Abstract
The microscopic observation of lung tissue is challenging due to its fragile nature. Xylene and isopropanol are common tissue-clearing reagents used before paraffin embedding, yet no studies have compared these two reagents in lung tissue processing. Due to the well-known health risks xylene could introduce to operators, as well as its environmental hazards, it has long been desired that a less harmful alternative to xylene with the same staining effects be introduced. Thus, we systematically assessed the efficacy of isopropanol as a substitution for xylene. Lung tissue obtained from diseased donors and explanted lungs from recipients were processed simultaneously using either xylene or isopropanol prior to paraffin embedding. Scoring of the overall staining quality after H&E staining, along with the ease of sectioning, was compared systematically. Fluorescent staining was performed to explore alveolar morphology and the overall lectin fluorescence signal between groups. To understand differences in antibody staining, the signal-to-noise ratio (SNR) of smooth muscle actin (SMA) and elastin was examined. No difference was observed with regard to ease of sectioning, staining quality, alveolar circularity, alveolar wall thickness or the SNR between slides processed with xylene or isopropanol. This study demonstrated comparable outcomes of isopropanol and xylene in lung tissue processing, suggesting isopropanol as a more favorable, operator- and environment-friendly substitute for xylene with regards to tissue processing.
1. Introduction
Histology plays a crucial role in medical research, primarily focusing on the detailed microscopic examination of cells and tissues [1]. Tissue processing involves tissue preservation, dehydration, clearing, and paraffin infiltration, which is fundamental for the preparation of fixed tissues [2]. The essential purpose of this tissue processing is to provide structural support for morphological preservation during sectioning, and it can be achieved via embedding the tissue in a liquid paraffin. This also acts to protect the tissue for long-term storage for future analysis [3]. In contrast to solid organ tissue, lung tissue processing can be challenging owing to its structural fragility. This necessitates elevated standards for providing comprehensive and uniform support during the sectioning procedure, thereby imposing heightened demands on the quality of tissue processing.
An essential and efficient factor contributing to paraffin penetration into tissues is tissue clearing. Therefore, the selection of a tissue-clearing agent holds particular significance in this context.
Xylene and isopropanol have traditionally been used as clearing reagents in tissue processing with xylene achieving more widespread use [4,5]. Xylene is an aromatic hydrocarbon compound solvent that is widely used in industry and medicine [6]. It has a crucial role in histological laboratories, aiming to render alcohol to be removed from tissue, enhancing tissue transparency, and contributing to better paraffin penetration and the observation of the morphological structure of the tissues, for which is it widely employed in tissue processing [4,7,8]. However, xylene also has a detrimental impact on the environment, potentially introducing a hazard to human beings and pollutions to our ecosystem [6,9,10,11,12]. Moreover, prolonged exposure, particularly through occupational means, like researchers and technicians in the lab, leads to inhalation risk, skin and eye irritation, central nervous system depression, and even long-term health impacts [6,13,14,15,16,17,18]. Isopropanol is a clear, colorless, flammable liquid with an odor similar to a mixture of ethanol and acetone [19]. It is metabolized mainly in the liver by alcohol dehydrogenase to acetone, which is the primary metabolite of isopropanol [20,21], and it is less toxic than xylene [22,23].
The aim of our study was to evaluate the differences, if any, between xylene and isopropanol for tissue processing and the imaging of human lung tissue, including brightfield imaging of hematoxylin and eosin (H&E) stains, epi-fluorescent imaging of a molecular lectin stain, and confocal imaging of a multiplex antibody stain.
2. Materials and Methods
2.1. Histological Processing and Ease of Sectioning Analysis
Samples were collected from donor lungs not used for transplantation, and explanted diseased lungs from patients who had received transplants, at Lund University Hospital. Biopsies were taken at the same location from each donor (n = 6) or recipient (n = 6) lung, leading to a n = 12 for comparing the impact of xylene versus isopropanol clearing. This was followed by 48 h fixation in 10% neutral buffered formalin solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) at 4 °C. After the completion of fixation, 2 tissue blocks were randomly allocated into xylene and isopropanol groups for further tissue processing (Table 1). One group was processed via xylene and the other was processed via isopropanol. All the tissues were thereafter embedded in paraffin wax for tissue sectioning, which is conducted via a rotary microtome (Leica, Germany). Finally, 5 µm paraffin sections were cut for H&E staining, and 10 µm paraffin sections were cut for immunofluorescent staining. The ease of sectioning was evaluated via rating from a scale of 1 to 5 (1 for poor; 2 for average; 3 for good; 4 for very good; 5 for excellent) by the operator [23].

