Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
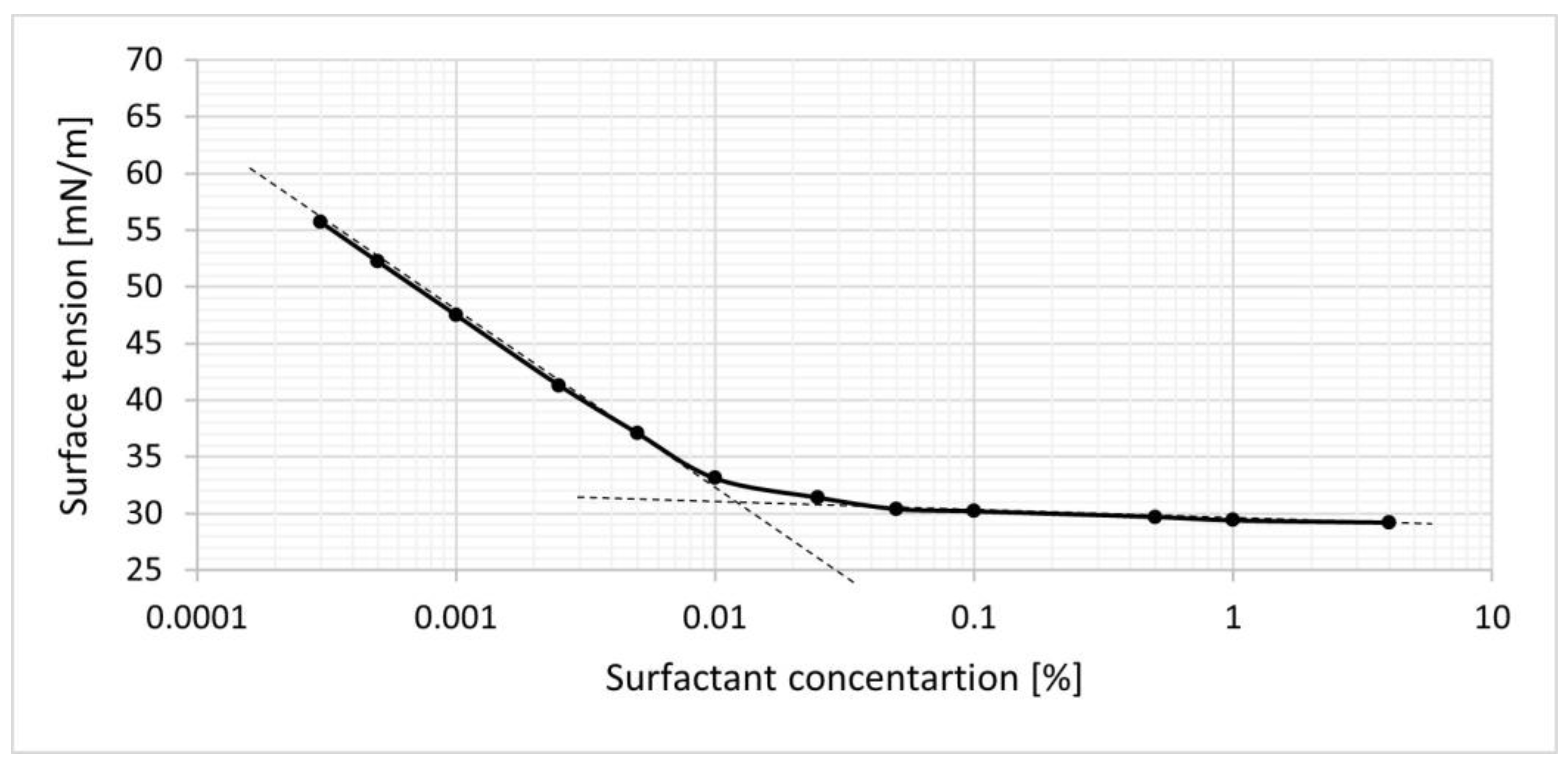
2.3. Micellar-extraction medium Characterization
Critical Micellar Concentration (CMC)
2.4. Solvent and Micellar LCE of Grapevine Buds
2.5. Determination of Bioactive Compounds
Quantitative Analysis of Selected Compounds in Grapevine Bud Extracts Using Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry (UPLC-MS/MS)
2.6. Determination of Antioxidant Properties
2.6.1. Total Phenolics Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.6.3. Antioxidant Activity (DPPH Test)
2.6.4. Antioxidant Activity (ABTS Test)
2.7. Color Parameters Determination of the Grapevine Bud extracts
2.8. Model Facial Serum Preparation
2.9. Assessment of the Cytotoxicity of the Tested Facial Serums on Keratinocytes
2.9.1. Cell Culture
2.9.2. Alamar Blue Assay (AB)
2.9.3. Neutral Red Assay (NR)
2.10. Rheological Behavior
2.11. Microbiological Stability
2.12. Chemical Stability
2.13. Statistical Analysis
3. Results
3.1. Development of Micellar and Solvent Loan Chemical Extraction for Obtaining Cosmetically Valuable Compounds from Grapevine Buds
3.2. Determination of Selected Compounds via UPLC-MS/MS
3.3. Total Phenolic, Flavonoids Content, and Antioxidant Activity (DPPH and ABTS)
3.4. Determination of the Color Parameters of Grapevine Bud Extracts
3.5. Application Analysis
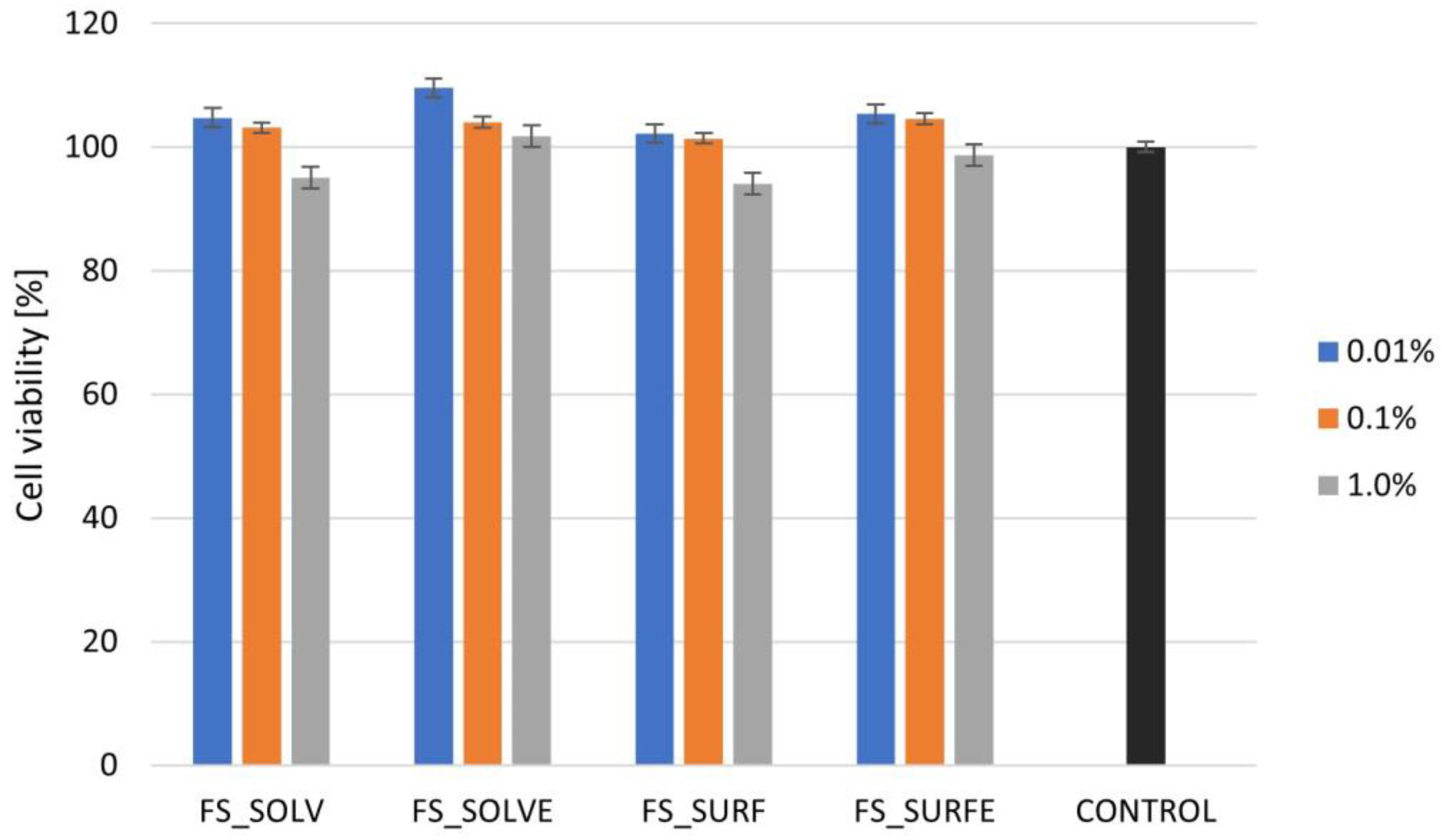
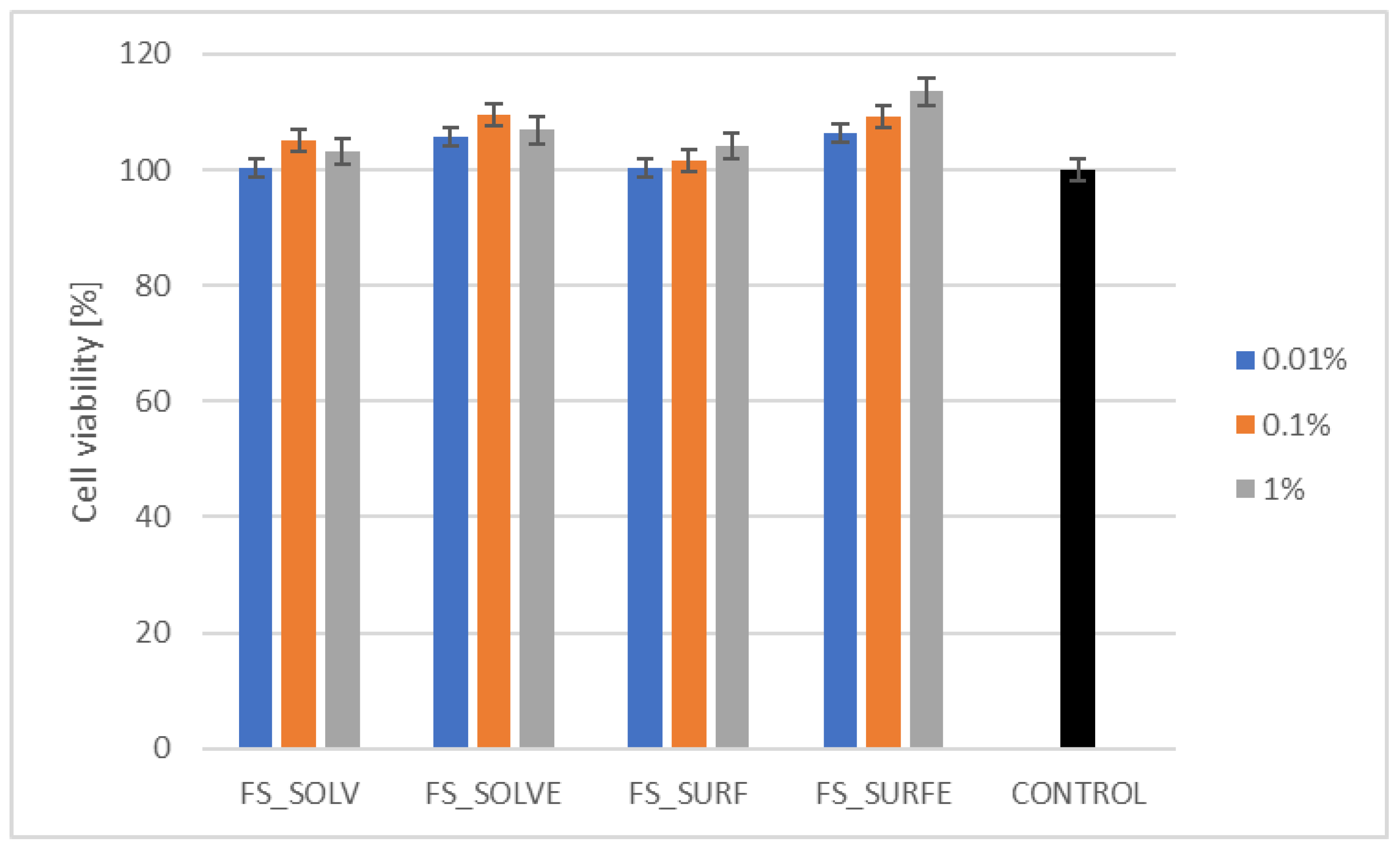
3.5.1. Cytotoxicity Assessment
