1. Introduction
Head and neck cancer is the sixth most common neoplasm in the world [
1], with some known risk factors such as smoking, oral health, genetics, and the presence or not of human papilloma virus infection (HPV) [
2,
3]. Nevertheless, the presence of HPV has been identified as a good prognostic factor, since HPV-positive cases show a reduction in mortality by 58% and a better response to treatment than HPV-negative patients [
2]. This is especially relevant in patients with oropharyngeal cancer, where the latest AJCC classification discriminates according to HPV status, due to its prognostic nature [
4]. Diet, ultraviolet radiation, oral health, or genetic predisposition are other risk factors with lower incidence [
3].
Despite its high incidence, the survival rate has been increasing in recent years due to early diagnosis and the improvement in therapeutic strategies [
1]. Functional images using Positron Emission Tomography (PET) with
18F-2-fluorine-2-deoxy-D-glucose ([
18F]FDG) in combination with computed tomography (CT) are a useful tool to assess the recurrence before the treatment or in the case of relapse thanks to the high negative predictive value [
1], especially during the first two years post-treatment due to the high risk of relapse (50–60%) [
5,
6] in locoregional advanced oropharyngeal cancer (stage III or IV). [
18F]FDG PET/CT images help to evaluate efficiently the changes produced in tissues after therapies and treatments, discriminating between active residual disease, recurrent disease, and post-treatment fibrosis. As a result of such high levels of sensibility and specificity, PET-CT findings lead to a change in management for up to 25% of patients [
7,
8,
9].
[
18F]FDG PET images are usually analyzed quantitatively through the Standardized Uptake Value (SUV), which represents the relative radiotracer uptake in the lesion [
1]. The [
18F]FDG uptake in head and neck cancer was evaluated by Torizuka et al. [
2], where lower SUVs were related to greater survival rates and disease control. In addition, the evaluation of volumetric parameters, such as the Metabolic Tumor Volume (MTV) and Total Lesion Glycolysis (TLG), has shown promising results. Pak et al. [
3] have demonstrated that higher MTV and TLG are related to tumor recurrence. Creff et al. [
10] proved the prognostic effectiveness of [
18F]FDG PET parameters among patients with head and neck squamous cell cancer. Additionally, Bonomo et al. [
7] demonstrated the prognostic relevance of [
18F]FDG PET parameters in the context of locally advanced head and neck squamous cell carcinoma.
Radiomics is an evolving field that uses a non-invasive methodology to characterize tissues and organs based on a large number of features extracted from the medical images. The underlying hypothesis of radiomics is that genomic cancer subtypes are reflected in image-based features. Subsequently, recent techniques such as textural analysis have demonstrated to be optimal to quantify these cancer phenotype properties [
11,
12,
13]. These sub-visual features can be grouped into intensity, shape, and textural relationships between pixels. The textural features, or second order, represent the spatial distribution relationship between voxel intensities, such as the gray level co-occurrence matrix (GLCM), which calculates the correlation between two gray levels at a certain distance and a certain direction in an image.
In the last years, the use of radiomics for the analysis of [
18F]FDG PET/CT images in oncology has increased [
8,
11,
14,
15]. In head and neck cancer, intratumoral heterogeneity is considered one of the main factors that influences treatment progression and chemotherapy resistance [
16]. Molecular heterogeneity is reflected macroscopically through these textural image features [
14]. Consequently, the relationship between genetic and metabolic heterogeneities and its ability of prognosis in [
18F]FDG PET images was evidenced in recent studies [
8,
13,
17,
18,
19,
20]. These studies demonstrated that the greater the homogeneity is within an FDG-avid region, the better the disease prognosis.
Several studies have revealed that textural parameters in [
18F]FDG PET images, as tumor heterogeneity indicators, have shown to provide additional prognostic value for treatment outcomes [
12,
19,
21,
22,
23,
24,
25,
26]. Furthermore, the combination of clinical and textural parameters has shown complementary predictive value for locoregional recurrence and Overall Survival (OS) [
12] in head and neck cancer. The ability of radiomics to stratify patients into potential risk cancer phenotypes may be of great use in clinical decision support systems. Radiomics can be used as a tool to enhance personalized medicine, evaluating patient-specific treatment efficacy and optimal treatment strategies during follow-up.