Table 1.
Protocol for xylene/isopropanol methods on tissue processing.
2.2. H&E Staining and Quality Evaluation
For H&E staining, all the slides were deparaffinized, rehydrated and washed in compliance with standard protocols (Table 2). H&E staining was subsequently conducted using hematoxylin and eosin (Merck Millipore, Darmstadt, Germany) consecutively, which was followed by dehydration and the mounting of slides via Pertex (Histolab, Askim, Sweden) (Table 2). The overnight air-dried slides were observed under light microscope (Olympus Life Sciences, Tokyo, Japan) and bright-field images were acquired for quality evaluation by three experienced observers independently. The three observers were blinded to tissue sources and processing information. Samples were scored from a scale of 0 to 3 (Table 3) in terms of the following aspects: overall quality of tissue, quality of staining, tissue architecture, clarity of nucleus–cytoplasmic differentiation/integrity, and vascular tissue [22].

Table 2.
Protocol for xylene and isopropanol H&E staining sections.

Table 3.
The criteria for scoring tissue sections.
2.3. Fluorescent Staining Effect Evaluation
For fluorescent imaging, slides were deparaffinized, rehydrated and washed according to standard protocols. Fluorescent staining was thereafter conducted via a mixed dye of DAPI (4′,6-diamidino-2-phenylindole, 1:1000, ThermoFisher Scientific, Waltham, MA, USA) and Lycopersicon esculentum lectin (LEA) DyLight488 (1:500, ThermoFisher Scientific, Waltham, MA, USA) diluted in PBS for 30 min (Table 4), which was followed by image acquirement via confocal (Nikon A1RHD, Tokyo, Japan) and Ti2 microscope (Nikon Eclipse, Tokyo, Japan). Alveolar wall thickness was evaluated on confocal images by comparing the ratio of the inner and outer alveolar circumference difference to the outer circumference. Lectin intensity was evaluated on Ti2 images, as the overall lectin intensity of the image adjusted by the entire tissue-covered area. A polygon selection tool was applied along to aforementioned analyses for depiction of lung tissues. For multiplex stains, sections were incubated in a blocking/permeabilization buffer (5% BSA, 5% normal goat serum, 0.5% triton-x-100) at 4 degrees for 45 min. Primary antibodies (SMA 1:500, elastin 1:250) were incubated in PBS at 4 degrees overnight. Secondary antibodies (goat anti-rabbit 568, goat anti-mouse 647) were incubated on sections for 90 min at 4 degrees at a dilution of 1:1000. Finally, sections were incubated with DAPI (1:1000) and lectin (1:500) for 30 min and then washed in PBS solution before mounting with Fluoromount G (Table 4). Bronchioles in sections were then imaged with a confocal (Nikon A1RHD, Japan). To assess SNR, peak fluorescent values were divided by the averaged background signal.

Table 4.
Protocol for fluorescent staining sections.
2.4. Calculations and Statistics
Data collection was conducted via Microsoft Excel 2023. Image analysis for fluorescent staining was conducted via Fiji ImageJ (Version: 2.14.0/1.54f). Normally distributed variables were reported as mean and standard deviation (SD) and were assessed via Student’s t-test for statistical significance. A Wilcoxon signed-rank test was conducted for statistical significance assessment for categorical data and numeric data failing to pass a normal distribution test. All statistical analysis was conducted using GraphPad Prism 9.1. Statistical significance was defined as p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), or p > 0.05 (not significant).
3. Results
3.1. Ease of Sectioning
As tissue processing factors may impact tissue hardness and thus hinder section quality, the ease of sectioning was compared within a total of 24 tissue blocks processed via either xylene or isopropanol. As presented in Table 5, the ease of sectioning paraffin blocks processed via xylene and isopropanol were scored 4.167 ± 0.577 and 4.083 ± 0.669, respectively, according to the previously mentioned protocols [23]. No statistically significant difference was identified, supporting equivalent effects on tissue hardness or other factors correlating to the ease of tissue sectioning lung tissue despite the utilization of different clearing reagents.

Table 5.
Ease of sectioning.
3.2. H&E Histological Observation
The general morphological features of lung tissue processed via xylene and isopropanol were observed utilizing brightfield microscopy for slides stained with H&E (Figure 1). In H&E slides processed via both xylene and isopropanol, properly constructed mesh architecture could be clearly observed with flat-shaped epithelial cells neatly lining up the alveolar walls. Surrounding the alveoli, capillaries were observable, and the interstitium was typically thin. Extensive observation of H&E staining slides did not yield naked-eye recognizable differences between overall readouts of tissue processed via the two methods.
