Winter Carbon Dioxide Measurement in Honeybee Hives
Abstract
1. Introduction
2. Materials and Methods
2.1. The Hives
2.2. Carbon Dioxide Sensor Electronics
2.3. Vibration Measurements
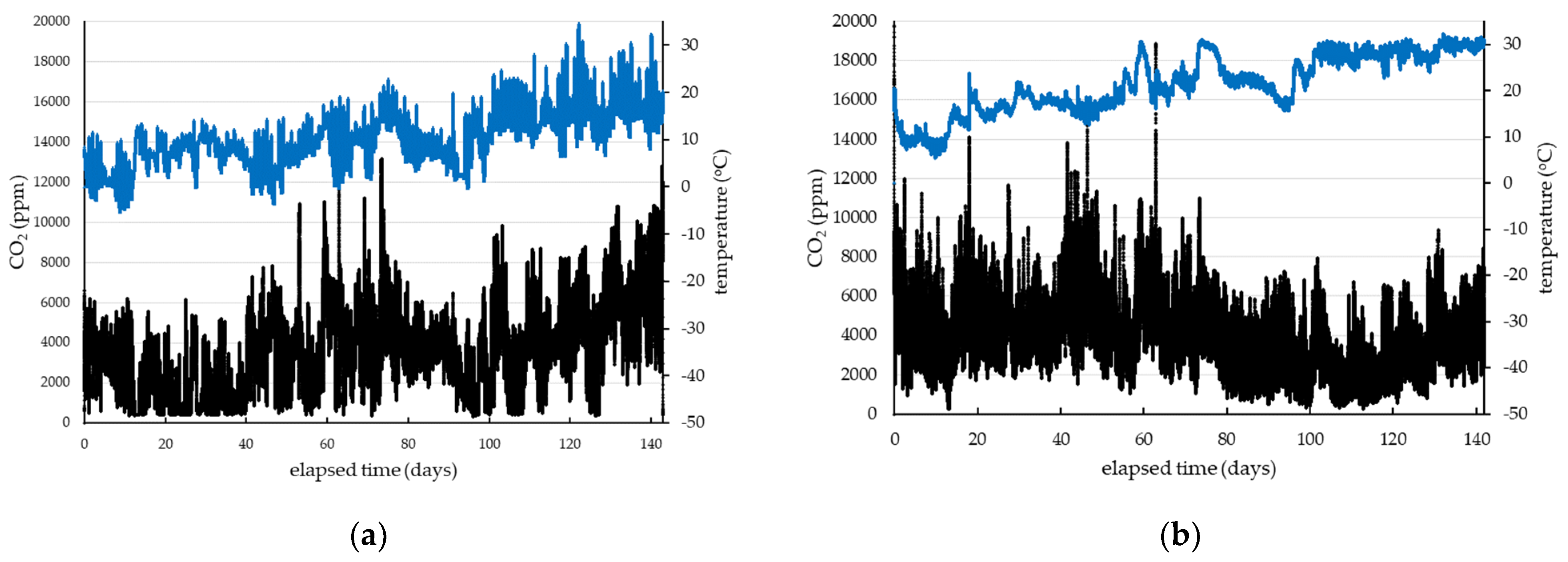
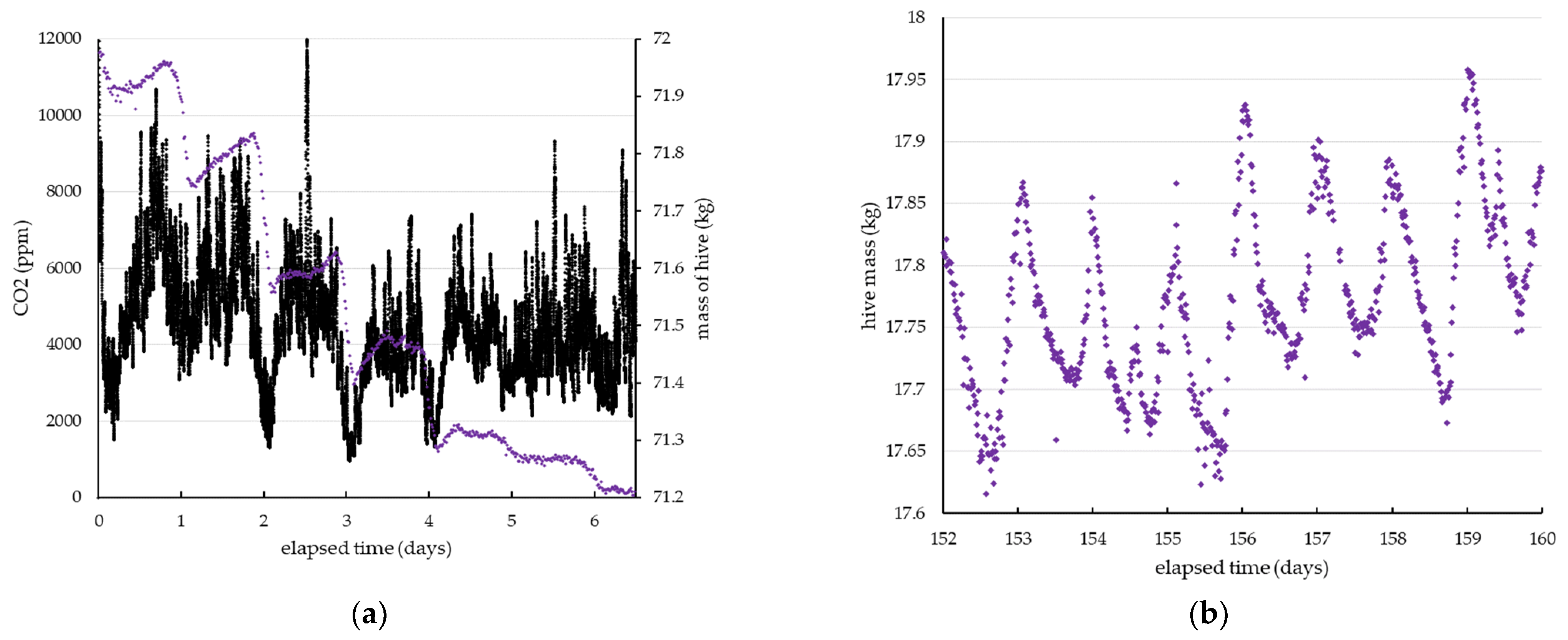
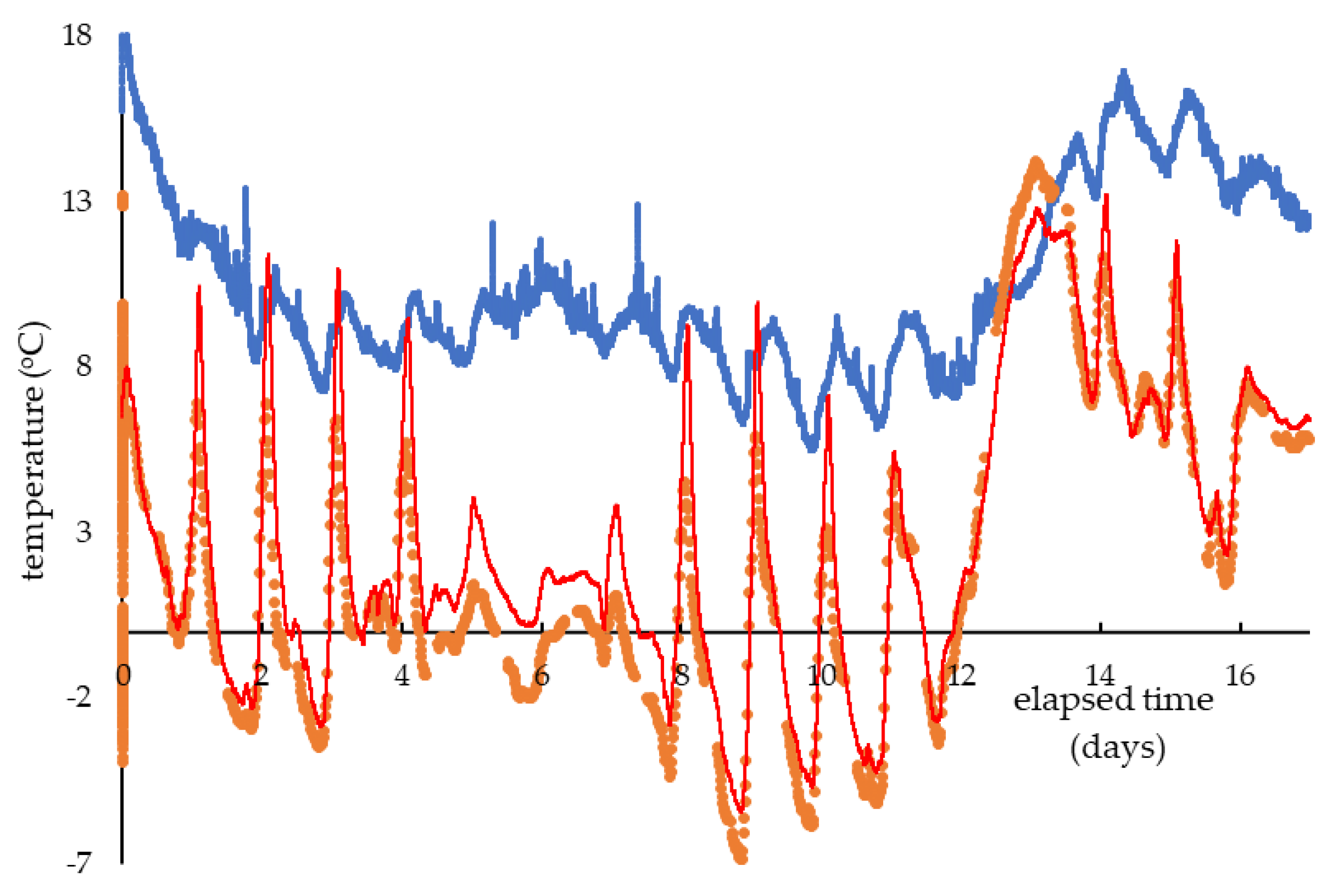
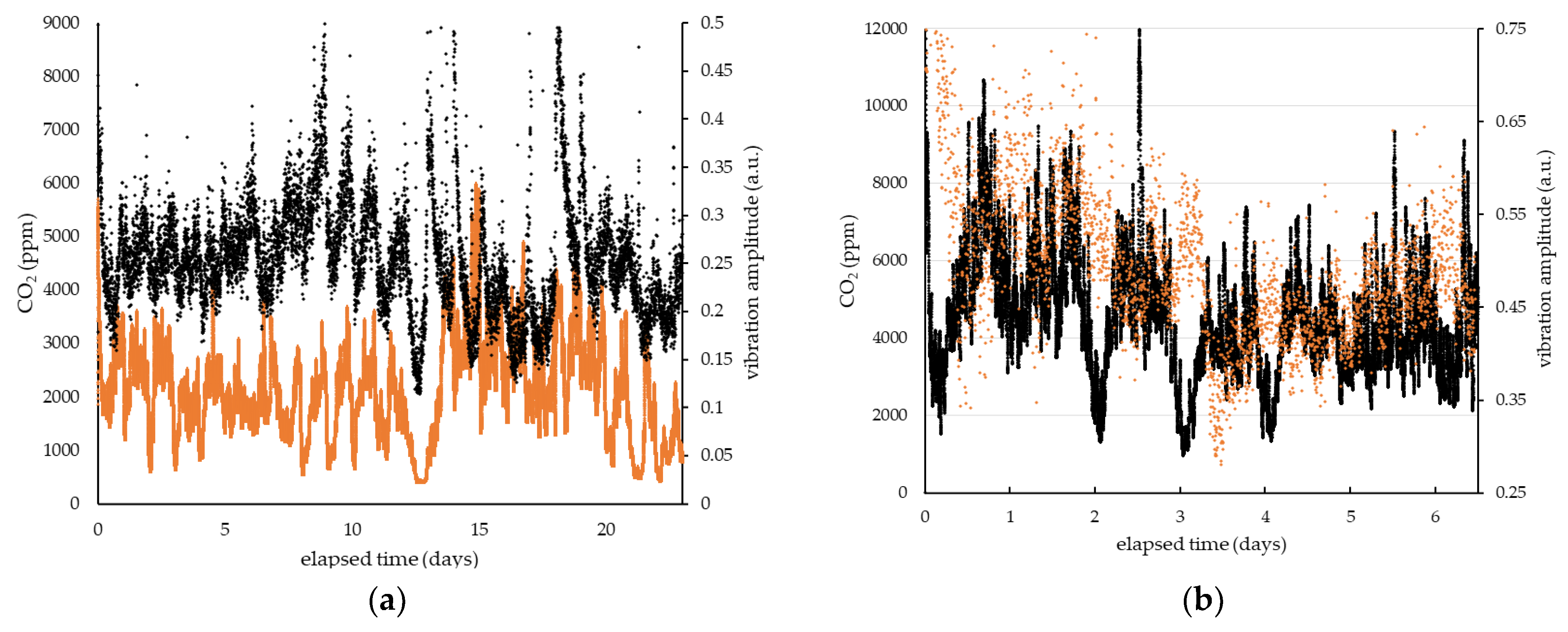