In this study, the aim was to evaluate the potential of radiomics analysis using [18F]FDG PET/CT images. The purpose was to identify predictive [18F]FDG PET radiomic features to predict recurrence and OS in patients with stage III and IV oropharyngeal cancer. The prognostic value of the combination of conventional and textural features was evaluated. We studied the relationship of these features, as well as clinical and pathological characteristics, especially HPV status, to treatment response and patient survival.
2. Materials and Methods
2.1. Study Cohort
Eligibility criteria included histologically confirmed HPV status oropharyngeal squamous cell carcinoma; American Joint Committee on Cancer (AJCC) 7th edition clinical categories T1–T2, N2a–N3 M0 or T3–T4, N0–N3 M0 (stage III or IV); Eastern Cooperative Oncology Group (ECOG) performance status 0–2; age of at least 18 years; and chemoradiotherapy concurrent treatment. Exclusion criteria included concomitant tumor present in another location or incomplete treatment. Lastly, due to software limitations, patients with tumor sizes smaller than 64 voxels were excluded.
Importantly, HPV-related oropharyngeal cancer patients’ staging should be done following the AJCC TNM 8th edition, whereas clinical decision making should follow the AJCC TNM 7th edition. This is the reason why we decided to unify our sample following the AJCC TNM 7th edition.
After applying the exclusion criteria, 39 patients (median age, 66; range, 53–82) were included in the study population. It must be noted that the vast majority of patients of this database are smokers (36 of 39 patients), being a risk factor for head and neck cancer [
22], making it impossible to establish differences between smokers and non-smokers. Patient demographics are summarized in
Figure 1, and the followed workflow is described in
Table 1.
2.2. [18F]FDG PET/CT Acquisition
Patients were subject to a 4 to 6 h fasting before the images were taken. Glucose levels were all below 200 mg/dL. Images were analyzed by experienced nuclear medicine physicians, and all primary lesions were defined through their evaluation. Selective head and neck images were acquired using a Siemens Biograph TruePoint PET/CT Model 1093 (Siemens Healthineers). Patients were weighted before the study, and a dose of 5 Mbq per kg was administered, which resulted in a mean dose of 341.14 ± 67.43 MBq of [18F]FDG intravenously administered. Images were acquired 73.02 ± 21.80 min post injection. Imaging protocol included a dedicated head and neck image from the top of the skull to the sternum manubrium with arms down, and a whole-body image from the base of the skull to the mid-thighs with arms up. Images were reconstructed using a Point Spread Function (PFS), with 3 iterations and 21 subsets. Attenuation correction was performed using simultaneously acquired low-dose CT scans. Additionally, scatter and random corrections were performed. The reconstructed PET images had a matrix size of 168 × 168 and voxel size of 4.07 × 4.07 mm.
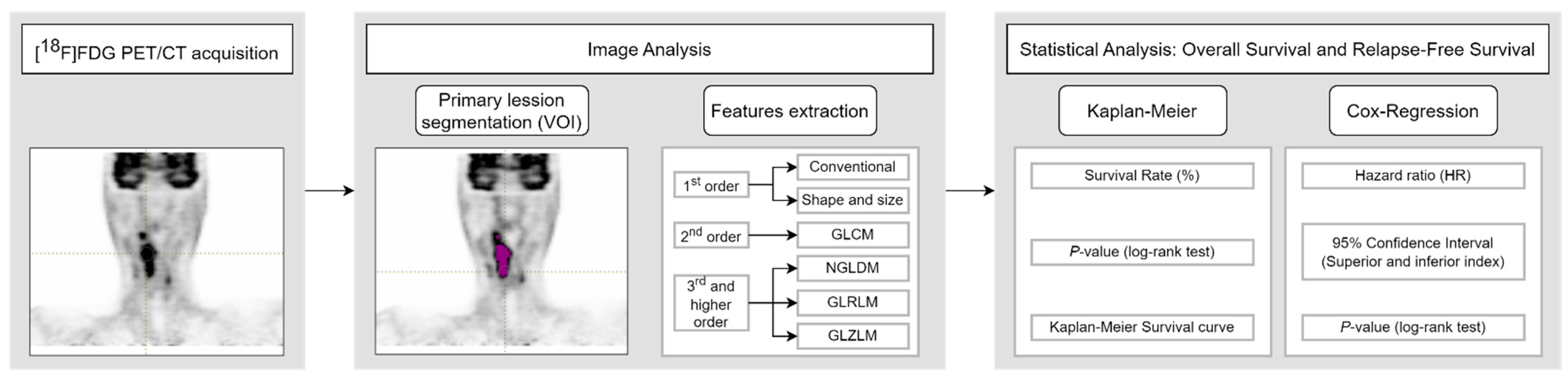
2.3. Image Analysis
Segmentation of the primary tumors was performed using the LIFEx v6.30 software (
https://www.lifexsoft.org (accessed on 9 February 2024) [
27]. An initial volume encompassing the whole tumor was manually drawn on the [
18F]FDG PET images according to clinical data. Images were read by an experienced nuclear medicine physician (example in
Figure 2). Specifically, the SUV
max from the corresponding initial clinical records was used to distinguish the primary lesion from secondary lymph nodes. The segmentation of the tumor, i.e., the primary lesion, was computed applying a threshold of 41% of the SUV