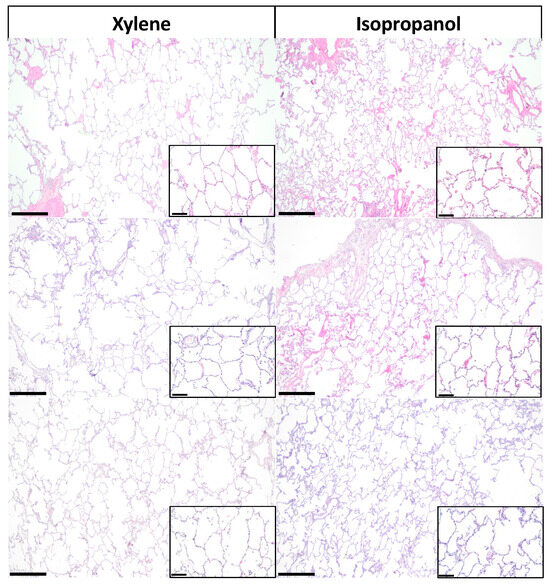
Figure 1.
Hematoxylin and eosin staining images of lung tissue in xylene and isopropanol. Representative images of hematoxylin and eosin (H&E) staining of lung tissue demonstrate the staining effects of xylene (left) and isopropanol (right). Scale bar in the larger image represents 500 µm, and the callout shows a magnified portion of the tissue where the bar represents 100 µm.
3.3. H&E Histological Evaluation
Given the aforementioned identical features in gross histology observation, intensive histological evaluation concerning more detailed aspects of tissue morphology were then conducted. Donor and recipient lung slides processed via xylene and isopropanol and stained with H&E were evaluated and scored by three independent observers blinded to slide classification and the processing method. Tissues were evaluated and scored with regard to overall quality of tissue (Figure 2a), quality of staining (Figure 2b), tissue architecture (Figure 2c), clarity of nucleus–cytoplasmic differentiation/integrity (Figure 2d), and vascular tissue (Figure 2e) in accordance with the pre-discussed protocol, as illustrated in Table 3 [22]. As demonstrated by corresponding statistical analysis between slides processed via two different methods, no significant difference was observed in terms of any of the aspects previously described (Table 6), thus indicating comparable readouts given the replacement of xylene with isopropanol in lung H&E histological evaluation.
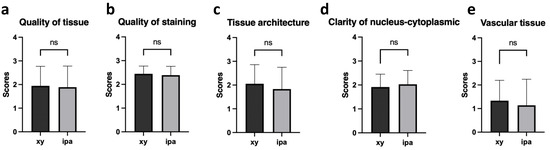
Figure 2.
Scoring results of H&E staining characteristics in lung tissue sections processed with xylene and isopropanol. Histological analysis of H&E staining was performed on tissue sections obtained from donor and recipient lung biopsies. The scoring results were assessed based on the following criteria: overall quality of tissue (a); quality of staining (b); tissue architecture (c); clarity of nucleus–cytoplasmic differentiation/integrity (d); vascular tissue (e). n = 12. ns: nonsignificant (p > 0.05).

Table 6.
Comparison of the scoring results of H&E staining characteristic in lung tissue sections processed via xylene and isopropanol methods.
3.4. Automated Immunofluorescence Image Evaluation
For the purpose of generating evaluation and comparison of the effect by xylene and isopropanol in a more objective and quantified manner minimizing risks of human-associated bias, automated immunofluorescent image evaluation on Lycopersicon esculentum lectin (LEA) DyLight488 and 4′,6-diamidino-2-phenylindole (DAPI) stained slides were consequently conducted. Type I alveolar epithelial cells were depicted with green color (produced by LEA) (Figure 3a). Gross assessments in accordance with protocols previously described identified no significant difference between slides undergoing the two methods in terms of alveolar circularity and alveolar wall thickness (% of total alveolar surface area), suggesting statistically identical gross histological morphology structure features in fluorescent staining regardless of whether xylene or isopropanol was used as the clearing reagent during tissue processing (Figure 3b, p = 0.916; Figure 3c, p = 0.535). An increment in the density of lectin in recipient lung tissue could be clearly observed no matter whether processed via xylene or isopropanol (Figure 3d,e). Statistically speaking, a significant elevation in lectin intensity was identified in the recipient tissues regardless of tissue processing reagents applied (Figure 3h, p = 0.0015, Figure 3i, p < 0.0001), and such significance was seemingly maintained even when tissues processed via the two methods were combined to form pooled donor and recipient groups for comparison (Figure 3j, p < 0.0001). Moreover, no significant differences were identified regarding lectin intensity between donor and recipient slides processed via xylene and isopropanol (Figure 3f, p = 0.744; Figure 3g, p = 0.780), providing favorable evidence supporting a comparable presentation power for image readouts yielded by xylene and isopropanol.
