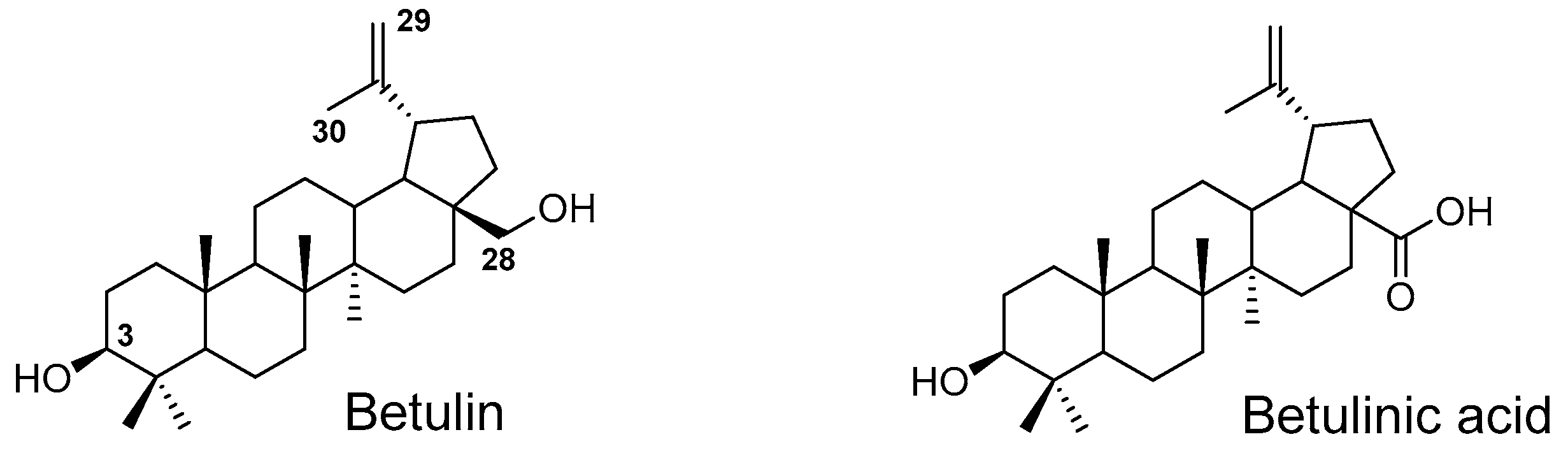
3.3.1. Assessment of ADMET Properties and Drug Likeness of the Screened Molecules
Modern research aimed at searching for new medicinal substances uses many computational methods that enable preliminary prediction of properties essential for assessing the probability that a chemical compound may become a drug [
63]. As part of our work, for the tested compounds, pharmacokinetics and toxicity were assessed using in silico methods. Calculations of ADMET parameters for the tested compounds were performed using the pkCSM (2015) software and are presented in
Table 6.
In order to initially assess the ease of absorption of a substance, various parameters should be considered. Both human intestinal absorption (HIA), human intestinal permeability (Caco-2), and the ability to interact with P-glycoprotein, the biological barrier responsible for the excretion of toxins and xenobiotics (PgP parameter), determine potential drug absorption difficulties. The HIA values obtained for the tested compounds
1,
3–
9, defining the percentage of the substance that will be absorbed by the human intestine, range from 52.222 to 100.000 and indicate good absorption (a value less than 30% indicates poor absorption). The highest Caco-2 permeability expired as logPapp values were calculated for compounds
1 and
3. They indicate high and good permeability (respectively, the values equal 1.321 for betulin
1 and 0.742 for compound
3). According to the pkCSM prediction model, high Caco-2 permeability can be expected with a predicted value > 0.90. The logPapp value for the remaining compounds is in the range 0.228–0.660, which defines moderate Caco-2 permeability [
45].
The tested derivatives may have an inhibitory effect on P-glycoprotein, while only compound
3 can be both its substrate and inhibitor. P-gp transport protein participates in the protection of cells against the effects of potentially harmful factors; however, the therapeutic effect of orally taken drugs that are Pgp substrates is also reduced. By reducing its activity, Pgp inhibitors may increase the bioavailability of other drugs. This causes the concentration of these drugs to increase to toxic levels, resulting in the occurrence of many side effects. Knowledge of this type of relationship requires caution and monitoring of the applied therapy [
64].