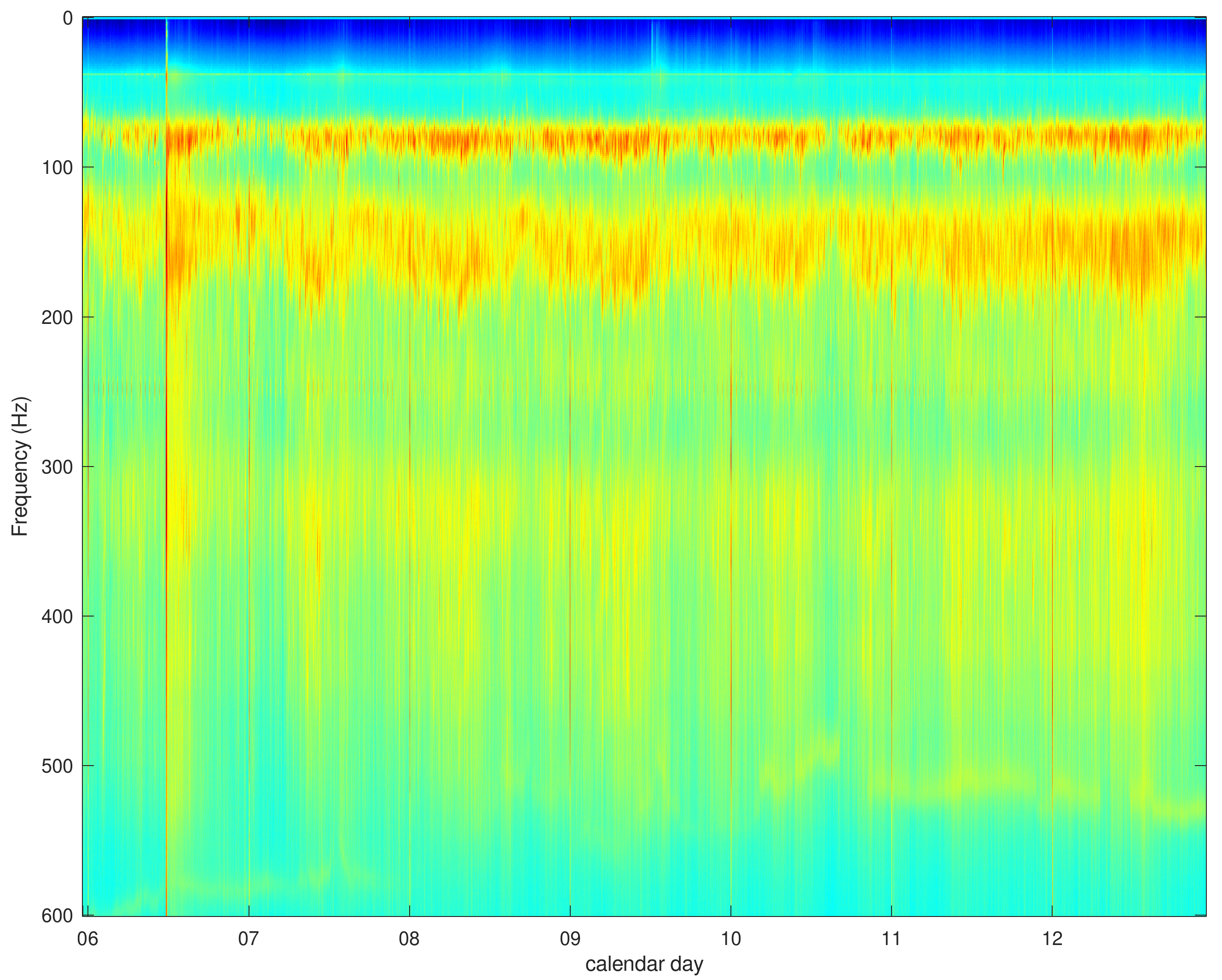
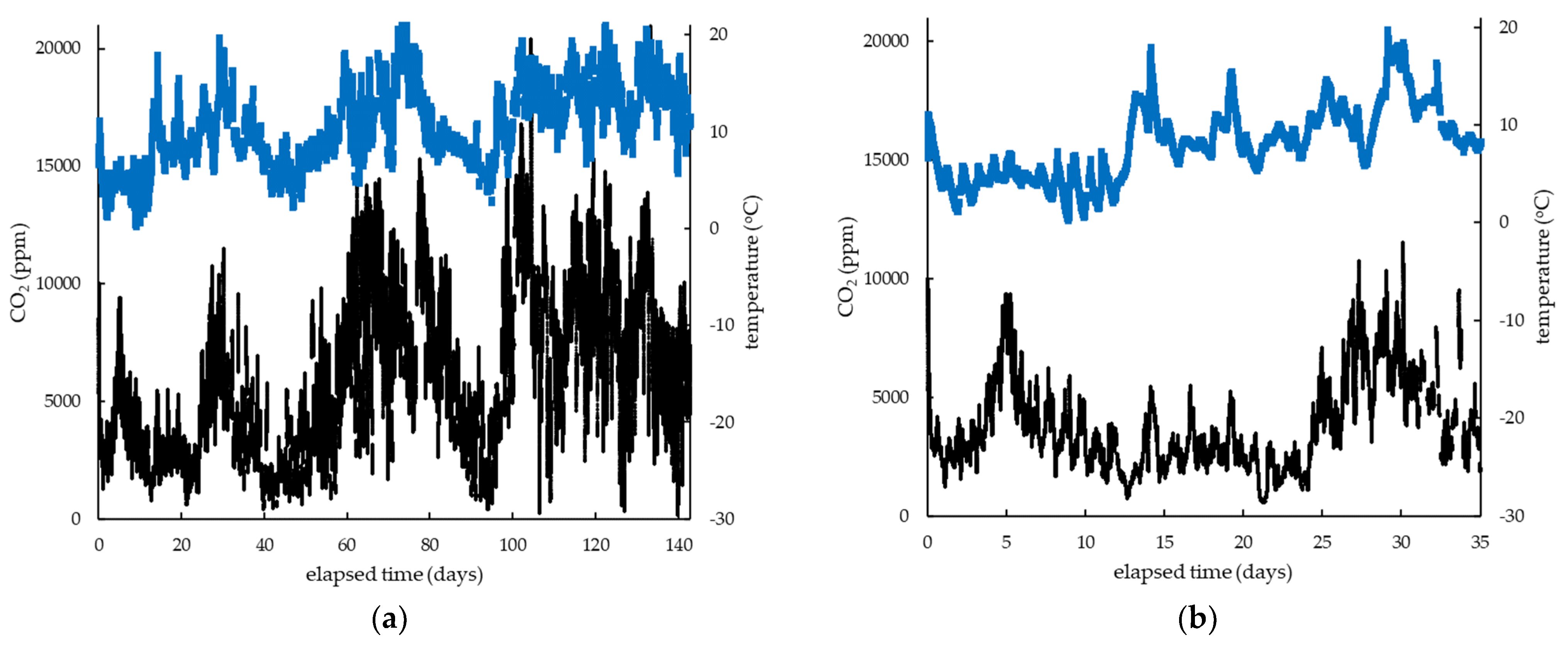
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissiere, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2010; Available online: https://www.cabidigitallibrary.org/doi/book/10.1079/9780851994482.0000 (accessed on 22 January 2024).
- Kaplan, J.K. Colony Collapse Disorder: A Complex Buzz. Agric. Res. 2008, 56, 8–11. Available online: https://agresearchmag.ars.usda.gov/ar/archive/2008/may/colony0508.pdf (accessed on 22 January 2024).