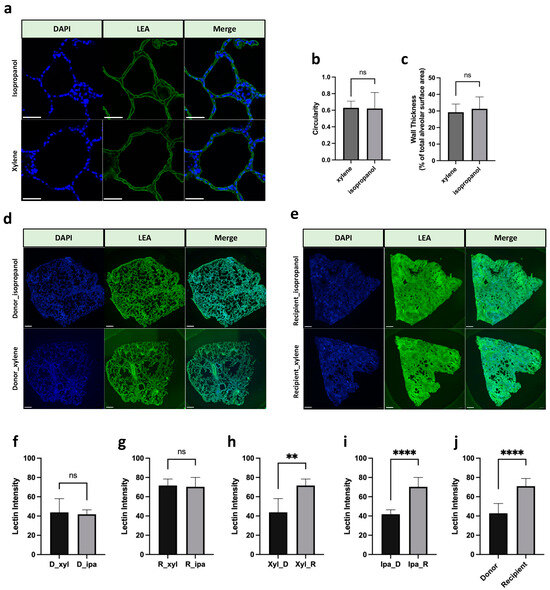
Figure 3.
Fluorescent staining of lung tissue in xylene and isopropanol. (a) Representative images of fluorescent staining of LEA (green, 1:500) and DAPI (blue, 1:1000) staining in lung slides. Scale bar represents 50 µm. (b,c) Quantification of alveolar morphology metrics at xylene and isopropanol groups, ns: nonsignificant (p > 0.05). (d,e) Representative fluorescent staining images by DAPI and LEA. Scale bar in the pictures represents 1000 μm. (f) Lectin intensity of donor lung slides (xylene vs. isopropanol). (g) Lectin intensity of recipient lung slides (xylene vs. isopropanol). (h) Lectin intensity of xylene processed slides (donor vs. recipient). (i) Lectin intensity of isopropanol processed slides (donor vs. recipient). (j) Lectin intensity of both xylene and isopropanol processed slides combined (donor vs. recipient). n = 12. p > 0.05; ** p < 0.01, **** p < 0.0001. ns: nonsignificant. D, donor; R, recipient; Xyl, xylene; Ipa, isopropanol. DAPI, 4′,6-diamidino-2-phenylindole; LEA, Lycopersicon esculentum lectin.
3.5. Multiplex Staining Evaluation
To explore whether tissue clearing with xylene or isopropanol had any impact on antibody labeling and the subsequent fluorescence signal, tissue sections were stained with primary antibodies against smooth muscle actin (SMA) and elastin (Figure 4a,b). To assess staining efficacy, we analyzed the signal-to-noise ratio (SNR) of the secondary antibody–fluorophore conjugate, which was bound to the target primary antibodies. The signal from both SMA, with a 568-secondary conjugate, and elastin, with a 647-secondary conjugate, revealed comparable SNR between clearing methods (Figure 4c,d). Taken together, these data convey that tissue clearing with xylene or isopropanol yields similar immunofluorescence staining efficacy with regard to the protein–conjugate pairs tested.
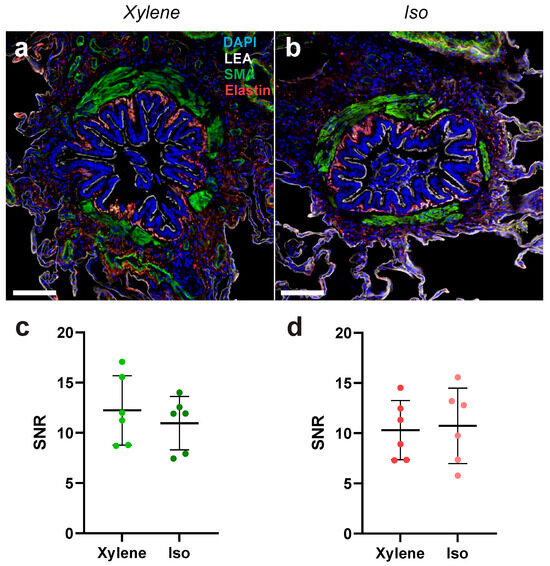
Figure 4.