max inside the previously drawn volume [
28], obtaining the volume of interest (VOI). LIFEx was set up using the following input parameters for calculation of textural features: 64 gray levels for intensity discretization; and absolute resampling between a minimum of 0 and a maximum of 39 (the maximum SUV of the segmented VOIs for the whole study cohort) for intensity rescaling [
29]. A total of 48 features were extracted. These included 13 conventional parameters, 4 shape and size features, 6 second-order textural features (from the gray-level co-occurrence matrix, GLCM), and 25 third and higher order textural features (3 from the neighborhood gray-level different matrix, NGLDM; 11 from the gray-level run-length matrix, GLRLM; and 11 from the gray-level zone-length matrix, GLZLM) [
30,
31,
32,
33]. Shape and size features include the MTV, Sphericity, Compacity, and Surface. Texture indices were computed for each of the gray-level matrixes mentioned above.
2.4. Statistical Analysis
OS and Relapse-Free Survival (RFS) of oropharyngeal stage III and IV cancer patients were analyzed for a follow-up period of 24 months. OS was defined as the time from the date of diagnosis to date of death (all causes) or date of last contact. RFS was defined as the time between the end of treatment (radiotherapy or surgery) date to the date of relapse, date of cancer-specific death, or date of last contact. Patients in the database with disease persistence were also included in RFS analysis, considering persistence as a relapse event at the start of the survival analysis.
The optimal cut-off values of continuous variables to define high- and low-value subgroups in Kaplan–Meier analysis were obtained using the receiver operating characteristic (ROC) and the closest-to-(0,1) criterion. Univariate survival rates (SRs) were calculated using Kaplan–Meier analysis, using a log-rank test to compare between high and low value subgroups, reporting the p-value. Association between features and survival (OS and RFS) was also evaluated using univariate Cox proportional hazards regression analysis. Statistically significant features in univariate analysis were included in a multivariate Cox proportional hazards regression analysis, using the stepwise backwards conditional regression model. The associated p-values and hazard ratio (HR) coefficients, with a 95% confidence interval (CI), were computed. A p-value less than 0.05 was considered statistically significant. p-values were corrected for multiple testing with the false discovery rate method by Benjamini–Hochberg. In addition, the univariate Cox proportional hazards regression analysis, with the same criterion, was performed, adjusting each variable by the HPV status to evaluate its importance in survival outcomes. Statistical analysis was performed using SPSS software version 26.0 (IBM Corp.).
4. Discussion
New cancer treatment techniques are currently being studied to improve patient survival [
34,
35,
36]. However, the same technique may have different results in patients diagnosed with the same cancer. For this reason, studying the possible therapeutic strategies based on the clinical and image characteristics of the patient at the time of diagnosis is a matter of great importance. In this study, cancer lesions from [
18F]FDG PET/CT images of patients with oropharyngeal cancer are analyzed to assess the predictive value of clinical, quantitative, and textural features, assessing OS and RFS over a 2-year follow-up period. Concretely, 48 parameters are extracted, including 13 conventional parameters, 4 shape and size features, and 31 textural features. Kaplan–Meier curves and Cox proportional hazards regression analyses are computed.