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; A Brown, M.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honeybees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Ellis, J.D.; Evans, J.D.; Pettis, J.S. Colony losses, managed colony population decline and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Gallai, N.; Garibaldi, L.A.; Kuhlmann, M.; Klein, A.M. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl. Ecol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Pettis, J.S.; Delaplane, K.S. Coordinated responses to honey bee decline in the USA. Apidologie 2010, 41, 256–263. [Google Scholar] [CrossRef]
- Grzeda, U.; Gajda, A.; Topolska, G. Chronic bee paralysis and sacbrood: Virus infections not associated with other bee diseases. Vet. Med.-Sci. Pract. 2014, 70, 729–734. [Google Scholar]
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef]
- Forsgren, E. European Foulbrood in honeybees. J. Invertebr. Pathol. 2010, 103, S5–S9. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honeybees. J. Invertebr. Pathol. 2010, 103, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Sammataro, D.; Gerson, U.; Needham, G. Parasitic mites of honeybees: Life History, Implications, and Impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Imdorf, A.; Gerig, L. Course in Determination of Colony Strength; Swiss Bee Research Centre, Swiss Federal Dairy Research Institute: Liebefeld, Switzerland, 2001; CH-3003 Bern. [Google Scholar]
- Meikle, W.G.; Rector, B.G.; Mercadier, G.; Holst, N. Within-day variation in continuous hive weight data as a measure of honey bee colony activity. Apidologie 2008, 39, 694–707. [Google Scholar] [CrossRef]
- Meikle, W.G.; Weiss, M.; Stilwell, A.R. Monitoring colony phenology using within-day variability in continuous weight and temperature of honey bee hives. Apidologie 2016, 47, 1–14. [Google Scholar] [CrossRef]
- Cook, D.; Tarlinton, B.; McGree, J.M.; Blackler, A.; Hauxwell, C. Temperature Sensing and Honey Bee Colony Strength. J. Econ. Entomol. 2022, 115, 715–723. [Google Scholar] [CrossRef]
- Czekońska, K.; Łopuch, S.; Miścicki, S.; Bańkowski, J.; Szabla, K. Monitoring of hive weight changes in various landscapes. Apidologie 2023, 54, 30. [Google Scholar] [CrossRef]
- Arias-Calluari, K.; Colin, T.; Latty, T.; Myerscough, M.; Altmann, E.G. Modelling daily weight variation in honey bee hives. PLoS Comput. Biol. 2023, 19, e1010880. [Google Scholar] [CrossRef]
- Zacepins, A.; Brusbardis, V.; Meitalovs, J.; Stalidzans, E. Challenges in the development of Precision Beekeeping. Biosyst. Eng. 2015, 130, 60–71. [Google Scholar] [CrossRef]
- Zaman, A.; Dorin, A. A framework for better sensor-based beehive health monitoring. Comput. Electron. Agric. 2023, 210, 107906. [Google Scholar] [CrossRef]
- Cecchi, S.; Terenzi, A.; Orcioni, S.; Spinsante, S.; Primiani, V.M.; Moglie, F.; Ruschioni, S.; Mattei, C.; Riolo, P.; Isidoro, N. Multi-sensor platform for real time measurements of honey bee hive parameters. IOP Conf. Ser. Earth Environ. Sci. 2019, 275, 012016. [Google Scholar] [CrossRef]
- Cecchi, S.; Spinsante, S.; Terenzi, A.; Orcioni, S. A Smart Sensor-Based Measurement System for Advanced Bee Hive Monitoring. Sensors 2020, 20, 2726. [Google Scholar] [CrossRef]
- Available online: https://beehivemonitoring.com/en/ (accessed on 16 January 2024).
- Available online: https://beemate.buzz/ (accessed on 16 January 2024).
- Available online: https://solutionbee.com/ (accessed on 16 January 2024).
- Available online: https://apisprotect.com/technology/ (accessed on 16 January 2024).
- Available online: https://beep.nl/index.php/home-english (accessed on 16 January 2024).
- Kraus, B.; Velthuis, H.H.W. High humidity in the honey bee (Apis mellifera L.) brood nest limits reproduction of the parasitic mite Varroa jacobsoni Oud. Naturwissenschaften 1997, 84, 217–218. [Google Scholar] [CrossRef]
- Ellis, M.B. Homeostasis: Humidity and Water Relations in Honeybee Colonies (Apis mellifera); Magister Scientiae University of Pretoria: Pretoria, South Africa, 2008; Available online: https://repository.up.ac.za/bitstream/handle/2263/28357/dissertation.pdf (accessed on 22 January 2024).
