A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy
Abstract
1. Introduction
2. Thermal Signature of Malignancy
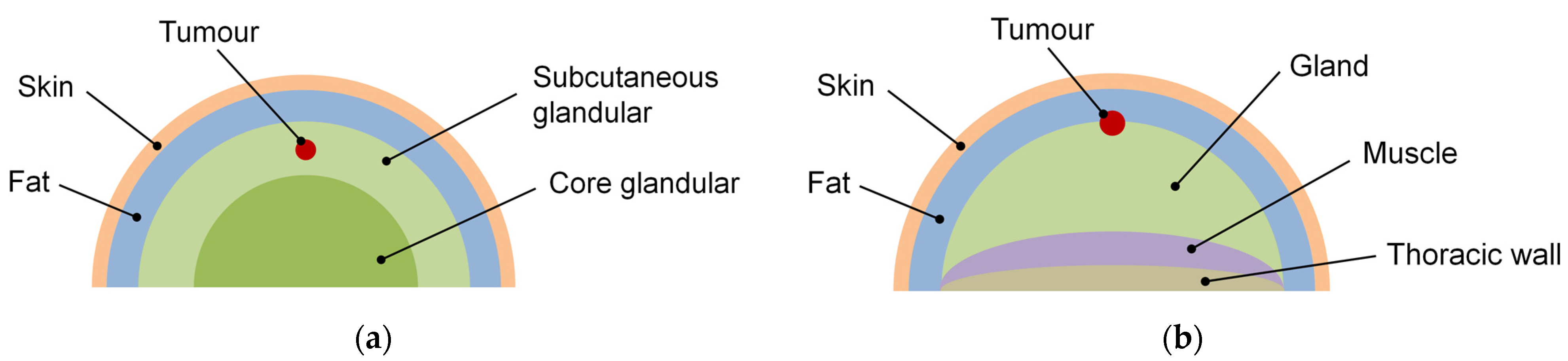
2.1. Skin Cancers
2.2. Breast Cancers
3. Thermographic Techniques for Skin Cancer Diagnosis
3.1. Steady-State Thermography
3.2. Dynamic Infrared Thermography
3.2.1. Cold Stimuli for IR Imaging
3.2.2. Dynamic IR Thermography Using a Cold Provocation
3.2.3. Lock-in Thermography
3.2.4. Thermal Wave Imaging
3.2.5. Literature Survey on Skin Cancer Diagnosis
4. Thermographic Techniques for Breast Cancer Detection
4.1. Steady-State Thermography
4.2. Dynamic Infrared Thermography
4.2.1. Cold Stimulus and Patient Comfort
4.2.2. Dynamic IR Thermography Using a Cold Provocation
4.2.3. Rotational Breast Thermography
4.2.4. Literature Survey on Breast Cancer Diagnosis
5. Skin Tissue Modeling
5.1. Forward Modeling
5.2. Inverse Modeling
6. Mammary Tissue Modeling
6.1. Forward Modeling
6.2. Inverse Modeling
7. Post-Processing Images
7.1. Skin Thermograms
7.2. Breast Thermograms
8. Conclusions
- Steady-state thermography is suited to detecting shallower tumors.
- Dynamic thermography can detect deeper tumors using cold/hot stress.
- Lock-in thermography and frequency-modulated thermal wave imaging represent
- New promising methods for the detection of skin lesions.
- Rotational breast thermography has the potential to overcome the limits of conventional breast thermography by allowing the whole breast surface to be analyzed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund International. Available online: https://www.wcrf.org (accessed on 18 December 2023).
- European Cancer Information System (ECIS). Available online: https://ecis.jrc.ec.europa.eu (accessed on 18 December 2023).
- Herman, C. Emerging technologies for the detection of melanoma: Achieving better outcomes. Clin. Cosmet. Investig. Dermatol. 2012, 5, 195–212. [Google Scholar]
- Akhter, N.; Manza, R.; Shaikh, S.; Gawali, B.; Yannawar, P.; Shaikh, S. Diagnosis of melanoma using thermography: A review. In Proceedings of the International Conference on Applications of Machine Intelligence and Data Analytics (ICAMIDA 2022), Aurangabad, India, 22–24 December 2022. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.K. A review of thermography as promising non-invasive detection modality for breast tumors. Int. J. Therm. Sci. 2009, 48, 849–859. [Google Scholar] [CrossRef]
- Lawson, R. Implications of surface temperatures in the diagnosis of breast cancer. Can. Med. Assoc. J. 1956, 75, 309–310. [Google Scholar] [PubMed]
- Maillard, G.F.; Hessler, C. La thermographie des melanomes malins cutanes. Dermatologica 1969, 139, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Pederson, C.O.; Chato, J.C. On the feasibility of obtaining three dimensional information from thermographic measurements. J. Biomech. Eng. 1977, 99, 58–64. [Google Scholar] [CrossRef]
- Cristofolini, M.; Perani, B.; Piscioli, F.; Recchia, G.; Zumiani, G. Uselessness of thermography for diagnosis and follow-up of cutaneous malignant melanomas. Tumori 1981, 67, 141–143. [Google Scholar] [CrossRef]
- Amalric, R.; Altschuler, C.; Giraud, D.; Spitalier, J.M. Value of infrared thermography in the assessment of malignant melanoma of the skin. In Recent Advances in Medical Thermology; Ring, E.F.J., Phillips, B., Eds.; Springer: Boston, MA, USA, 1984; pp. 623–629. [Google Scholar]
- Di Carlo, A. Thermography and the possibilities for its applications in clinical and experimental dermatology. Clin. Dermatol. 1995, 13, 329–336. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Perez-Raya, I.; Raghupathi, P.A.; Gonzalez-Hernandez, J.L.; Dabydeen, D.; Medeiros, L.; Phatak, P. Infrared imaging technology for breast cancer detection—Current status, protocols and new directions. Int. J. Heat Mass Transf. 2017, 108, 2303–2320. [Google Scholar] [CrossRef]
- Mashekova, A.; Zhao, Y.; Ng, E.Y.K.; Zarikas, V.; Fok, S.C.; Mukhmetov, O. Early detection of the breast cancer using infrared technology—A comprehensive review. Therm. Sci. Eng. Prog. 2022, 27, 101142. [Google Scholar] [CrossRef]
- Verstockt, J.; Verspeek, S.; Thiessen, F.; Tjalma, W.A.; Brochez, L.; Steenackers, G. Skin cancer detection using infrared thermography: Measurement setup, procedure and equipment. Sensors 2022, 22, 3327. [Google Scholar] [CrossRef] [PubMed]