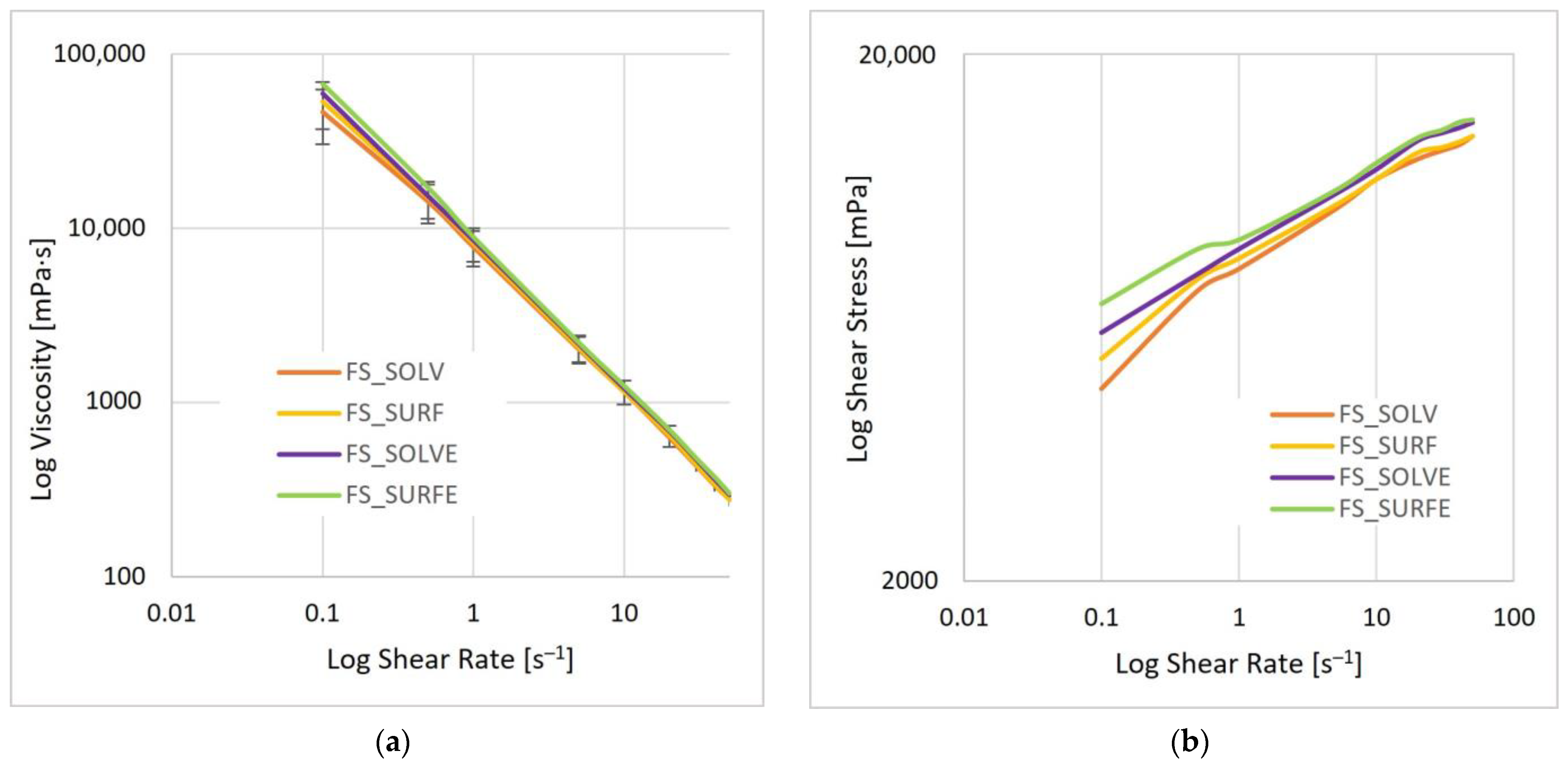
3.5.2. Rheological Behavior
3.5.3. Microbiological Stability
3.5.4. Chemical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FS_Solv | Facial Serum without extract (solvent extraction) |
| FS_SolvE | Facial Serum with extract (solvent extraction) |
| FS_Surf | Facial Serum without extract (surfactant extraction) |
| FS_SurfE | Facial Serum with extract (surfactant extraction) |
References
- Dorni, A.C.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants. 2017, 7, 1–26. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Lal, K.; Wasilewski, T.; Hordyjewicz-Baran, Z. Flower Extracts as Multifunctional Dyes in the Cosmetics Industry. Molecules 2022, 27, 922. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. Extracts as Bioactive Ingredients That Increase Safety of Body Wash Cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Gaweł-Bęben, K.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–292. [Google Scholar] [CrossRef]
- Ramawat, K.; Dass, S.; Mathur, M. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–32. [Google Scholar]
- Verpoorte, R. Medicinal plants: A renewable resource for novel leads and drugs. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–5. [Google Scholar]
- Pal, S.K.; Shukla, Y. Herbal medicine: Current status and the future. Asian Pac. J. Cancer Prev. 2003, 4, 281–288. [Google Scholar]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Panizzi, L.; Caponi, C.; Catalano, S.; Cioni, P.L.; Morelli, I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2015, 53, 1071–1083. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud extracts as new phytochemical source for herbal preparations: Quality control and standardization by analytical fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; Volume 1, pp. 187–218. [Google Scholar]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and natural medicine preparations: Analysis of new sources of bioactive compounds from Ribes and Rubus spp. buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Bonvegna, L.; Bounous, G. Castanea spp. Buds as a phytochemical source for herbal preparations: Botanical fingerprint for nutraceutical identification and functional food standardisation. J. Sci. Food Agric. 2014, 94, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Knoss, W.; Chinou, I. Regulation of medicinal plants for public health—European community monographs on herbal substances. Planta Med. 2012, 78, 1311–1316. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.L.; Yuan, Q.P.; Su, D.H.; Liu, W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Medicinal plants, chemical composition and quality: May blackcurrant buds and blackberry sprouts be a new polyphenol source for herbal preparations? J. Appl. Bot. Food Qual. 2013, 86, 79–89. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, G.M.; Cerutti, A.K.; Canterino, S.; Bounous, G. Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Vegetos Int. J. Plant Res. 2012, 25, 21–29. [Google Scholar]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Liang, Y.-Z.; Xie, P.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Stanek-Wandzel, N.; Zarębska, M.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Zajszły-Turko, E.; Tomaka, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Kombucha fermentation as a modern way of processing vineyard by-products into cosmetic raw materials. Int. J. Cosmet. Sci. 2023, 45, 834–850. [Google Scholar] [CrossRef]
- Brito, A.G.; Peixoto, J.; Oliveria, J.M.; Oliveria, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and winery wastewater treatment: Some focal points of design and operation. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer Science + Business Media LLC.: New York, NY, USA, 2007; pp. 109–131. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J Clean Prod 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant activities of grape Vitis vinifera pomace extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Kumar, S.J.; Kumar, G.V.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Oluwaseun, R.A.; Nour, H.A.; Chinonso, I.U. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar]
- Venkatesan, T.; Choi, Y.; Kim, Y. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Effect of solvents on the antioxidant activity of walnut (Juglans regia L.) shell extracts. J. Food Nutr. Res. 2014, 2, 621–626. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Sobuj, M.K.A.; Islam, M.A.; Islam, M.S.; Islam, M.M.; Mahmud, Y.; Rafiquzzaman, S.M. Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci. Rep. 2021, 11, 19082. [Google Scholar] [CrossRef]
- Wasilewski, T.; Hordyjewicz-Baran, Z.; Zarębska, M.; Stanek, N.; Zajszły-Turko, E.; Tomaka, M.; Bujak, T.; Nizioł-Łukaszewska, Z. Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics. Molecules 2022, 27, 2444. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the extraction of antioxidants from winery wastes using cloud point extraction and a surfactant of natural origin (lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Kumar, B.; Tikariha, D.; Ghosh, K.K. Effects of Electrolytes on Micellar and Surface Properties of Some Monomeric Surfactants. J. Dispers. Sci. Technol. 2012, 33, 265–271. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements in Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Tomaka, M.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Zarębska, M.; Stanek, N.; Zajszły-Turko, E.; Tomaka, K. The role of commodity science in quality management in a knowledge-based economy. In Proceedings of the 11th International Scientific Conference, Gdynia, Poland, 7–9 September 2022. [Google Scholar]
- Parus, A. Antioxidant and Pharmacological Properties of Phenolic Acids. Postępy Fitoter. 2012. Available online: https://www.czytelniamedyczna.pl/4338,przeciwutleniajce-i-farmakologiczne-waciwoci-kwaslw-fenolowych.html (accessed on 1 December 2023).