Multiplex antibody labeling efficacy of lung tissue in xylene and isopropanol clearing. (a) Representative images of bronchioles stained for SMA and elastin, as well as lectin and DAPI, from a tissue sample cleared with xylene. (b) Representative images of bronchioles stained for SMA and elastin, as well as lectin and DAPI, from a tissue sample cleared with isopropanol. Scale bar in the pictures represents 100 μm. (c) Quantification of the SNR between xylene and isopropanol tissue clearing for an SMA primary antibody stain with a 568-secondary conjugate. (d) Quantification of the SNR between xylene and isopropanol tissue clearing for an elastin primary antibody stain with a 647-secondary conjugate. n = 6. DAPI, 4′,6-diamidino-2-phenylindole; LEA, Lycopersicon esculentum lectin; SMA, smooth muscle actin. SNR, signal-to-noise ratio.
4. Discussion
The primary purpose of tissue processing is to efficiently replace water content present in the tissues with another medium, enhancing tissue support and solidification [24]. In histology laboratories, the most frequently utilized clearing reagent for tissue processing is xylene, as it facilitates paraffin infiltration [2,25]. Nevertheless, it is well established that xylene poses potential hazards to the health of researchers and the environment [18,26]. There is sufficient evidence supporting significant toxicity with exposure, which has widely provoked the desire for optimization [17,18,27]. Efforts to achieve this purpose led to proposals for several clearing agents of less toxicity, better environmental friendliness, and higher cost-effectiveness, like isopropanol, which facilitates paraffin infiltration and the procedures thereafter. This study confirmed that the staining and sectioning of the tissue processed via isopropanol were comparable to that via xylene in human lung tissue samples [28,29,30,31,32]. Previous studies have been conducted comparing various tissue processing reagents, yet few were conducted in a systematic manner with objective evaluation methods, which was particularly notable in the context of lung tissue [32,33]. In light of this, our study employed a systematic comparison of the staining effects of xylene and isopropanol on lung tissue, adopting both H&E and immunofluorescent staining methods in our research.
Both blinded observation and the evaluation of H&E staining indicated the comparable overall quality of slides processed via xylene and isopropanol. This is in keeping with previous studies conducted by Metgud et al. [22] and Adediran et al. [32] who both identified no significant difference between slides processed via xylene and isopropanol in kidney, liver, stomach and adenocarcinoma samples; however, their studies lacked systematic evaluation as well as the inclusion of lung tissue. Our study employed routine lung tissue assessments for a more comprehensive and thorough comparison of the possible different effects the two processing methods might introduce to final readouts. Although seemingly comprehensive, popularly applied H&E histological evaluations are mainly based on subjective notions relating to observer knowledge and proficiency, producing risks of introducing potential and inevitable bias especially if implemented alone as in some of the previous studies. Therefore, the incorporation of fluorescent staining, both molecular and antibody-based, coupled with automated confocal and Ti2 microscopic imaging and analysis, was conducted here to diminish human-based limitations.
Automated evaluations yielded comparable readouts of slides of the same origin (donor-originated or recipient originated) regarding alveolar circularity and the percentage of alveolar surface area, lectin intensity and SNR of SMA and elastin, whilst the only significant differences were identified between slides of different origins (donor vs. recipient), demonstrating the attributability of such differences to tissue origin (patient factors) instead of alteration of reagents and thus indicating the comparable effects of xylene and isopropanol. Our study indicates a comparable staining effect on LEA, a histochemical marker for type I alveolar epithelial cells, irrespective of whether the tissues were processed via xylene or isopropanol [34,35,36]. Furthermore, in the current study, we show that there is no significant difference in the SNR in SMA and elastin antibody stains between xylene and isopropanol. This suggests that these antigen epitopes in lung tissue remain unaffected regardless of whether the lung tissue is processed via either of these two reagents. The ease of tissue sectioning was evaluated for the tissues processed via xylene and isopropanol, revealing no difference in these two groups. Furthermore, this implies the comparable effects of these two clearing reagents on the quality of paraffinization and embedding before the sectioning process. As an alternate clearing agent, previous studies have also investigated the viability of coconut oil as a potential alternative for xylene in tissue processing [23,33,37]. These studies found no difference in the quality of staining effects, whereas a significantly higher difficulty during the sectioning of coconut oil-processed tissue was observed, thereby limiting the potential of coconut oil as a replacement for xylene [33].