- González, F.J.; Castillo-Martínez, C.; Valdes-Rodríguez, R.; Kolosovas-Machuca, E.S.; Villela-Segura, U.; Moncada, B. Thermal signature of melanoma and non-melanoma skin cancers. In Proceedings of the 11th International Conference on Quantitative InfraRed Thermography QIRT 2012, Naples, Italy, 11–14 June 2012. [Google Scholar]
- Head, J.F.; Wang, F.; Lipari, C.A.; Elliott, R.L. The important role of infrared imaging in breast cancer. IEEE Eng. Med. Biol. 2000, 19, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.A.A.; do Nascimento, J.G.; Malheiros, F.C.; da Silva Ignacio, L.H.; Fernandes, H.C.; Guimaraes, G. Breast tumor localization using skin surface temperatures from a 2D anatomic model without knowledge of the thermophysical properties. Comput. Methods Programs Biomed. 2019, 172, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Yahara, T.; Koga, T.; Yoshida, S.; Nakagawa, S.; Deguchi, H.; Shirouzu, K. Relationship between microvessel density and thermography hot areas in breast cancer. Surg. Today 2003, 33, 243–248. [Google Scholar] [PubMed]
- Francis, S.V.; Sasikala, M.; Bharathi, G.B.; Jaipurkar, S.D. Breast cancer detection in rotational thermography images using texture features. Infrared Phys. Technol. 2014, 67, 490–496. [Google Scholar] [CrossRef]
- Keyserlingk, J.R.; Ahlgren, P.D.; Yu, E.; Belliveau, N. Infrared imaging of the breast: Initial reappraisal using high-resolution digital technology in 100 successive cases of stage I and II breast cancer. Breast J. 1998, 4, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, M.; Gershenson, J. Dynamic vascular imaging using active breast thermography. Sensors 2023, 23, 3012. [Google Scholar] [CrossRef] [PubMed]
- Buzug, T.M.; Schumann, S.; Pfaffmann, L.; Reinhold, U.; Ruhlmann, J. Functional infrared imaging for skin-cancer screening. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBS’06, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Solivetti, F.M.; Desiderio, F.; Guerrisi, A.; Bonadies, A.; Maini, C.L.; Di Filippo, S.; D’Orazi, V.; Sperduti, I.; Di Carlo, A. HF ultrasound vs PET-CT and telethermography in the diagnosis of In-transit metastases from melanoma: A prospective study and review. J. Exp. Clin. Cancer Res. 2014, 33, 96. [Google Scholar] [CrossRef]
- Magalhaes, C.; Vardasca, R.; Rebelo, M.; Valenca-Filipe, R.; Ribeiro, M.; Mendes, J. Distinguishing melanocytic nevi from melanomas using static and dynamic infrared thermal imaging. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1700–1705. [Google Scholar] [CrossRef]
- Flores-Sahagun, J.H.; Vargas, J.V.C.; Mulinari-Brenner, F.A. Analysis and diagnosis of basal cell carcinoma (BCC) via infrared imaging. Infrared Phys. Technol. 2011, 54, 367–378. [Google Scholar] [CrossRef]
- Vargas, J.V.C.; Brioschi, M.L.; Dias, F.G.; Parolin, M.B.; Mulinari-Brenner, F.A.; Ordonez, J.C.; Colman, D. Normalized methodology for medical infrared imaging. Infrared Phys. Technol. 2009, 52, 42–47. [Google Scholar] [CrossRef]
- Shada, A.L.; Dengel, L.T.; Petroni, G.R.; Smolkin, M.E.; Acton, S.; Slingluff, C.L., Jr. Infrared thermography of cutaneous melanoma metastases. J. Surg. Res. 2013, 182, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Vardasca, R.; Esteves, L.; Rebelo, M.; Gabriel, J. Malignant melanoma characterization with thermal and visual imaging. In Infrared Imaging a Casebook in Clinical Medicine; Ring, A., Jung, A., Zuber, J., Eds.; IOP Publishing: Bristol, UK, 2015; pp. 9-1–9-3. [Google Scholar]
- Stringasci, M.D.; Salvio, A.G.; Sbrissa Neto, D.; Vollet-Filho, J.D.; Bagnato, V.S.; Kurachi, C. Discrimination of benign-versus malignant skin lesions by thermographic images using support vector machine classifier. J. Appl. Phys. 2018, 124, 044701-1–044701-8. [Google Scholar] [CrossRef]
- Zenzie, H.H.; Altshuler, G.B.; Smirnov, M.Z.; Anderson, R.R. Evaluation of cooling methods for laser dermatology. Lasers Surg. Med. 2000, 26, 130–144. [Google Scholar] [CrossRef]
- Deng, Z.S.; Liu, J. Enhancement of thermal diagnostics on tumors underneath the skin by induced evaporation. In Proceedings of the 27th Annual Conference of IEEE Engineering in Medicine and Biology, Sanghai, China, 1–4 September 2005. [Google Scholar]
- Cheng, T.Y.; Herman, C. Analysis of skin cooling for quantitative dynamic infrared imaging of near-surface lesions. Int. J. Therm. Sci. 2014, 86, 175–188. [Google Scholar] [CrossRef]