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. Agrobiodiversity Conservation: Genotype Influence on Chemical and Sensorial Traits of Cultivars Grown on the Same Clonal Rootstock. Foods 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Bononi, I.; Tedeschi, P.; Mantovani, V.; Maietti, A.; Mazzoni, E.; Pancaldi, C.; Brandolini, V.; Tognon, M. Antioxidant Activity of Resveratrol Diastereomeric Forms Assayed in Fluorescent-Engineered Human Keratinocytes. Antioxidants 2022, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Shiu, P.H.-T.; Zheng, C.; Rangsinth, P.; Wang, W.; Li, J.; Li, R.; Leung, G.P.-H. Anti-inflammatory effect of gallic acid on HaCaT keratinocytes through the inhibition of MAPK-, NF-kB-, and Akt-dependent signaling pathways. Bangladesh J. Pharmacol. 2023, 18, 24–32. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Protective effect of 3,5-dicaffeoyl-epi-quinic acid against UVB-induced photoaging in human HaCaT keratinocytes. Mol. Med. Rep. 2019, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Han, J.H.; Hwang, E.J.; Seo, J.Y.; Cho, K.H.; Kim, K.H.; Youn, J.I.; Eun, H.C. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003, 17, 1913–1915. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; De Bellis, R.; Calcabrini, C.; Potenza, L.; Cucchiarini, L.; Mancini, U.; Dachà, M.; Ricci, D. Aqueous extract from Vitis vinifera tendrils is able to enrich keratinocyte antioxidant defences. Nat. Prod. Commun. 2011, 6, 1315–1319. [Google Scholar]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Amin, S. Rheology of Cosmetic Products: Surfactant Mesophases, Foams and Emulsions. J. Cosmet. Sci. 2020, 71, 481–496. [Google Scholar] [PubMed]
- Gräbner, D.; Hoffmann, H. Rheology of Cosmetic Formulations. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 471–488. [Google Scholar]
- Moravkova, T.; Stern, P. Rheological and Textural Properties of Cosmetic Emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar]
- Sulek, M.; Wasilewski, T.; Zieba, M. Tribological and physical-chemical properties of aqueous solutions of cationic surfactants. Ind. Lubr. Tribol. 2010, 62, 279–284. [Google Scholar] [CrossRef]
- Sarkar, D.K. Creams and Ointments. In Pharmaceutical Emulsions: A Drug Developer’s Toolbag; Sarkar, D.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 69–76. [Google Scholar]
- Fan, Y.; Zhang, L.; Wang, X.; Wang, K.; Wang, L.; Wang, Z.; Xue, F.; Zhu, J.; Wang, C. Rheological thixotropy and pasting properties of food thickening gums orienting at improving food holding rate. Appl. Rheol. 2022, 32, 100–121. [Google Scholar] [CrossRef]
- García-Ochota, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]



| Ingredient (INCI Name) | SurfE [wt. %] | SolvE [wt. %] | |
|---|---|---|---|
| 1 | polyglyceryl-4 laurate/sebacate (and) polyglyceryl-6 caprylate/caprate | 4 | - |
| 1,3-propanediol | - | 4 | |
| 2 | benzyl alcohol, benzoic acid, dehydroacetic acid, tocopherol | 0.5 | 0.5 |
| 3 | aqua | 85.5 | 85.5 |
| 4 | grapevine buds | 10 | 10 |
| FS_Solv Facial Serum Based on Solvent without Extract | FS_SolvE Facial Serum Based on Solvent with Extract | FS_Surf Facial Serum Based on Surfactants without Extract | FS_SurfE Facial Serum Based on Surfactants with Extract | ||