Viral diseases often attack patients with weakened immunity, who are already receiving treatment for other ailments. Therefore, with the applied pharmacotherapy, one can expect not only side effects from the drug used, but also the possibility of drug–drug interactions. Mutual drug interactions also depend on the activity of cytochrome P450 enzymes responsible for the metabolism of a given drug (mainly the CYP3A4 isoenzyme) [
65]. All tested compounds may be substrates of the CYP3A4 isoenzyme; however, their inhibitory effect on CYP3A4 and other isoforms (CYP1A2, CYP2C19, CYP2C9, CYP2D6) is not expected.
The blood–brain barrier (BBB) not easily penetrated by tested compounds. The calculated logBB values range from −0.083 for compound 9 to −1.373 for the derivative 7b (log BB < −1 are poorly distributed to the brain, logBB > 0.3 indicates readily cross the blood–brain barrier).
The predicted total clearance (expressed as log (mL/min/kg) defines the volume of plasma that is completely cleared of drug per unit of time by the organ that eliminates the drug from the body. Knowledge of this parameter is essential for determining the size of the maintenance dose of the drug. The highest value was calculated for betulin 1, the values for the other compounds are in the range −0.199 to −0.556.
The toxicity of the tested compounds was determined based on the Ames test, inhibition of the potassium channels (hERG; human ether-a-go-go gene), calculation of the maximum human-tolerated dose (MRTD)) and oral rat acute toxicity (LD50—the amount of compound administered at a time that causes death to 50% of a group of test animals). For all tested compounds, the result of the Ames test, which evaluated the mutagenic potential of compounds using bacteria, was negative, which suggests a lack of carcinogenic activity. These compounds are also not hERG I and II inhibitors, so they should not be the cause of the development of acquired long QT syndrome, which leads to lethal ventricular arrhythmias. The maximum recommended tolerated dose (MRTD) of the test compound less than or equal to 0.477 log (mg/kg/day) is considered low and high above 0.477 log (mg/kg/day). For the compounds tested, the calculated values range from -0.433 to 0.533. The MRTD determines the size of the maximum recommended starting dose for pharmaceuticals in phase I clinical trials. Lethal dose values (LD50) are a standard measure of acute toxicity. For compounds 1, 2–9, the LD50 ranged from 2.348 to 3.267 (mol/kg).
Drug-induced liver damage is a major safety concern for drugs. pkCSM classifies a compound as hepatotoxic if it has shown at least one pathological or physiological event strongly associated with disruption of normal liver function [
45]. Compounds
3–
9 showed no hepatotoxicity.
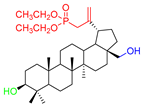
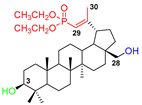
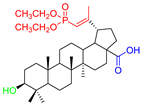
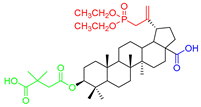
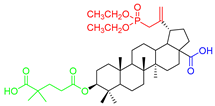
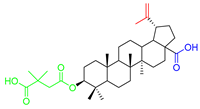
For compounds
3 and
8a, which showed activity in in vitro studies, and for the starting betulin
1, physicochemical parameters helpful in assessing bioavailability were determined (
Table 7) and drug similarity was analyzed [
46].
Considering two in vitro active semi-synthetic betulin derivatives, 3 and 8a, and the initial betulin 1, it can be seen that the introduction of new functional groups resulted in an increase in molar mass (MW; g/mol) from 442.72 (for betulin 1) to the value of 578.80 and 720.91, respectively. Another consequence was to increase the number of rotational bonds (nRt) and the number of acceptors (nHA) of hydrogen binding as well as a topologic polar surface (TPSA; Å2). The introduced structural changes did not affect the number of hydrogen bond donors (nHD).
The theoretical values of the lipophilicity of the tested compounds calculated using various algorithms are in the range of 5.29–9.74 and all go beyond the criteria of drug-likeness formulated by Lipinski, Weber, and Egan. Compound
8a showed the most violations of these rules. Betulin
1 and its derivative
3 met the Veber criteria without any violations. The number and type of violated criteria for the tested compounds are summarized in
Table 8.