- Gomboc, T.; Iljaž, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. Design of constant temperature cooling device for melanoma screening by dynamic thermography. Eng. Anal. Bound. Elem. 2021, 125, 66–79. [Google Scholar] [CrossRef]
- Verstockt, J.; Thiessen, F.E.F.; Hoorens, I.; Brochez, L.; Steenackers, G. Comparative analysis of cooling methods for dynamic infrared thermography (DIRT)-based skin cancer diagnosis. Appl. Sci. 2023, 13, 10105. [Google Scholar] [CrossRef]
- Verstockt, J.; Somers, R.; Thiessen, F.E.F.; Hoorens, I.; Brochez, L.; Steenackers, G. Finite element skin models as additional data for dynamic infrared thermography on skin lesions. Quant. InfraRed Thermogr. J. 2023. [Google Scholar] [CrossRef]
- Santa Cruz, G.A.; Bertotti, J.; Marin, J.; Gonzalez, S.J.; Gossio, S.; Alvarez, D.; Roth, B.M.C.; Menendez, P.; Pereira, M.D.; Albero, M.; et al. Dynamic infrared imaging of cutaneous melanoma and normal skin in patients treated with BNCT. Appl. Radiat. Isot. 2009, 67, S54–S58. [Google Scholar] [CrossRef]
- Cetingul, M.P.; Herman, C. The assessment of melanoma risk using the dynamic infrared imaging technique. J. Therm. Sci. Eng. Appl. 2011, 3, 031006-1–031006-9. [Google Scholar] [CrossRef]
- Di Carlo, A.; Elia, F.; Desiderio, F.; Catricalà, C.; Solivetti, F.M.; Laino, L. Can video thermography improve differential diagnosis and therapy between basal cell carcinoma and actinic keratosis? Dermatol. Ther. 2014, 27, 290–297. [Google Scholar] [CrossRef]
- Baek, Y.S.; Kim, A.; Seo, J.Y.; Jeon, J.; Oh, C.H.; Kim, J. Dynamic thermal imaging for pigmented basal cell carcinoma and seborrheic keratosis. Int. J. Hyperth. 2021, 38, 1462–1468. [Google Scholar] [CrossRef]
- Godoy, S.E.; Ramirez, D.A.; Myers, S.A.; von Winckel, G.; Krishna, S.; Berwick, M.; Padilla, R.S.; Sen, P.; Krishna, S. Dynamic infrared imaging for skin cancer screening. Infrared Phys. Technol. 2015, 70, 147–152. [Google Scholar] [CrossRef]
- Laino, L.; Elia, F.; Desiderio, F.; Scarabello, A.; Sperduti, I.; Cota, C.; Di Carlo, A. The efficacy of a photolyase-based device on the cancerization field: A clinical and thermographic study. J. Exp. Clin. Cancer Res. 2015, 34, 84. [Google Scholar] [CrossRef] [PubMed]
- Cholewka, A.; Stanek, A.; Kwiatek, S.; Cholewka, A.; Cieslar, G.; Straszak, D.; Gibinska, J.; Sieron-Stołtny, K. Proposal of thermal imaging application in photodynamic therapy—Preliminary report. Photodiagn. Photodyn. Ther. 2016, 14, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bonmarin, M.; Le Gal, F.-A. A lock-in thermal imaging setup for dermatological applications. Ski. Res. Technol. 2015, 21, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Repaka, R.; Mulaveesala, R.; Mishra, S.C. Suitability of frequency modulated thermal wave imaging for skin cancer detection—A theoretical prediction. J. Therm. Biol. 2015, 51, 65–82. [Google Scholar] [CrossRef]
- Ohashi, Y.; Uchida, I. Applying dynamic thermography in the diagnosis of breast cancer. IEEE Eng. Med. Biol. 2000, 19, 42–51. [Google Scholar] [CrossRef]
- Vreugdenburg, T.D.; Willis, C.D.; Mundy, L.; Hiller, J.E. A systematic review of elastography, electrical impedance scanning, and digital infrared thermography for breast cancer screening and diagnosis. Breast Cancer Res. Treat. 2013, 137, 665–676. [Google Scholar] [CrossRef]
- Ng, E.Y.K.; Chen, Y.; Ung, L.N. Computerized breast thermography: Study of image segmentation and temperature cyclic variations. J. Med. Eng. Technol. 2001, 25, 12–16. [Google Scholar]
- Zeng, J.; Lin, L.; Deng, F. Infrared thermal imaging as a nonradiation method for detecting thermal expression characteristics in normal female breasts in China. Infrared Phys. Technol. 2020, 104, 103125. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, K.J.; Chen, C.Y.; Chien, K.L.; Tsai, Y.S.; Wu, Y.M.; Teng, Y.C.; Shih, T.T.F. Evaluation of the diagnostic performance of infrared imaging of the breast: A preliminary study. Biomed. Eng. Online 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Kontos, M.; Wilson, R.; Fentiman, I. Digital infrared thermal imaging (DITI) of breast lesions: Sensitivity and specificity of detection of primary breast cancers. Clin. Radiol. 2011, 66, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Morais, K.C.C.; Vargas, J.V.C.; Reisemberger, G.G.; Freitas, F.N.P.; Oliari, S.H.; Brioschi, M.L.; Louveira, M.H.; Spautz, C.; Dias, F.G.; Gasperin, P., Jr.; et al. An infrared image based methodology for breast lesions screening. Infrared Phys. Technol. 2016, 76, 710–721. [Google Scholar]