|---|---|---|---|---|---|
| Ingredient (INCI Name) | [wt. %] | [wt. %] | [wt. %] | [wt. %] | |
| 1 | aqua | 60 | 60 | 60 | 60 |
| 2 | xanthan gum | 0.6 | 0.6 | 0.6 | 0.6 |
| 3 | benzyl alcohol, benzoic acid, dehydroacetic acid, tocopherol | 0.5 | 0.45 | 0.5 | 0.45 |
| 4 | sodium hydroxide/lactic acid | to pH 5.5 | to pH 5.5 | to pH 5.5 | to pH 5.5 |
| 5 | polyglyceryl-4 laurate/sebacate (and) polyglyceryl-6 caprylate/caprate | - | - | 0.4 | - |
| 6 | propanediol | 0.4 | - | - | - |
| 7 | extract with surfactant | - | - | - | 10.0 |
| 8 | extract with solvent | - | 10.0 | - | - |
| 9 | aqua | to 100 | to 100 | to 100 | to 100 |
| Compound | SurfE | SolvE |
|---|---|---|
| Quinic acid | 78.8 ± 0.8 a | 54.5 ± 0.6 b |
| Gallic acid | 10.8 ± 0.4 a | 9.0 ± 0.4 b |
| Rutin | 8.43 ± 0.28 a | 3.43 ± 0.41 b |
| Apigenin | 4.91 ± 0.05 a | 5.07 ± 0.06 a |
| Vanillic acid | 1.09 ± 0.06 a | 1.08 ± 0.15 a |
| trans-Resveratrol | 1.67 ± 0.09 a | 1.65 ± 08 a |
| (−)-Epicatechin 3-gallate | 1.07 ± 0.07 | nd |
| Quercetin | 0.46 ± 0.09 | nd |
| (+)-Catechin | 0.31 ± 0.02 | nd |
| trans-Ferulic acid | 0.35 ± 0.01 | nd |
| Sum of polyphenols | 107.9 | 74.8 |
| Grapevine Buds Extract | TPC [mg GAE/L] | TFC [mg QE/L] | DPPH [%] | ABTS [%] |
|---|---|---|---|---|
| SurfE | 722 ± 13 | 57.6 ± 1.1 | 69.8 ± 1.7 | 42.4 ± 1.9 |
| SolvE | 486 ± 10 | 32.6 ± 0.7 | 60.1 ± 2.5 | 34.6 ± 1.4 |
| L* | a* | b* | C* | ho | Color | |
|---|---|---|---|---|---|---|
| SolvE | 53.65 ± 0.04 a | −7.40 ± 0.01 a | 19.29 ± 0.03 b | 20.66 ± 0.03 b | 111.00 ± 0.04 b | more greener yellow |
| SurfE | 45.06 ± 0.02 b | −7.18 ± 0.01 b | 29.52 ± 0.01 a | 30.38 ± 0.01 a | 103.66 ± 0.01 a | less greener yellow |
| Sample | n | K | R2 |
|---|---|---|---|
| FS_Solv | 0.17 | 7.508 | 0.9848 |
| FS_SolvE | 0.15 | 8.476 | 0.9953 |
| FS_Surf | 0.15 | 7.966 | 0.9895 |
| FS_SurfE | 0.13 | 9.115 | 0.9952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hordyjewicz-Baran, Z.; Wasilewski, T.; Zarębska, M.; Stanek-Wandzel, N.; Zajszły-Turko, E.; Tomaka, M.; Zagórska-Dziok, M. Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds. Appl. Sci. 2024, 14, 1420. https://doi.org/10.3390/app14041420
Hordyjewicz-Baran Z, Wasilewski T, Zarębska M, Stanek-Wandzel N, Zajszły-Turko E, Tomaka M, Zagórska-Dziok M. Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds. Applied Sciences. 2024; 14(4):1420. https://doi.org/10.3390/app14041420
Chicago/Turabian StyleHordyjewicz-Baran, Zofia, Tomasz Wasilewski, Magdalena Zarębska, Natalia Stanek-Wandzel, Ewa Zajszły-Turko, Magdalena Tomaka, and Martyna Zagórska-Dziok. 2024. "Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds" Applied Sciences 14, no. 4: 1420. https://doi.org/10.3390/app14041420
APA StyleHordyjewicz-Baran, Z., Wasilewski, T., Zarębska, M., Stanek-Wandzel, N., Zajszły-Turko, E., Tomaka, M., & Zagórska-Dziok, M. (2024). Micellar and Solvent Loan Chemical Extraction as a Tool for the Development of Natural Skin Care Cosmetics Containing Substances Isolated from Grapevine Buds. Applied Sciences, 14(4), 1420. https://doi.org/10.3390/app14041420