Compounds with a high molecular weight and higher lipophilicity are characterized by poor solubility in water, which results in low oral bioavailability. The choice of a compound that meets the criteria of drug similarity increases the chances of success in further stages of research (clinical trials). On the other hand, 16% of orally administered drugs do not meet at least one of Lipinski’s rule of five criteria; moreover, 6% of them violate more than two [
66]. Moreover, new methods are currently being introduced to reduce the difficulties resulting from the limited oral bioavailability of drugs of natural origin. For this purpose, various types of carriers are used: polymeric nanoparticles, liposomes, dendrimers, micelles, and inorganic nanoparticles [
67,
68].
Based on the analysis of the pharmacokinetic profile (ADMET), as well as the drug-likeness, compound 3 was chosen for the next few studies.
3.3.2. Study on Quantum Descriptors
The energy of HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied MO) orbital determines the quantum chemical properties of molecules [
69]. The energy gap (E
LUMO-E
HOMO) makes it possible to designate the kinetic stability and reactivity of a compound [
69,
70,
71,
72]. The energy gap (E
LUMO-E
HOMO) makes it possible to designate the kinetic stability and reactivity of a compound [
69,
70,
71,
72]. The HOMO and LUMO orbitals and their energy gap were calculated using the Gaussian09 package at the B3LYP level using the 6-311G + (d,p) basis set [
47].
As seen in
Figure 5, the HOMO orbital is delocalized near the phosphonate group and E ring of betulin
1, while the LUMO orbital is delocalized on betulin scaffold.
The influence of the energy gap (ΔE) on the reactivity of the compound; decreasing the ΔG value increased the reactivity of the compound. Comparing the energy gap of compound
3 and betulin
1 [
73] shows that the introduction of a phosphonate group led to an increase in the reactivity of the compound.
The energies of the HOMO-LUMO orbital enable the calculation of the global reactivity descriptors, such as the ionization potential (I), electron affinity (A), hardness (η), softness (s), chemical potential (µ), electronegativity (χ), and electrophilicity index (ω) [
74]. Global reactivity descriptors are presented in
Table 9.
The values of ionization potential (I) and electron affinity (A) show that the derivatives have low reactivity with the electrophilic molecules. The high values of electronegativity (χ) and electrophilicity index (ω) indicate that compounds are good acceptors for the nucleophilic target [
69,
74].
The molecular electrostatic potential map (MEP) is a useful tool to designate the electrophilic and nucleophilic region of the compound [
75]. Surfaces with different charges are marked with different colors (negative—red, positive—blue, and neutral—green) (
Figure 6) [
76].
For compound
3, the nucleophilic regions are localized in three main areas. The first is localized near the phosphonate group. The second and third area include the oxygen atoms at positions C28 and C3, respectively. The electrophilic regions are localized near the hydrogen atom of the hydroxyl group at positions C3 and C28, respectively. The betulin scaffold is neutral (green color). Determination of the potential indicating the places or regions of the molecule to which an approaching electrophile is initially attracted can be useful in studying interactions related to the relative orientation of reactants; for example, between a drug and its cell receptor [
76].
3.3.3. Molecular Docking
Molecular docking was performed to propose a probable mechanism of action of active compounds. The use of this method allows for the determination of possible interactions with selected cellular proteins without the need for expensive and time-consuming experimental methods. The obtained results may allow for further optimization of the structure of active compounds in order to improve biological activity [
54].