- Amri, A.; Pulko, S.H.; Wilkinson, A.J. Potentialities of steady-state and transient thermography in breast tumour depth detection: A numerical study. Comput. Methods Programs Biomed. 2016, 123, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Herman, C. Optimization of skin cooling by computational modeling for early thermographic detection of breast cancer. Int. J. Heat Mass Transf. 2018, 126, 864–876. [Google Scholar] [CrossRef]
- Sadeghi, M.; Boese, A.; Maldonado, I.; Sauerhering, J.; Schlosser, S.; Wehberg, H.; Wehberg, K.; Friebe, M. Feasibility test of dynamic cooling for detection of small tumors in IR thermographic breast imaging. Curr. Dir. Biomed. Eng. 2019, 5, 397–400. [Google Scholar] [CrossRef]
- Parisky, Y.R.; Sardi, A.; Hamm, R.; Hughes, K.; Esserman, L.; Rust, S.; Callahan, K. Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. Am. J. Roentgenol. 2003, 180, 263–269. [Google Scholar] [CrossRef]
- Arora, N.; Martins, D.; Ruggerio, D.; Tousimis, E.; Swistel, A.J.; Osborne, M.P.; Simmons, R.M. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am. J. Surg. 2008, 196, 523–526. [Google Scholar] [CrossRef]
- Wishart, G.C.; Campisi, M.; Boswell, M.; Chapman, D.; Shackleton, V.; Iddles, S.; Hallett, A.; Britton, P.D. The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. EJSO Eur. J. Surg. Oncol. 2010, 36, 535–540. [Google Scholar] [CrossRef]
- Sarigoz, T.; Ertan, T. Role of dynamic thermography in diagnosis of nodal involvement in patients with breast cancer: A pilot study. Infrared Phys. Technol. 2020, 108, 103336. [Google Scholar] [CrossRef]
- Deng, Z.-S.; Liu, J. Mathematical modeling of temperature mapping over skin surface and its implementation in thermal disease diagnostics. Comput. Biol. Med. 2004, 34, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Cetingul, M.P.; Herman, C. A heat transfer model of skin tissue for the detection of lesions: Sensitivity analysis. Phys. Med. Biol. 2010, 55, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Cetingul, M.P.; Herman, C. Quantification of the thermal signature of a melanoma lesion. Int. J. Therm. Sci. 2011, 50, 421–431. [Google Scholar] [CrossRef]
- Bhowmik, A.; Repaka, R.; Mishra, S.C. Thermographic evaluation of early melanoma within the vascularized skin using combined non-Newtonian blood flow and bio heat models. Comput. Biol. Med. 2014, 53, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Bonmarin, M.; Le Gal, F.-A. Lock-in thermal imaging for the early-stage detection of cutaneous melanoma: A feasibility study. Comput. Biol. Med. 2014, 47, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Iljaž, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. Subdomain BEM formulations for the solution of bio-heat problems in biological tissue with melanoma lesions. Eng. Anal. Bound. Elem. 2017, 83, 25–42. [Google Scholar] [CrossRef]
- Agyingi, E.; Wiandt, T.; Maggelakis, S. A quantitative model of cutaneous melanoma diagnosis using thermography. In Mathematical and Computational Approaches in Advancing Modern Science and Engineering; Bélair, J., Frigaard, I.A., Kunze, H., Makarov, R., Melnik, R., Spiteri, R.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 167–175. [Google Scholar] [CrossRef]
- Greene, F.L.; Compton, C.C.; Fritz, A.G.; Shah, J.P.; Winchester, D.P. Melanoma of the Skin. In AJCC Cancer Staging Atlas, Part V; Greene, F.L., Compton, C.C., Fritz, A.G., Shah, J.P., Winchester, D.P., Eds.; Springer: New York, NY, USA, 2006; pp. 207–216. [Google Scholar]
- Partridge, P.W.; Wrobel, L.C. An inverse geometry problem for the localisation of skin tumours by thermal analysis. Eng. Anal. Bound. Elem. 2007, 31, 803–811. [Google Scholar] [CrossRef]
- Agnelli, J.P.; Barrea, A.A.; Turner, C.V. Tumor location and parameter estimation by thermography. Math. Comput. Model. 2011, 53, 1527–1534. [Google Scholar] [CrossRef]
- Bhowmik, A.; Repaka, R. Estimation of growth features and thermophysical properties of melanoma within 3D human skin using genetic algorithm and simulated annealing. Int. J. Heat Mass Transf. 2016, 98, 81–95. [Google Scholar] [CrossRef]
- Strąkowska, M.; Strąkowski, R.; Strzelecki, M.; de Mey, G.; Więcek, B. Evaluation of perfusion and thermal parameters of skin tissue using cold provocation and thermographic measurements. Metrol. Meas. Syst. 2016, 23, 373–381. [Google Scholar] [CrossRef][Green Version]