- LiI, X.; Ma, W.; Shen, J.; Long, D.; Feng, Y.; Su, W.; Xu, K.; Du, Y.; Jiang, Y. Tolerance and response of two honeybee species Apis cerana and Apis mellifera to high temperature and relative humidity. PLoS ONE 2019, 14, e0217921. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Shen, J.; Du, Y.; Xu, K.; Jiang, Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019, 184, 109599. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Weiss, M.; Maes, P.W.; Fitz, W.; Snyder, L.A.; Sheehan, T.; Mott, B.M.; Anderson, K.E. Internal hive temperature as a means of monitoring honey bee colony health in a migratory beekeeping operation before and during winter. Apidologie 2017, 48, 666–680. [Google Scholar] [CrossRef]
- Seeley, T.D. Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. J. Insect Physiol. 1974, 20, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.; Kuszewska, K. CO2 narcosis influences the memory of honey bees. J. Apic. Res. 2020, 59, 663–668. [Google Scholar] [CrossRef]
- Meikle, W.G.; Adamczyk, J.J.; Weiss, M.; Ross, J.; Werle, C.; Beren, E. Sublethal concentrations of clothianidin affect honey bee colony growth and hive CO2 concentration. Sci. Rep. 2021, 11, 4364. [Google Scholar] [CrossRef]
- Available online: https://www.amphenol-sensors.com/en/telaire/co2 (accessed on 16 January 2024).
- Available online: https://sensirion.com/products/catalog/?category=CO%E2%82%82 (accessed on 16 January 2024).
- Available online: https://www.figarosensor.com/ (accessed on 16 January 2024).
- Meikle, W.G.; Barg, A.; Weiss, M. Honey bee colonies maintain CO2 and temperature regimes in spite of change in hive ventilation characteristics. Apidologie 2022, 53, 51. [Google Scholar] [CrossRef]
- Bencsik, M.; McVeigh, A.; Tsakonas, C.; Kumar, T.; Chamberlain, L.; Newton, M.I. A Monitoring System for Carbon Dioxide in Honeybee Hives: An Indicator of Colony Health. Sensors 2023, 23, 3588. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, A.; Cecchi, S.; Spinsante, S. On the Importance of the Sound Emitted by Honey Bee Hives. Vet. Sci. 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.; Bencsik, M.; Newton, M.I. Extensive vibrational characterisation and long-term monitoring of honeybee dorso-ventral abdominal vibration signals. Sci. Rep. 2018, 8, 14571. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.-T.; Bencsik, M.; Newton, M.I.; Reyes, M.; Pioz, M.; Crauser, D.; Delso, N.S.; Le Conte, Y. The prediction of swarming in honeybee colonies using vibrational spectra. Sci. Rep. 2020, 10, 9798. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, M.; Newton, M.I. Honey Bee Vibration Monitoring Using the 805M1 Accelerometer. Proceedings 2019, 4, 42. [Google Scholar] [CrossRef]
- Peters, J.M.; Gravish, N.; Combes, S.A. Wings as impellers: Honey bees co-opt flight system to induce nest ventilation and disperse pheromones. J. Exp. Biol. 2017, 220, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. The Honey Bee Colony as a Superorganism. Am. Sci. 1989, 77, 546–555. [Google Scholar]
- Southwick, E.E. The honey bee cluster as a homeothermic superorganism. Camp. Biochem. Physiol. 1983, 75, 641–645. [Google Scholar] [CrossRef]
- Heinrich, B. How the honey bee regulates its body temperature. Bee World 1996, 77, 130–137. [Google Scholar] [CrossRef]
- Harrison, J.M. Roles of individual honeybee workers and drones in colonial thermogenesis. J. Exp. Biol. 1987, 129, 53–61. [Google Scholar] [CrossRef]
- Langstroth, L.L. Bee Hive. U.S. Patent US9300A, 5 October 1852. Available online: https://patentimages.storage.googleapis.com/71/b8/4e/0122ed14d4ec59/US9300.pdf (accessed on 22 January 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newton, M.I.; Chamberlain, L.; McVeigh, A.; Bencsik, M. Winter Carbon Dioxide Measurement in Honeybee Hives. Appl. Sci. 2024, 14, 1679. https://doi.org/10.3390/app14041679
Newton MI, Chamberlain L, McVeigh A, Bencsik M. Winter Carbon Dioxide Measurement in Honeybee Hives. Applied Sciences. 2024; 14(4):1679. https://doi.org/10.3390/app14041679
Chicago/Turabian StyleNewton, Michael I., Luke Chamberlain, Adam McVeigh, and Martin Bencsik. 2024. "Winter Carbon Dioxide Measurement in Honeybee Hives" Applied Sciences 14, no. 4: 1679. https://doi.org/10.3390/app14041679
APA StyleNewton, M. I., Chamberlain, L., McVeigh, A., & Bencsik, M. (2024). Winter Carbon Dioxide Measurement in Honeybee Hives. Applied Sciences, 14(4), 1679. https://doi.org/10.3390/app14041679