In the process of tissue processing, substantial quantities of xylene are often required for dehydrating and clearing tissues. For instance, our group approximately consumed a total of 43.2 L xylene per year in the large animal experiment. This large volume of xylene must then be safely discarded, as the inappropriate disposal of waste can result in a detrimental environmental pollutant, posing potential hazards to human indirectly [38,39]. Therefore, finding and using alternatives to xylene for tissue processing in histological research has a profoundly positive impact on health and the environment. Due to its widespread application and the potential for adverse effects resulting from exposure to xylene, it is currently listed in the European Chemical Agency’s “Community Rolling Action Plan (CoRAP)” [40]. Studies show that short-term exposure to xylene results in irritation of the respiratory mucosa, which may lead to lung edema [41]. Furthermore, the symptoms of acute dermal exposure to xylene includes skin erythema, dryness, and scaling [42], whereas prolonged exposure to low concentrations of xylene may lead to hazardous effects on the respiratory and cardiovascular system, central nervous system, digestive system, reproductive system, and renal system, eventually resulting in chronic, long-term health problems [13,14,41,42,43,44]. Consequently, in consideration of user safety, isopropanol emerges as a less toxic and harmful alternative for the human body. It proves to be more environmentally sustainable, economically friendly, and affordable [45].
It is noteworthy that technological advancements have ushered in the standardization of automated tissue processors in most laboratories, addressing the need to reduce operators’ exposure to chemical reagents. This development not only markedly enhances the efficiency of experiments but also aligns with the broader objective of minimizing potential bio-hazardous effects associated with chemical reagents. Moreover, the consideration of isopropanol as a viable alternative clearing reagent to replace xylene reflects a strategic move toward safer practices. The integration of automated equipment, coupled with the adoption of more environmentally friendly reagents, positions laboratories to establish a healthy and efficient working environment for operators, thereby mitigating the potential bio-hazardous effects associated with chemical reagents and ensuring the well-being of laboratory personnel.
5. Conclusions
The results of this study revealed that the fluorescence staining effects and the ease of sectioning of lung tissue processed with both xylene and isopropanol are comparable. Given the consideration of the aspects of safety, health, environmental friendliness, and cost-effectiveness of isopropanol, and the health hazards, potential environmental pollutant risks, higher costs, and other undesired adverse aspects of xylene, isopropanol due to its favorable attributes can therefore be a prioritized option for tissue-clearing reagent in lung tissue processing. The future value of this study can provide us with a reasonable and reliable reference for choosing tissue-clearing reagents particularly in processing lung tissue.
Author Contributions
Conceptualization, Q.W., N.B.B. and S.L.; Methodology, Q.W., N.B.B., F.O. and R.G.; Formal Analysis, Q.W. and N.B.B.; Writing—Original Draft Preparation, Review and Editing, all authors; Project Administration, Q.W., N.B.B. and S.L.; Funding Acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Wallenberg Molecule Medicine Fellowship, Knut and Alice Wallenberg Foundation, ALF Foundation, and the Wallenberg Clinical Fellowship from the Marianne and Marcus Wallenberg Foundation.
Institutional Review Board Statement
Lung tissue obtained from diseased donors and lung tissue from explanted lungs from lung transplant recipients were used. Ethical approval was granted by Swedish National Ethics Committee (Dnr 2021-00016 (Date: 27 January 2021), Dnr 2017/396, 2018/845, 2022-05028-02 (Date: 13 June 2017 and 16 October 2018 and 14 February 2023)).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge funding received from the Wallenberg Molecular Medicine Foundation and Lund University Bioimaging Centre (LBIC) for access to the Nikon A1RHD.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gurina, T.S.; Simms, L. Histology, Staining; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aziz, S.J.; Zeman-Pocrnich, C.E. Tissue Processing. In Immunohistochemistry and Immunocytochemistry: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2422, pp. 47–63. [Google Scholar] [CrossRef]
- Ganjali, H. Tissue processing: An Overview. Sch. Res. Libr. Ann. Biol. Res. 2012, 3, 5374–5378. Available online: https://www.scholarsresearchlibrary.com/articles/tissue-processing-an-overview.pdf (accessed on 15 February 2024).