- Strąkowska, M.; Strąkowski, R.; Strzelecki, M.; de Mey, G.; Więcek, B. Thermal modelling and screening method for skin pathologies using active thermography. Biocybern. Biomed. Eng. 2018, 38, 602–610. [Google Scholar] [CrossRef]
- Iljaž, J.; Wrobel, L.C.; Gomboc, T.; Hriberšek, M.; Marn, J. Solving inverse bioheat problems of skin tumour identification by dynamic thermography. Inverse Probl. 2020, 36, 035002. [Google Scholar] [CrossRef]
- Iljaž, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. Numerical modelling of skin tumour tissue with temperature-dependent properties for dynamic thermography. Comput. Biol. Med. 2019, 112, 103367. [Google Scholar] [CrossRef] [PubMed]
- Iljaž, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. The use of Design of Experiments for steady-state and transient inverse melanoma detection problems. Int. J. Therm. Sci. 2019, 135, 256–275. [Google Scholar] [CrossRef]
- Chen, H.; Wang, K.; Du, Z.; Liu, W.; Liu, Z. Predicting the thermophysical properties of skin tumor based on the surface temperature and deep learning. Int. J. Heat Mass Transf. 2021, 180, 121804. [Google Scholar] [CrossRef]
- Jiang, L.; Zhan, W.; Loew, M.H. Modeling static and dynamic thermography of the human breast under elastic deformation. Phys. Med. Biol. 2011, 56, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Mukhmetov, O.; Igali, D.; Mashekova, A.; Zhao, Y.; Ng, E.Y.K.; Fok, S.C.; Teh, S.L. Thermal modeling for breast tumor detection using thermography. Int. J. Therm. Sci. 2021, 161, 106712. [Google Scholar] [CrossRef]
- Ng, E.Y.K.; Sudharsan, N.M. Effect of blood flow, tumour and cold stress in a female breast: A novel time-accurate computer simulation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 393–404. [Google Scholar] [CrossRef]
- Lozano, A.; Hayes, J.C.; Compton, L.M.; Azarnoosh, J.; Hassanipour, F. Determining the thermal characteristics of breast cancer based on high-resolution infrared imaging, 3D breast scans, and magnetic resonance imaging. Sci. Rep. 2020, 10, 10105. [Google Scholar] [CrossRef]
- Amri, A.; Saidane, A.; Pulko, S. Thermal analysis of a three-dimensional breast model with embedded tumour using the transmission line matrix (TLM) method. Comput. Biol. Med. 2011, 41, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.A.; Salim, M.I.M.; Ahamat, M.A.; Manaf, N.A.; Yunus, J.; Lai, K.W. Thermal distribution analysis of three-dimensional tumor-embedded breast models with different breast density compositions. Med. Biol. Eng. Comput. 2016, 54, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Al Husaini, M.A.S.; Habaebi, M.H.; Suliman, F.M.; Islam, R.; Elsheikh, E.A.A.; Muhaisen, N.A. Influence of tissue thermophysical characteristics and situ-cooling on the detection of breast cancer. Appl. Sci. 2023, 13, 8752. [Google Scholar] [CrossRef]
- Paruch, M.; Majchrzak, E. Identification of tumor region parameters using evolutionary algorithm and multiple reciprocity boundary element method. Eng. Appl. Artif. Intell. 2007, 20, 647–655. [Google Scholar] [CrossRef]
- Das, K.; Mishra, S.C. Simultaneous estimation of size, radial and angular locations of a malignant tumor in a3-D human breast—A numerical study. J. Therm. Biol. 2015, 52, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Mishra, S.C. Non-invasive estimation of size and location of a tumor in a human breast using a curve fitting technique. Int. Commun. Heat Mass Transf. 2014, 56, 63–70. [Google Scholar] [CrossRef]
- Hossain, S.; Mohammadi, F.A. Tumor parameter estimation considering the body geometry by thermography. Comput. Biol. Med. 2016, 76, 80–93. [Google Scholar] [CrossRef]
- Ferreira de Melo, J.R.; Queiroz, J.R.A.; Bezerra, L.A.; de Lima, R.C.F. Development of a three-dimensional surrogate geometry of the breast and its use in estimating the thermal conductivities of breast tissue and breast lesions based on infrared images. Int. Commun. Heat Mass Transf. 2019, 108, 104279. [Google Scholar] [CrossRef]
- Bezerra, L.A.; Oliveira, M.M.; Rolim, T.L.; Conci, A.; Santos, F.G.S.; Lyra, P.R.M.; Lima, R.C.F. Estimation of breast tumor thermal properties using infrared images. Signal Process. 2013, 93, 2851–2863. [Google Scholar] [CrossRef]