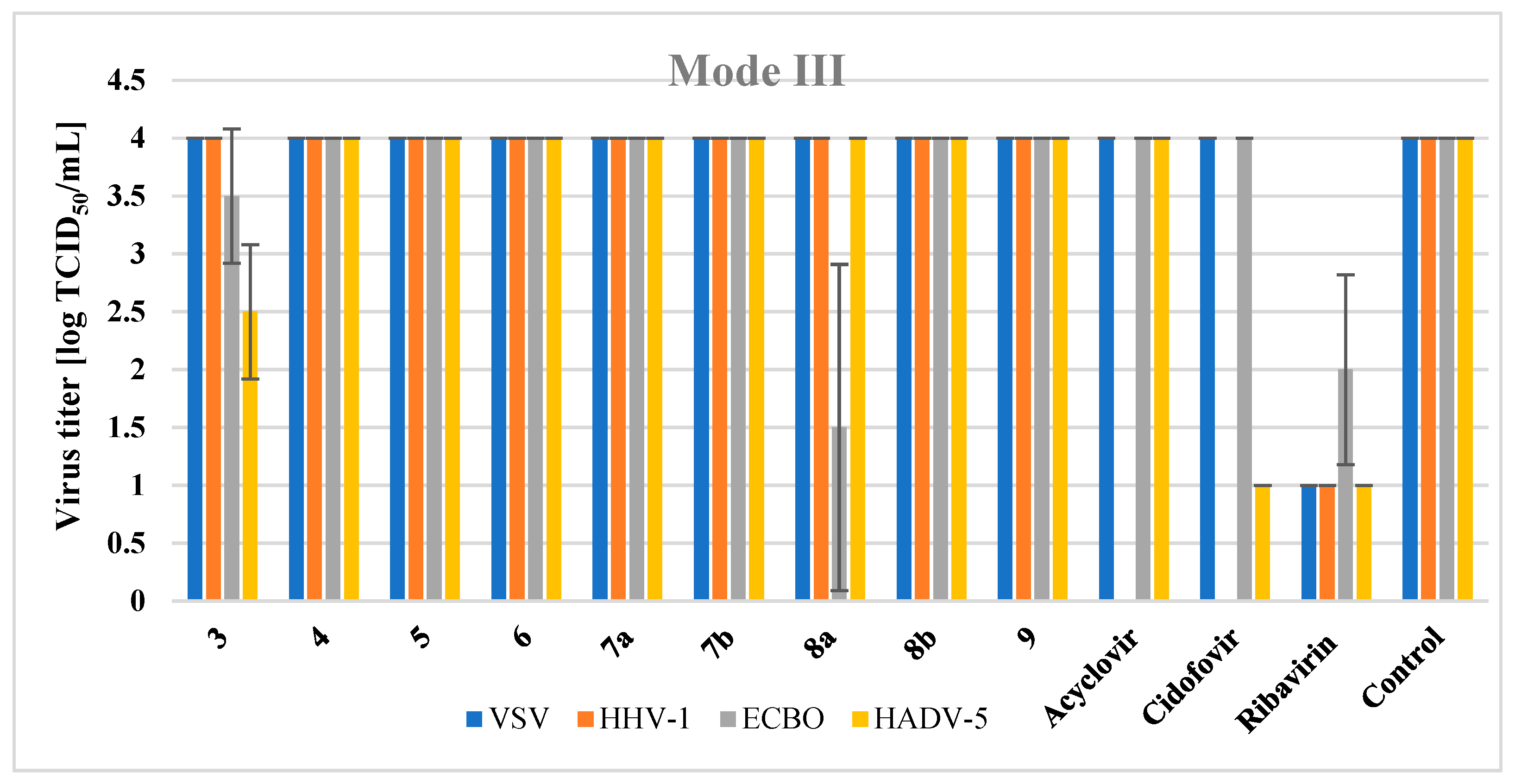
The time-of-addition experiment showed the activity of compound
3 in the third mode, where the final stages of assembly and maturation of new virions take place. One of the key replication enzymes of adenoviruses is adenaine cysteine protease. It plays a critical role in the formation of important core and capsid proteins, which then form the mature, infectious virus particles [
77].
The target crystal structure of adenain was obtained from Protein Data Bank (PDB ID: 4PIE) [
50]. Betulin
1 and active compound
3 were ranked by their binding energy with protein (
Table 10). The lower binding energy, represented as ΔG, corresponds to a stronger binding affinity. Compound
3 exhibited a lower ΔG value compared with reference betulin (–4.8 and –3.4 kcal/mol, respectively).