- Buesa, R.J.; Peshkov, M.V. Histology without xylene. Ann. Diagn. Pathol. 2009, 13, 246–256. [Google Scholar] [CrossRef]
- Chaudhari, K.; Chattopadhyay, A.; Dutta, S.K. Microwave technique in histopathology and its comparison with the conventional technique. Indian J. Pathol. Microbiol. 2000, 43, 387–394. [Google Scholar]
- Kandyala, R.; Raghavendra, S.P.; Rajasekharan, S. Xylene: An overview of its health hazards and preventive measures. J. Oral Maxillofac. Pathol. 2010, 14, 1. [Google Scholar] [CrossRef]
- Freida, L.; Carson, C.H.C. Histotechnology. A Self-Instructional Text, 5th ed.; American Society for Clinical Pathology(ASCP) Press: Chicago, IL, USA, 2020. [Google Scholar]
- Pandey, P.; Dixit, A.; Tanwar, A.; Sharma, A.; Mittal, S. A comparative study to evaluate liquid dish washing soap as an alternative to xylene and alcohol in deparaffinization and hematoxylin and eosin staining. J. Lab. Physicians 2014, 6, 084–090. [Google Scholar] [CrossRef] [PubMed]
- Zoveidavianpoor, M.; Samsuri, A.; Shadizadeh, S.R. Health, Safety, and Environmental Challenges of Xylene in Upstream Petroleum Industry. Energy Environ. 2012, 23, 1339–1352. [Google Scholar] [CrossRef]
- Chang, Y.C.; Fuzisawa, S.; Reddy, M.V.; Kobayashi, H.; Yoshida, E.; Yajima, Y.; Hoshino, T.; Choi, D. Degradation of Toxic Compounds at Low and Medium Temperature Conditions Using Isolated Fungus. Clean 2016, 44, 992–1000. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Li, A. Ecological risk assessment for xylenes and propylbenzenes in aquatic environment using a species sensitivity distribution approach. Ecotoxicol. Environ. Saf. 2023, 261, 115106. [Google Scholar] [CrossRef] [PubMed]
- Hutzinger, O. The Handbook of Environmental Chemistry, Volume 3-Part D: Anthropogenic Compounds; Springer New York Inc.: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Rajan, S.T. Health Hazards of Xylene: A Literature Review. J. Clin. Diagn. Res. 2014, 8, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Nakatsuka, H.; Ukai, H.; Watanabe, T.; Liu, Y.T.; Huang, M.Y.; Wang, Y.L.; Zhu, F.Z.; Yin, H.; Ikeda, M. Symptoms and signs in workers exposed predominantly to xylenes. Int. Arch. Occup. Environ. Health 1993, 64, 597–605. [Google Scholar] [CrossRef]
- Trujillo, F.; Dang, D.; Starck, T. Xylene Keratopathy. Cornea 2003, 22, 88–90. [Google Scholar] [CrossRef]
- Ansari, E.A. Ocular injury with xylene—A report of two cases. Hum. Exp. Toxicol. 1997, 16, 273–275. [Google Scholar] [CrossRef]
- Šedivec, V.; Flek, J. Exposure test for xylenes. Int. Arch. Occup. Environ. Health 1976, 37, 219–232. [Google Scholar] [CrossRef]
- Niaz, K.; Bahadar, H.; Maqbool, F.; Abdollahi, M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015, 14, 1167–1186. [Google Scholar] [CrossRef]
- Maryadele, J.O. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 1. [Google Scholar]
- Haviv, Y.S.; Safadi, R.; Osin, P. Accidental Isopropyl Alcohol Enema Leading to Coma and Death. Am. J. Gastroenterol. 1998, 93, 850–851. [Google Scholar] [CrossRef]
- Nordmann, R.; Ribiere, C.; Rouach, H.; Beauge, F.; Giudicelli, Y.; Nordmann, J. Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sci. 1973, 13, 919–932. [Google Scholar] [CrossRef]
- Metgud, R.; Astekar, M.; Soni, A.; Naik, S.; Vanishree, M. Conventional xylene and xylene-free methods for routine histopathological preparation of tissue sections. Biotech. Histochem. 2013, 88, 235–241. [Google Scholar] [CrossRef]
- Bordoloi, B.; Jaiswal, R.; Tandon, A.; Jayaswal, A.; Srivastava, A.; Gogoi, N. Evaluation and comparison of the efficacy of coconut oil as a clearing agent with xylene. J. Oral Maxillofac. Pathol. 2022, 26, 72. [Google Scholar] [CrossRef]
- Spencer, L.T.; Bancroft, J.D. Tissue processing. In Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 105–123. [Google Scholar] [CrossRef]
- Shah, A.; Kulkarni, D.; Ingale, Y.; Koshy, A.; Bhagalia, S.; Bomble, N. Kerosene: Contributing agent to xylene as a clearing agent in tissue processing. J. Oral Maxillofac. Pathol. 2017, 21, 367. [Google Scholar] [CrossRef]