- Hatwar, R.; Herman, C. Inverse method for quantitative characterization of breast tumours from surface temperature data. Int. J. Hyperth. 2017, 33, 741–757. [Google Scholar]
- Luna, J.M.; Romero-Mendez, R.; Hemandez-Guerrero, A.; Elizalde-Blancas, F. Procedure to estimate thermophysical and geometrical parameters of embedded cancerous lesions using thermography. J. Biomech. Eng. 2012, 134, 031008. [Google Scholar]
- Mitra, S.; Balaji, C. A neural network based estimation of tumour parameters from a breast thermogram. Int. J. Heat Mass Transf. 2010, 53, 4714–4727. [Google Scholar] [CrossRef]
- Mital, M.; Pidaparti, R.M. Breast tumor simulation and parameters estimation using evolutionary algorithms. Model. Simul. Eng. 2008, 2008, 756436. [Google Scholar] [CrossRef]
- Sudarshan, N.M.; Ng, E.Y.K.; Teh, S.L. Surface temperature distribution of a breast with and without tumour. Comput. Methods Biomech. Biomed. Eng. 1999, 2, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Saniei, E.; Setayeshi, S.; Akbari, M.E.; Navid, M. Parameter estimation of breast tumour using dynamic neural network from thermal pattern. J. Adv. Res. 2016, 7, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Akhter, N.; Manza, R. Application of image processing techniques for characterization of skin cancer lesions using thermal images. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Shaikh, S.; Akhter, N.; Gaike, V.; Manza, R. Boundary detection of skin cancer lesions using image processing techniques. J. Med. Chem. Drug Discov. 2016, 1, 381–388. [Google Scholar]
- Benjumea, E.; Morales, Y.; Torres, C.; Vilardy, J. Characterization of thermographic images of skin cancer lesions using digital image processing. In Proceedings of the IX International Congress of Physics Engineering, Mexico City, Mexico, 5–9 November 2018. [Google Scholar]
- Magalhaes, C.; Tavares, J.M.R.S.; Mendes, J.; Vardasca, R. Comparison of machine learning strategies for infrared thermography of skin cancer. Biomed. Signal Process. Control 2021, 69, 102872. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Yuan, Y.; Wang, Q. Morphological measurement of localized temperature increase amplitudes in breast infrared thermograms and its clinical application. Biomed. Signal Process. Control 2008, 3, 312–318. [Google Scholar] [CrossRef]
- Zadeh, H.G.; Pakdelazar, O.; Haddadnia, J.; Rezai-Rad, G.; Mohammad-Zadeh, M. Diagnosing breast cancer with the aid of fuzzy logic based on data mining of a genetic algorithm in infrared images. Middle East J. Cancer 2011, 3, 119–129. [Google Scholar]
- Krawczyk, B.; Schaefer, G. A hybrid classifier committee for analysing asymmetry features in breast thermograms. Appl. Soft Comput. 2014, 20, 112–118. [Google Scholar] [CrossRef]
- Mahmoudzadeh, E.; Montazeri, M.A.; Zekri, M.; Sadri, S. Extended hidden Markov model for optimized segmentation of breast thermography images. Infrared Phys. Technol. 2015, 72, 19–28. [Google Scholar] [CrossRef]
- Raghavan, K.; Balasubramanian, S.; Veezhinathan, K. IR-GAN: Improved generative adversarial networks for infrared breast image segmentation. Quant. InfraRed Thermogr. J. 2023, in press. [Google Scholar] [CrossRef]
- Gomathi, P.; Muniraj, C.; Periasamy, P.S. Breast thermography based unsupervised anisotropic- feature transformation method for automatic breast cancer detection. Microprocess. Microsyst. 2020, 77, 103137. [Google Scholar] [CrossRef]
- Ekici, S.; Jawzal, H. Breast cancer diagnosis using thermography and convolutional neural networks. Med. Hypotheses 2020, 137, 109542. [Google Scholar] [CrossRef]
- Mishra, V.; Rath, S.K. Detection of breast cancer tumours based on feature reduction and classification of thermograms. Quant. InfraRed Thermogr. J. 2021, 18, 300–313. [Google Scholar] [CrossRef]
- Mishra, V.; Rath, S.K.; Mohapatra, D.P. Thermograms-based detection of cancerous tumors in breasts applying texture features. Quant. InfraRed Thermogr. J. 2023; in press. [Google Scholar] [CrossRef]
- Mahoro, E.; Akhloufi, M.A. Breast cancer classification on thermograms using deep CNN and transformers. Quant. InfraRed Thermogr. J. 2024, 21, 30–49. [Google Scholar] [CrossRef]
- Silva, L.F.; Santos, A.A.S.M.D.; Bravo, R.S.; Silva, A.C.; Muchaluat-Saadea, D.C.; Conci, A. Hybrid analysis for indicating patients with breast cancer using temperature time series. Comput. Methods Programs Biomed. 2016, 130, 142–153. [Google Scholar] [CrossRef]