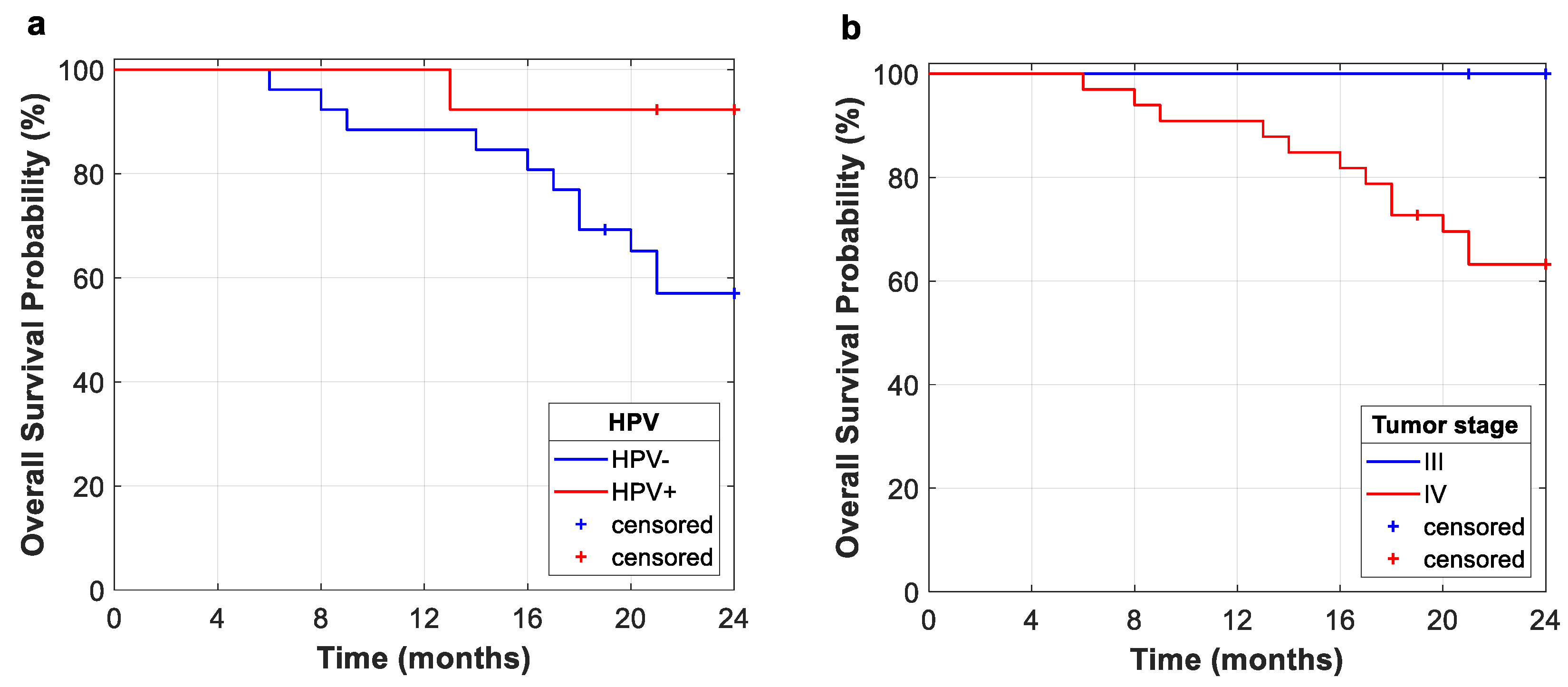
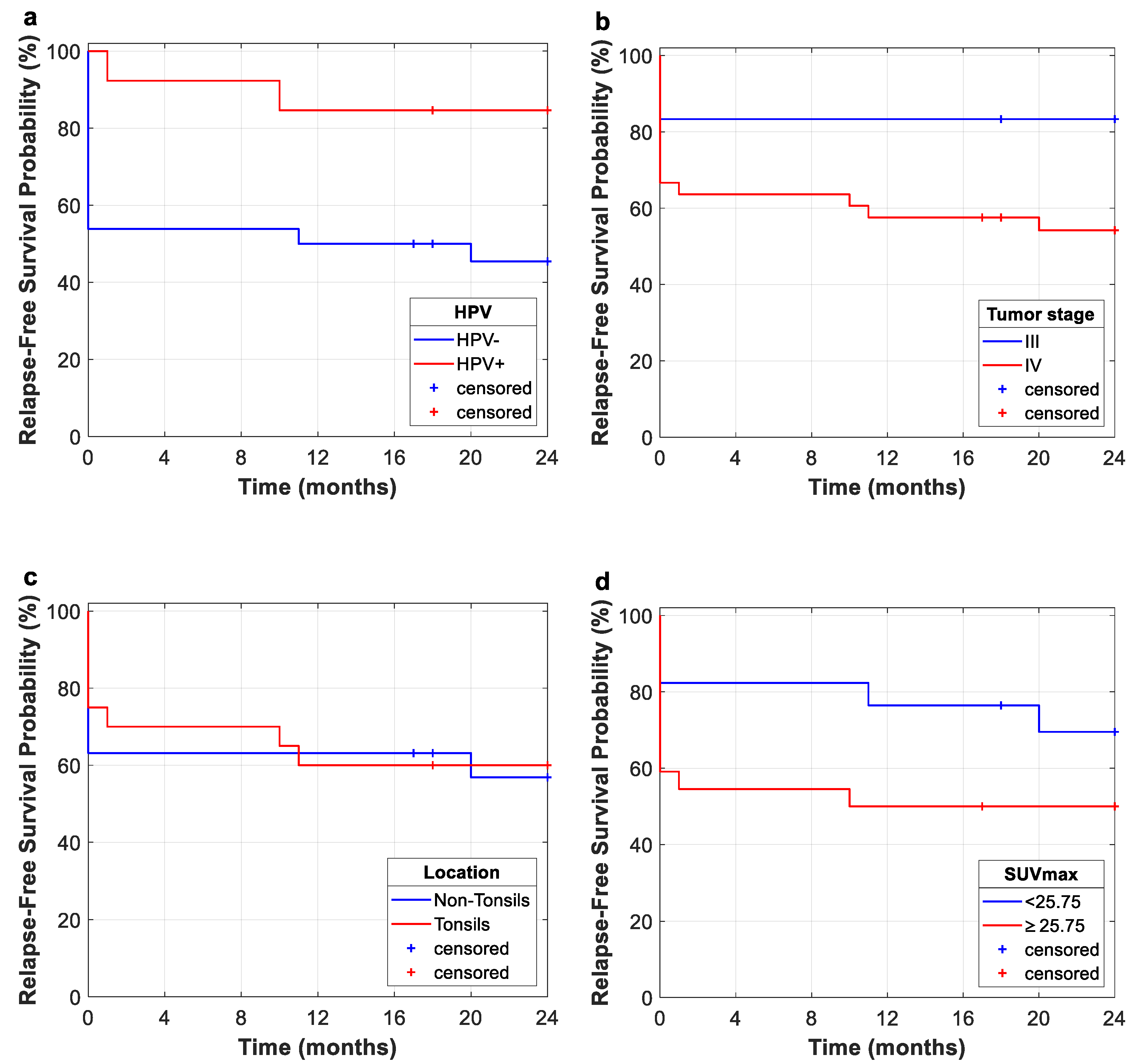
This study evidences the importance of HPV status for the prognosis of oropharyngeal cancer for OS and RFS. In this study, Kaplan–Meier curves for OS and RFS showed that HPV-negative diagnosis is related to poorer outcomes. However, future clinical trials should include a greater variety of patients in order to further study the implications of HPV status. This fact suggests that within the group of patients who respond satisfactorily, the vast majority achieve a sustained response, with low rates of relapse. As shown in Kaplan–Meier curves, several retrospective studies have demonstrated that patients with HPV-positive status have a better prognosis than patients with HPV-negative status, especially those patients with oropharyngeal carcinoma. This is due to the fact that HPV-positive cases are identified as a tumor entities with different biological, pathological, and clinical features [
37,
38]. Consequently, a better OS and RFS is expected in these patients.
The fact that most of our cohort are stage IV (84.64%) may explain the low rates of OS and RFS, along with the inclusion of tumor persistence as relapse cases. Nevertheless, the Kaplan–Meier curve for the entire study cohort shows that no relapse occurs after 20 months from the end of treatment (radiotherapy or radiotherapy plus surgery), which suggests that within the group of patients who respond satisfactorily, the vast majority achieve a sustained response, with low rates of relapse.
Most patients in our database are smokers (92.31%), which hinders finding differences between smokers and non-smokers in terms of survival, as well as differences between HPV-positive and HPV-negative smokers. Nevertheless, various studies have evidenced that HPV-positive patients with a smoking history have worse treatment outcomes and an increased risk of death. The specific impact of tobacco remains unknown, but it could have an effect by inducing additional genetic alterations or, indeed, provoking other disorders such as cardiac or respiratory diseases [
38,
39].