- Kapp Jr, R.W.; Bevan, C.; Gardiner, T.H.; Banton, M.I.; Tyler, T.R.; Wright, G.A. Isopropanol: Summary of TSCA Test Rule Studies and Relevance to Hazard Identification. Regul. Toxicol. Pharmacol. 1996, 23, 183–192. [Google Scholar] [CrossRef]
- Thetkathuek, A.; Jaidee, W.; Saowakhontha, S.; Ekburanawat, W. Neuropsychological Symptoms among Workers Exposed to Toluene and Xylene in Two Paint Manufacturing Factories in Eastern Thailand. Adv. Prev. Med. 2015, 2015, 183728. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, L.J. Isopropanol poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef]
- Ashurst, J.V.; Nappe, T.M. Isopropanol Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Viktorov, I.V.; Proshin, S.S. Use of Isopropyl Alcohol in Histological Assays: Dehydration of Tissue, Enbessing into Paraffin, and Processing of Paraffin Sections. Bull. Exp. Biol. Med. 2003, 136, 105–106. [Google Scholar] [CrossRef]
- Bettington, A. Xylene-free processing using isopropanol. Pathology 2011, 43, S68. [Google Scholar] [CrossRef]
- Adediran OAI, D.E. Evaluation of isopropanol as a better alternative to xylene in tissue processing by the paraffin wax method. Afr. J. Cell Pathol. 2016, 6, 74–79. [Google Scholar]
- Chandraker, R.; C.Rathod, V.; Chandraker, N.K.; Pundir, S.; Dixit, S.; Desai, V. Comparison Between Xylene and Coconut Oil in Tissue Processing. Mod. Med. Lab. J. 2019, 2, 96–99. [Google Scholar] [CrossRef]
- Kasper, M.; Singh, G. Epithelial lung cell marker: Current tools for cell typing. Histol. Histopathol. 1995, 10, 155–169. [Google Scholar]
- Togami, K.; Ozaki, H.; Yumita, Y.; Kitayama, A.; Tada, H.; Chono, S. Three-Dimensional Imaging of Pulmonary Fibrotic Foci at the Alveolar Scale Using Tissue-Clearing Treatment with Staining Techniques of Extracellular Matrix. Int. J. Biomed. Imaging 2020, 2020, 8815231. [Google Scholar] [CrossRef]
- Barkhordari, A.; Stoddart, R.W.; McClure, S.F.; McClure, J. Lectin Histochemistry of Normal Human Lung. J. Mol. Histol. 2003, 35, 147–156. [Google Scholar] [CrossRef]
- Sermadi, W.; Prabhu, S.; Acharya, S.; Javali, S. Comparing the efficacy of coconut oil and xylene as a clearing agent in the histopathology laboratory. J. Oral Maxillofac. Pathol. 2014, 18, 49. [Google Scholar] [CrossRef]
- Hernandes, E.; Pawloski Schoffen, R.; Conte, H. Xylene: Features, Risks and Management of Waste. Braz. J. Surg. Clin. Res. 2017, 17, 68–73. [Google Scholar]
- Giger, W.; Schaffner, C. Groundwater Pollution by Volatile Organic Chemicals. In Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1981; Volume 17, pp. 517–522. [Google Scholar] [CrossRef]
- Rocha, A.C.S.; Reis-Henriques, M.A.; Galhano, V.; Ferreira, M.; Guimarães, L. Toxicity of seven priority hazardous and noxious substances (HNSs) to marine organisms: Current status, knowledge gaps and recommendations for future research. Sci. Total Environ. 2016, 542, 728–749. [Google Scholar] [CrossRef] [PubMed]
- Morley, R.; Eccleston, D.W.; Douglas, C.P.; Greville, W.E.J.; Scott, D.J.; Anderson, J. Xylene Poisoning: A Report on One Fatal Case and Two Cases of Recovery after Prolonged Unconsciousness. BMJ 1970, 3, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Husman, K.; Engström, K.; Riihimäki, V. Percutaneous absorption of m-xylene in man. Int. Arch. Occup. Environ. Health 1977, 39, 181–189. [Google Scholar] [CrossRef]
- Reutman, S.R.; LeMasters, G.K.; Knecht, E.A.; Shukla, R.; Lockey, J.E.; Burroughs, G.E.; Kesner, J.S. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ. Health Perspect. 2002, 110, 805–811. [Google Scholar] [CrossRef]
- Franchini, I.; Cavatorta, A.; Falzoi, M.; Lucertini, S.; Mutti, A. Early indicators of renal damage in workers exposed to organic solvents. Int. Arch. Occup. Environ. Health 1983, 52, 1–9. [Google Scholar] [CrossRef]
- Rj, B.; Mv, P. Complete elimination of xylene in practice of a histology laboratory. Arkh Patol. 2011, 73, 54–60. Available online: https://api.semanticscholar.org/CorpusID:68033502 (accessed on 15 February 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).