- Hakim, A.S.; Awale, R.N. Extraction of hottest blood vessels from breast thermograms using state-of-the-art image segmentation methods. Quant. InfraRed Thermograms. J. 2022, 19, 347–365. [Google Scholar] [CrossRef]
- Chebbah, N.K.; Ouslim, M.; Benabid, S. New computer aided diagnostic system using deep neural network and SVM to detect breast cancer in thermography. Quant. InfraRed Thermogr. J. 2023, 20, 62–77. [Google Scholar] [CrossRef]
- Torres-Galván, J.C.; Guevara, E.; Kolosovas-Machuca, E.S.; Oceguera-Villanueva, A.; Flores, J.L.; González, F.J. Deep convolutional neural networks for classifying breast cancer using infrared thermography. Quant. InfraRed Thermogr. J. 2022, 19, 283–294. [Google Scholar] [CrossRef]


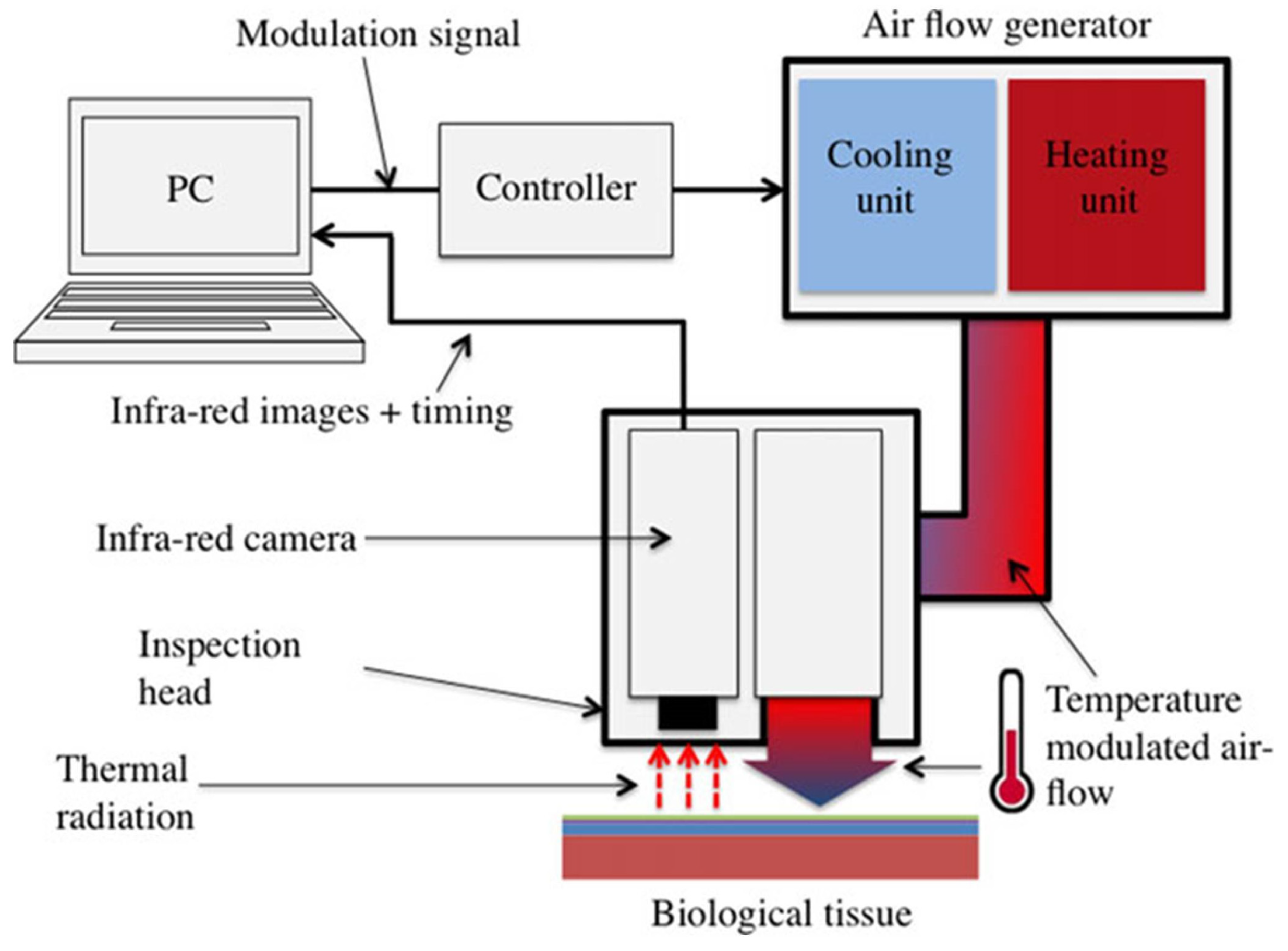
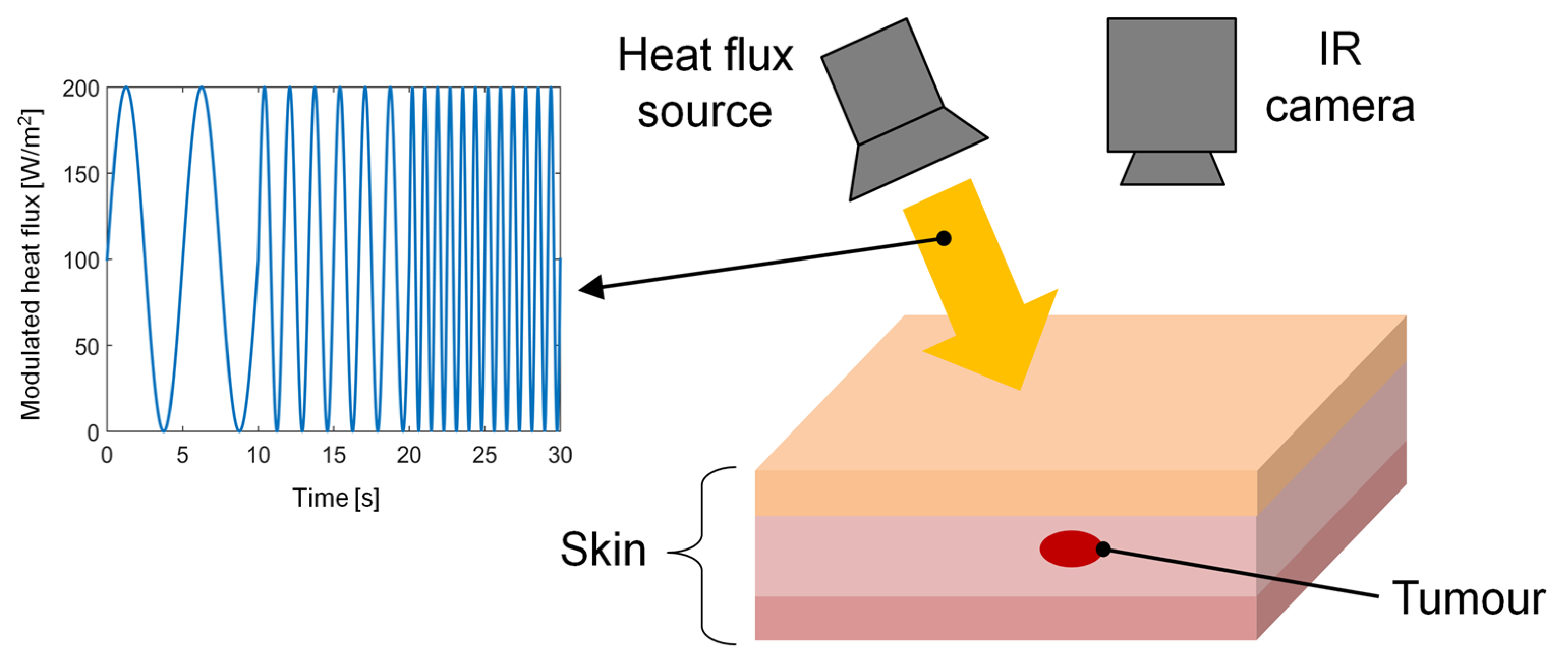
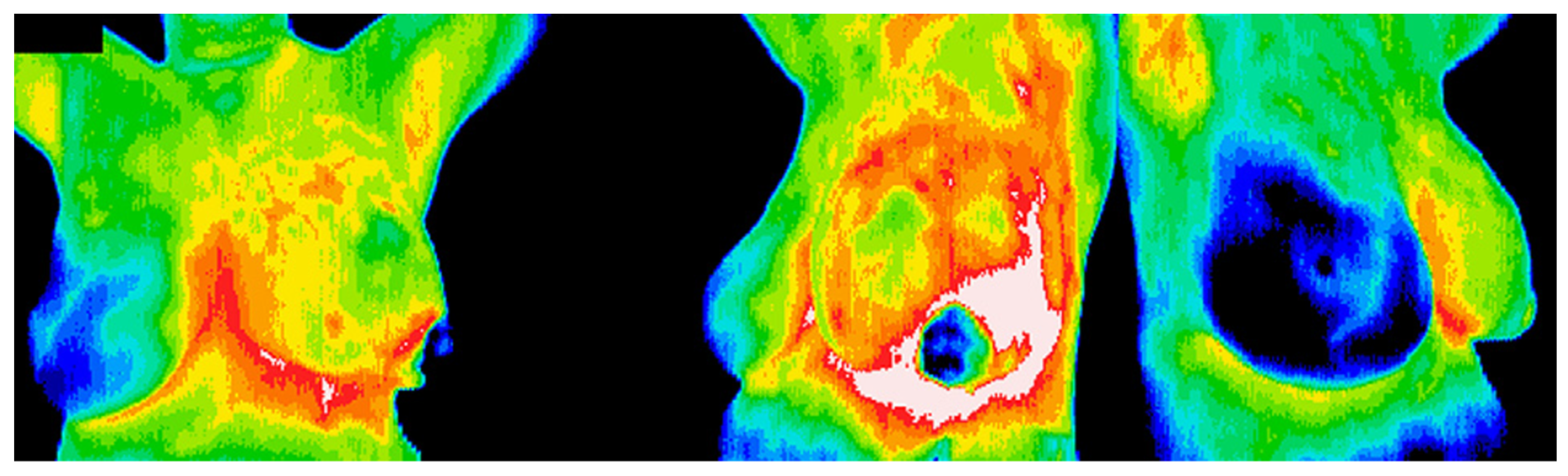

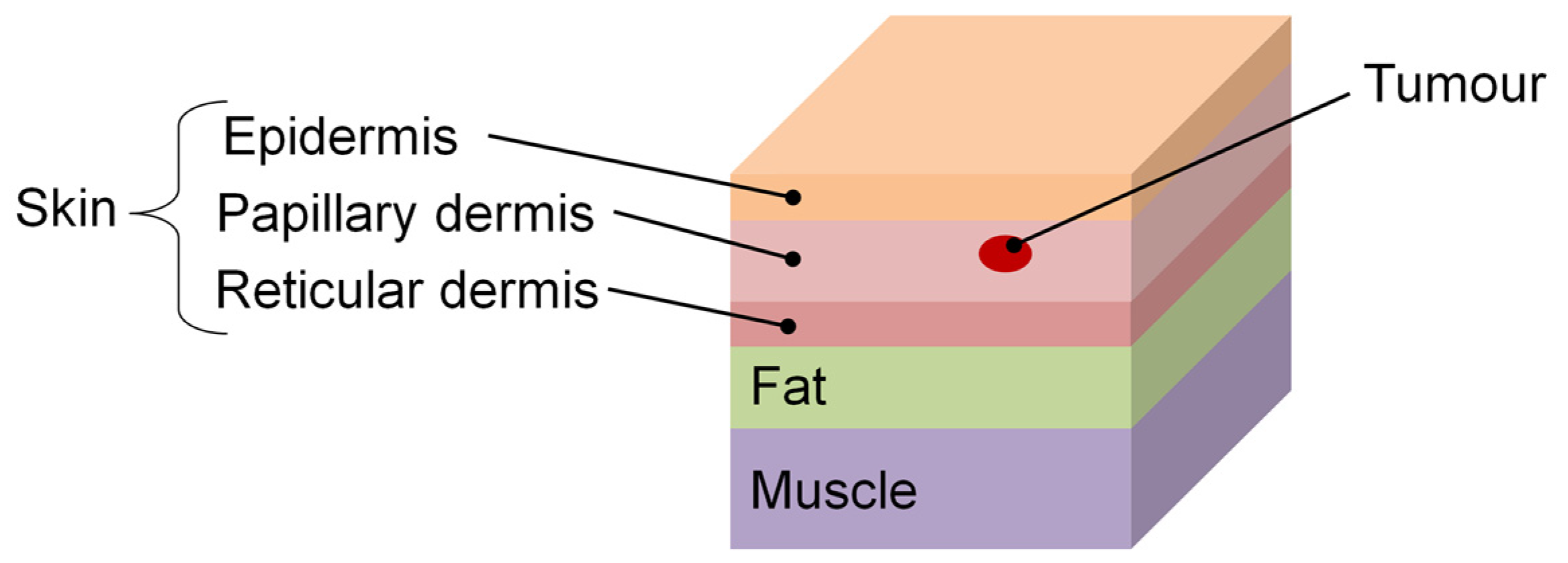
| Study | Thermal Imaging Technique | Sample Size and Lesion Type | Camera Specifications | Cooling/Heating Methods/ Acclimatization Information | Findings/Results |
|---|---|---|---|---|---|
| Gonzalez et al. [16] | Infrared Thermography. | 30 patients (6 melanomas, 18 BCC, and 6 SCC). | FLIR T400 IR camera (320 × 240 FPA, spectral range 7.5–13 μm, thermal sensitivity 50 mK at 30 °C). | … | Differentiated BCC, SCC, and melanoma based on temperature differences. Correlation with vascularity. |
| Flores -Sahagun et al. [26] | Steady-state Thermography. | 11 subjects (BCC). | SAT-S160 Infrared camera (160 × 120 pixels, temp. accuracy ± 2%, temp. resolution 0.1 °C). | … | Detected BCC despite low camera resolution. |
| Shada et al. [28] | Steady-state Thermography. | 74 patients (various lesions). | Camera specifications not provided. | … | Evaluated sensitivity and specificity based on lesion diameter. |
| Vardasca et al. [29] | Steady-state Thermography. | 58 patients (neoplasms). | FLIR A325sc camera (320 × 240 resolution, precision 70 mK). | 15 min acclimatization. | Distinguished benign from malignant skin cancers based on thermal contrast. |
| Stringasci et al. [30] | Steady-state Thermography. | 100 cases each (various lesions). | FlukeVR FLK-Ti400 IR camera (320 × 240 pixels, thermal sensitivity < 0.05 °C, precision ± 2 °C). | 10 min acclimatization. | Used SVM classifier to discriminate lesions; identified clear temperature differences for some lesions. |
| Buzug et al. [23] | Dynamic IR Thermography (cold). | … | FLIR SC 3000 camera (temp. resolution 0.03 K). | Cool gel pack. | Detected BCC using dynamic thermography with cold stimulus. |
| Santa Cruz et al. [37] | Dynamic IR Thermography (cold). | … | Camera specifications not provided. | Water immersion or alcohol spray with fan currents. | Used dynamic thermography to monitor melanoma patients during BNCT. |
| Cetingul and Herman [38] | Dynamic IR Thermography (Cold) | 37 patients (pigmented lesions). | Unspecified IR Camera (320 × 256 pixel InSb FPA and sensitivity of 0.025 °C). | Stream of cold air at 15 °C. | Detected early-stage melanoma cases with a stream of cold air as a cooling stimulus. |
| Di Carlo et al. [39] | Dynamic IR Thermography (cold). | 36 patients (87 actinic keratosis and 48 BCC). | FLIR3000 Thermocam. | Cold stress at 5 °C for 20 s. | Distinguished thermal patterns of actinic keratosis and BCC. |
| Baek et al. [40] | Dynamic IR Thermography (hot and cold). | 37 patients (22 BCC and 15 seborrheic keratosis). | FLIRVR A615 IR camera (640 × 480 pixels, noise equivalent temp. difference < 0.05 °C at 30 °C). | 5–10 min acclimatization, hot stress up to 40 °C, and cold stress to 15 °C after 5 min rest. | Distinguished pigmented BCC and seborrheic keratosis. |
| Godoy et al. [41] | Dynamic IR Thermography (cold). | About 100 subjects. | Long-wave infrared camera (320 × 256 FPA). | Cold air flow produced by Ranque–Hilsch vortex tube. | Successfully classified malignant cases using a cold air flow cooling stimulus. |
| Laino et al. [42] | Dynamic IR Thermography (cold). | 30 patients (actinic keratosis). | FLIR3000 IR camera. | Alcohol and water mixture cooling. | Monitored effectiveness of actinic keratosis treatment with dynamic IR thermography. |
| Cholewka et al. [43] | Dynamic IR Thermography (laser). | 6 patients (BCC). | FLIR Thermovision Camera E60 (sensitivity 50 mK). | Laser illumination (active thermography). | Investigated temperature gradient changes due to photodynamic therapy for BCC. |