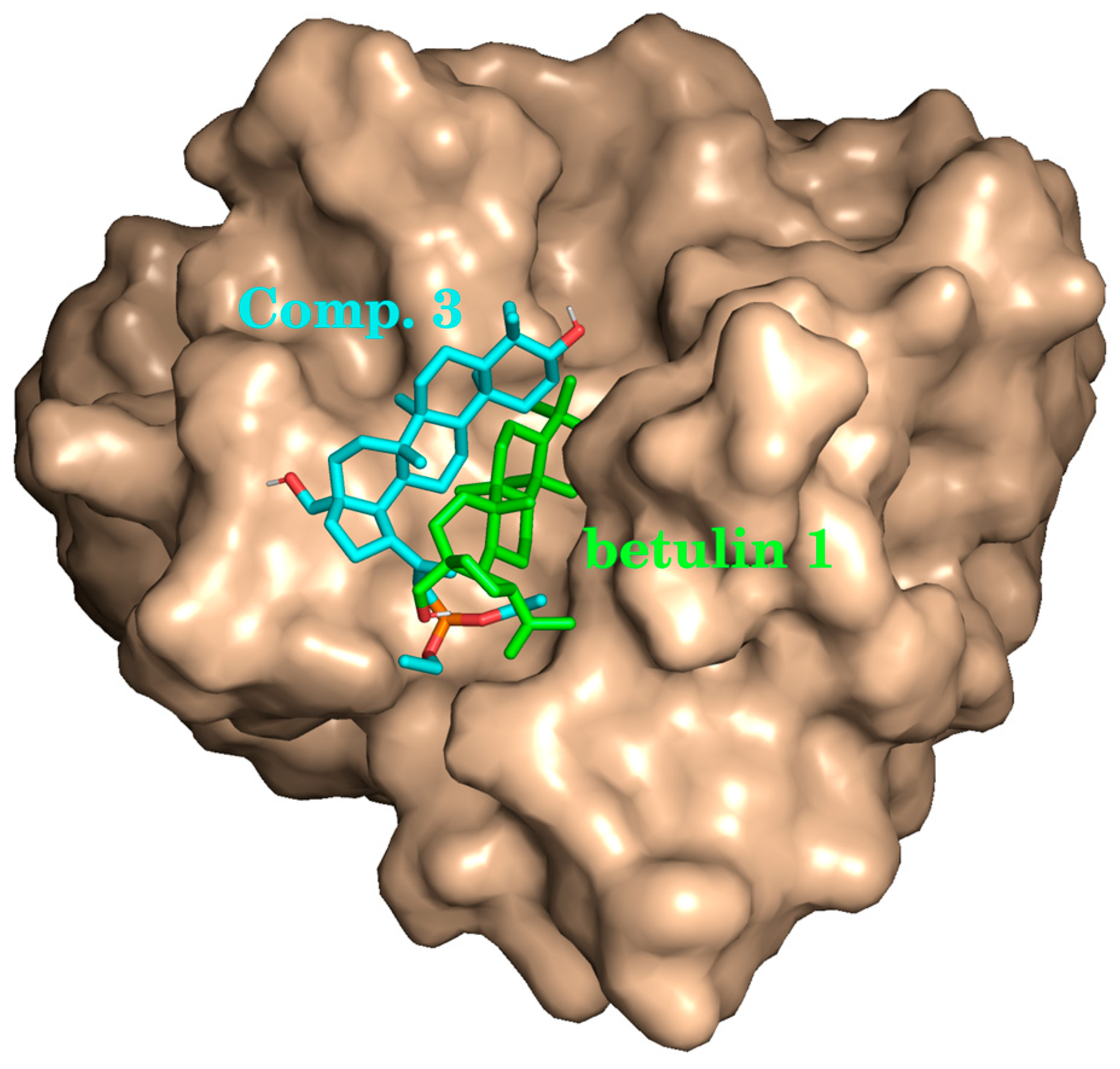
Visual inspection of the docking modes reveals that botulin
1, due its relative smaller hydrophilicity, binds deeper inside the protein pocket than compound
3 (
Figure 7).