As well as HPV, other clinical features are evaluated for survival analysis. Tumor stage and localization are important features to characterize the tumor obtaining high HR for every analysis, but no statistically significant relationship to survival rates was observed. On the other hand, greater values of some quantitative features, such as SUV
max, TLG, and MTV, are in all cases related to a poorer prognosis [
2,
3]. Nonetheless, volumetric features (MTV and TLG) turn out to be better predictors than SUV measures. The prognostic value of TLG can be highlighted, with higher values being related to lower survival rates in Kaplan–Meier (
p = 0.029) and Cox regression analyses (
p = 0.001) for OS. TLG demonstrated a prognostic value in RFS analysis as well, being related to higher relapse rates in Kaplan–Meier (
p = 0.010) and Cox regression analyses (
p = 0.026). It is worth mentioning that Surface resulted in a great prognostic feature in the OS Cox regression analysis (
p = 0.001), being, along with TLG, the unique feature that remained statistically significant after multiple-testing correction. This shape feature also was a statistically significant predictor for RFS Kaplan–Meier (
p = 0.004) and Cox regression (
p = 0.028) analyses, with higher values related to poorer prognosis.
Although several studies have demonstrated the utility of SUV metrics as well as metabolic features such as TLG and MTV to predict clinical outcomes in head and neck cancer [
3,
7,
10], they do not reflect information of intratumoral heterogeneity. Spatial distribution of metabolic activity in the tumor can be evaluated through textural image features describing spatial relationships between pixel intensities [
8,
14,
15]. Recent studies have shown the potential of texture analysis to reveal intratumoral heterogeneity, which can lead to poor prognosis in head and neck cancer [
8,
17,
18]. Our analyses suggest heterogeneity patterns with respect to gray-level non-uniformity matrixes (GLRLM and GLZLM).
Cox regression analyses for 2-year OS showed that gray-level texture indices (GLRLM_RLNU, GLZLM_GLNU and GLZLM_ZLNU) result in poorer prognosis for higher values of these indices. This fact is reflected by means of the HR for these image features (GLRLM_RLNU, HR = 1.002 (1.000–1.003),
p = 0.043; GLZLM_GLNU, HR = 1.069 (1.005–1.137),
p = 0.033; GLZLM_ZLNU, HR = 1.017 (1.005–1.030),
p = 0.007). Additionally, RFS Cox regression analysis revealed higher relapse rates for higher values of GLZLM_ZLNU (HR = 1.011 (1.001–1.022),
p = 0.031). As mentioned, recent studies have supported the assumption of higher intratumoral heterogeneity being related to poorer outcomes [
8,
17,
18]. Specifically, these three textural features have also been found to be prognostic factors in head and neck cancer by previous studies [
13,
19,
20]. This premise is reinforced in univariate RFS analysis. Log-rank tests for 2-year follow-up reflect differences in RFS rates for zone-length non-uniformity (GLZLM_ZLNU) (
p = 0.001), with the better prognosis related to lower values (SR: 78.4% vs. SR: 26.7%, low- and high-value subgroups, respectively), i.e., higher intratumoral heterogeneity means poorer prognosis. RFS Kaplan–Meier results remained statistically significant after multiple-testing correction. Additional textural features were statistically significant in RFS Kaplan–Meier (GLCM_Entropy,
p = 0.023; NGLDM_Busyness,
p = 0.040; GLZLM_GLNU,
p = 0.047) analysis. However, this may be due to the small size of the cohort; these results were not statistically significant after multiple-testing correction. Cox regression analyses adjusted by HPV status did not demonstrate the influence of HPV in the outcomes of OS or RFS, since the results showed that some variables are influenced by this factor, obtaining lower
p-values, while others are not, making it no longer significant. Further studies involving greater cohorts are needed to prove these findings. Limitations of this study include a small cohort of patients, considering that 7 patients out of the 46 total subjects were excluded due to tumor stage or size. In addition, the database is unbalanced (36 male and 3 female, 36 smokers and 3 non-smokers, and 26 HPV-negative and 13 HPV-positive). Therefore, no differences between smokers and non-smokers, nor differences between HPV-positive and HPV-negative smokers, could be evaluated. Another limitation is the number of radiomic features analyzed, as other studies evaluate hundreds of characteristics, such as in [
12,
25], while this study is limited to the LIFEx feature extraction. In addition, only survival and univariate analyses are performed, while machine learning models were developed previously by other authors [
40,
41]. For future works, it is proposed to analyze a larger and more balanced (sex, smoker, and HPV status) database and a larger group of radiomic characteristics, also by using machine learning models.