| Bonma- rine and Le Gal [44] | Lock-in Thermography. | … | IR camera and temperature-modulated airflow. | Temperature-modulated air flow synchronized with the camera. | Used lock-in thermography for dermatological applications, reported results for benign skin lesions. |
| Bhowmik et al. [45] | Thermal Wave Imaging (FMTWI). | … | IR camera and controlled heating. | Controlled heating of the skin surface. | Theoretical feasibility of FMTWI for detection and differentiation of melanoma stages. |
| Study | Thermal Imaging Technique | Sample Size and Lesion Type | Camera Specifications | Cooling/Heating Methods/ Acclimatization Information | Findings/Results |
|---|---|---|---|---|---|
| Head et al. [17] | Infrared Thermography. | 326 patients. | Camera specifications not provided. | … | Demonstrated increased risk of breast cancer in subjects with abnormal IR breast images. |
| Ohashi and Uchida [46] | Infrared Thermography. | 728 breast cancer patients. | Camera specifications not provided. | Cold air flow provided by a fan for 2 min. | Reached diagnostic accuracy of 82% in dynamic conditions. |
| Ng et al. [48] | Steady-state Thermography. | 50 healthy women. | Encapsulated liquid crystal SINOTEST MSP8. | 15–20 min acclimatization. | Investigated cyclic variation of temperature of normal breast thermograms. |
| Zeng et al. [49] | Steady-state Thermography. | 35 healthy women. | HYIR I-1206 (384 × 288 resolution, sensitivity of 0.08 °C). | 5–10 min acclimatization. | Demonstrated thermal symmetry in healthy breast. |
| Wang et al. [50] | Steady-state Thermography. | 276 women with previous diagnoses. | ATIR-M301 camera (320 × 240 FPA, temp. resolution < 0.1 °C). | 15 min acclimatization. | Investigated correlation between IR signs and final disease status. |
| Kontos et al. [51] | Steady-state Thermography. | 63 patients. | Meditherm med2000 thermal imaging system. | 10–15 min acclimatization. | Steady-state infrared imaging should not be used for breast cancer screening. |
| Morais et al. [52] | Steady-state Thermography. | 47 breast cancer patients. | ThermaCAM T400 FLIR E60 (320 × 240 resolution) SAT-S160 (160 × 120). | … | Identified breast cancer lesions using different resolution cameras |
| Parisky et al. [56] | Dynamic IR Thermography (cold). | 769 patients (187 malignant; 688 benign lesions). | Camera specifications not provided. | Stream of cold air supplied by a refrigeration chamber. | Distinguished between benign and malignant lesions. |
| Arora et al. [57] | Dynamic IR Thermography (cold). | 92 women (suspicious breast lesions). | Sentinel BreastScanTM (320 × 240 FPA, sensitivity of 0.08 °C). | Cold air flow supplied by an air cooler. | Obtained high sensitivity values. |
| Wishart et al. [58] | Dynamic IR Thermography (cold). | 100 women (suspicious breast lesions). | Sentinel BreastScanTM (320 × 240 FPA, sensitivity of 0.08 °C). | Cold air flow for 5 min. | Demonstrated effectiveness of IR imaging for breast cancer detection in women under 50 years old. |
| Sarigoz and Ertan [59] | Dynamic IR Thermography (cold). | 26 patients (IDC). | Fluke® Ti9 IR camera (640 × 480 resolution, sensitivity ≤ 0.20 °C). | Gel pack at 4–8 °C for 2 min. | Demonstrated superiority of IR imaging superior than other imaging techniques in detecting malignant lymph nodes. |
| Francis et al. [20] | Rotational Thermography. | 36 patients (24 healthy and 12 abnormal breast IR images). | Camera specifications not provided. | Cold air in a closed chamber. | Demonstrated the potential of rotational thermography. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, G.; Tavakolian, P.; Sfarra, S. A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy. Appl. Sci. 2024, 14, 1603. https://doi.org/10.3390/app14041603
D’Alessandro G, Tavakolian P, Sfarra S. A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy. Applied Sciences. 2024; 14(4):1603. https://doi.org/10.3390/app14041603
Chicago/Turabian StyleD’Alessandro, Giampaolo, Pantea Tavakolian, and Stefano Sfarra. 2024. "A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy" Applied Sciences 14, no. 4: 1603. https://doi.org/10.3390/app14041603
APA StyleD’Alessandro, G., Tavakolian, P., & Sfarra, S. (2024). A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy. Applied Sciences, 14(4), 1603. https://doi.org/10.3390/app14041603