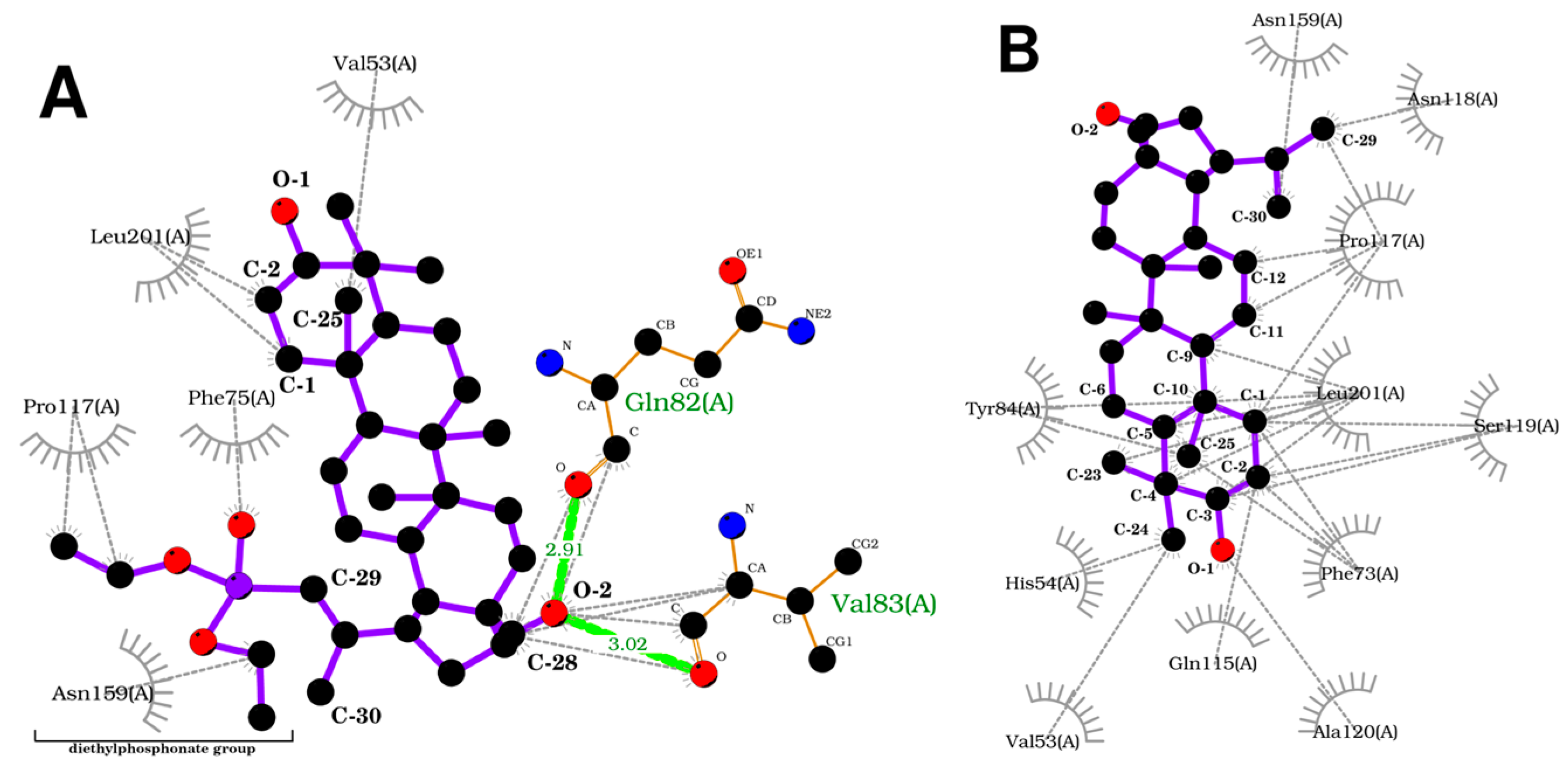
To acquire a more in-depth insight into the binding model, an analysis of protein–ligands bonding has been performed (
Figure 8). Compound
3 forms two hydrogen bonds between the oxygen atom at position C-28 of the betulin core and Gln82 and Val83 residues of protein. On the other hand, the betulin molecule lacks a hydrogen bond. Additionally, both ligands form numerous hydrophobic bonds with various residues. The introduction of a diethylphosphonate group to the betulin molecule changes the structure and influences the way compound
3 interacts with the receptor, increasing the stability of the formed complex.
In general, in silico analysis confirms in vitro data. The most active compound 3 exhibited stronger binding affinity both in terms of ΔG and hydrogen bonding. Further exploration of the molecular mechanism of action could lead to more potent anti-adenovirus compounds being obtained from phosphonate derivatives of betulin.