, 51, 95–107. [Google Scholar]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Oberley, R.E.; Ault, K.A.; Neff, T.L.; Khubchandani, K.R.; Crouch, E.C.; Snyder, J.M. Surfactant proteins A and D enhance the phagocytosis of Chlamydia into THP-1 cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L296–L306. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.; Ema, K.; Tokuda, Y.; Maeda-Yamamoto, M. Enhancement of phagocytic activity of macrophage-like cells by pyrogallol-type green tea polyphenols through caspase signaling pathways. Cytotechnology 2010, 62, 201–203. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Matsukawa, A.; Hogaboam, C.; Lukacs, N.; Kunkel, S. Chemokines and innate immunity. Rev. Immunogenet. 2000, 2, 339–358. [Google Scholar]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Grinstein, S.; Canton, J. The life cycle of phagosomes: Formation, maturation, and resolution. Immunol. Rev. 2016, 273, 156–179. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Liu, J.; Ding, X.; He, J.; Zhao, S.; Wu, L.; Gao, S.; Zhao, C.; Liu, D.; Zhang, J. Sargassum blooms in the East China Sea and Yellow Sea: Formation and management. Mar. Pollut. Bull. 2021, 162, 111845. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cheon, K.-S.; Kim, S.; Yun, S.-H.; Oh, H.-J.; Park, S.R.; Kim, T.-H.; Kim, J.K.; Lee, H.J. Comparative analysis of sequence polymorphism in complete organelle genomes of the ‘Golden Tide’ seaweed Sargassum horneri between Korean and Chinese forms. Sustainability 2020, 12, 7280. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Wang, Y.; Jin, Z.; Moejes, F.W.; Sun, S. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol. Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Lee, B.-J.; Lee, S.-M.; Hyun, J.-H.; Kim, Y.-Y. Durability Performances of Concrete Produced with Recycled Bio-Polymer Based on Sargassum honeri. J. Korean Recycl. Constr. Resour. Inst. 2019, 7, 445–451. [Google Scholar]
- Madhavaraj, L.; Lim, H.-D.; Kim, K.-M.; Kim, D.-H.; Han, G.H. Influence of Sargassum horneri Mitigating odorous gas emissions from swine manure storage facilities. Sustainability 2020, 12, 7587. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jayawardena, T.U.; Lee, H.G.; Herath, K.H.I.N.M.; Jee, Y.; Jeon, Y.-J. The protective effect of Sargassum horneri against particulate matter-induced inflammation in lung tissues of an in vivo mouse asthma model. Food Funct. 2019, 10, 7995–8004. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.-R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H. The anti-oxidative and anti-neuroinflammatory effects of sargassum horneri by heme oxygenase-1 induction in BV2 and HT22 cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Cho, J.; Kim, A.; Kim, H.-S.; Han, E.J.; Kim, H.J.; Kim, M.S.; Ahn, G.; Jeon, Y.-J.; Jee, Y. Differential modulation of immune response and cytokine profiles of Sargassum horneri ethanol extract in murine spleen with or without Concanavalin A stimulation. Biomed. Pharmacother. 2019, 110, 930–942. [Google Scholar] [CrossRef]
- South, P.M.; Floerl, O.; Forrest, B.M.; Thomsen, M.S. A review of three decades of research on the invasive kelp Undaria pinnatifida in Australasia: An assessment of its success, impacts and status as one of the world’s worst invaders. Mar. Environ. Res. 2017, 131, 243–257. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Cho, J.-Y.; Lee, M.-C.; Kang, J.-Y.; Park, N.G.; Fujii, H.; Hong, Y.-K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef]
- Han, J.; Kang, S.; Choue, R.; Kim, H.; Leem, K.; Chung, S.; Kim, C.; Chung, J. Free radical scavenging effect of Diospyros kaki, Laminaria japonica and Undaria pinnatifida. Fitoterapia 2002, 73, 710–712. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo 2003, 17, 245–249. [Google Scholar] [PubMed]
- Lee, S.J.; Lee, H.S.; Kim, S.Y.; Shin, K.-S. Immunostimulatory and anti-metastatic activity of polysaccharides isolated from byproducts of the corn starch industry. Carbohydr. Polym. 2018, 181, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, D.-S.; Jung, Y.; Sung, N.-Y.; Kim, M.; Han, I.-J.; Nho, E.Y.; Hong, J.H.; Lee, J.-K.; Boo, M. Immune-Enhancing Effect of Sargassum horneri on Cyclophosphamide-Induced Immunosuppression in BALB/c Mice and Primary Cultured Splenocytes. Molecules 2022, 27, 8253. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak–Vidarsson, M.M.; Gudjónsdóttir, M.; Marteinsdottir, G.; Sigurjonsson, O.E.; Kristbergsson, K. Evaluation of bioactivity of fucoidan from laminaria with in vitro human cell cultures (THP-1). Funct. Foods Health Dis. 2017, 7, 688–701. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Lee, H.; Kim, G.-Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef] [PubMed]
- Alomar, S.Y.; Gheit, R.E.A.E.; Enan, E.T.; El-Bayoumi, K.S.; Shoaeir, M.Z.; Elkazaz, A.Y.; Al Thagfan, S.S.; Zaitone, S.A.; El-Sayed, R.M. Novel mechanism for memantine in attenuating diabetic neuropathic pain in mice via downregulating the spinal HMGB1/TRL4/NF-kB inflammatory axis. Pharmaceuticals 2021, 14, 307. [Google Scholar] [CrossRef]
- Azam, S.; Jakaria, M.; Kim, I.-S.; Kim, J.; Haque, M.E.; Choi, D.-K. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: Focus on TLR4 signaling. Front. Immunol. 2019, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, C.; Zhuang, J.; Li, H.; Yao, Y.; Shao, C.; Wang, H. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: Role of the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2015, 11, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K.; Mori, N. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int. J. Oncol. 2012, 40, 251–260. [Google Scholar]
- Fajriah, S.; Handayani, S.; Sinurat, E.; Megawati, M.; Darmawan, A.; Hariyanti, H.; Dewi, R.T.; Septama, A.W. In vitro Immunomodulatory Effect from Edible Green Seaweed of Caulerpa lentillifera Extracts on Nitric Oxide Production and Phagocytosis Activity of RAW 264.7 Murine Macrophage Cells. J. Young Pharm. 2020, 12, 334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).