Immuno-PET for Glioma Imaging: An Update
Abstract
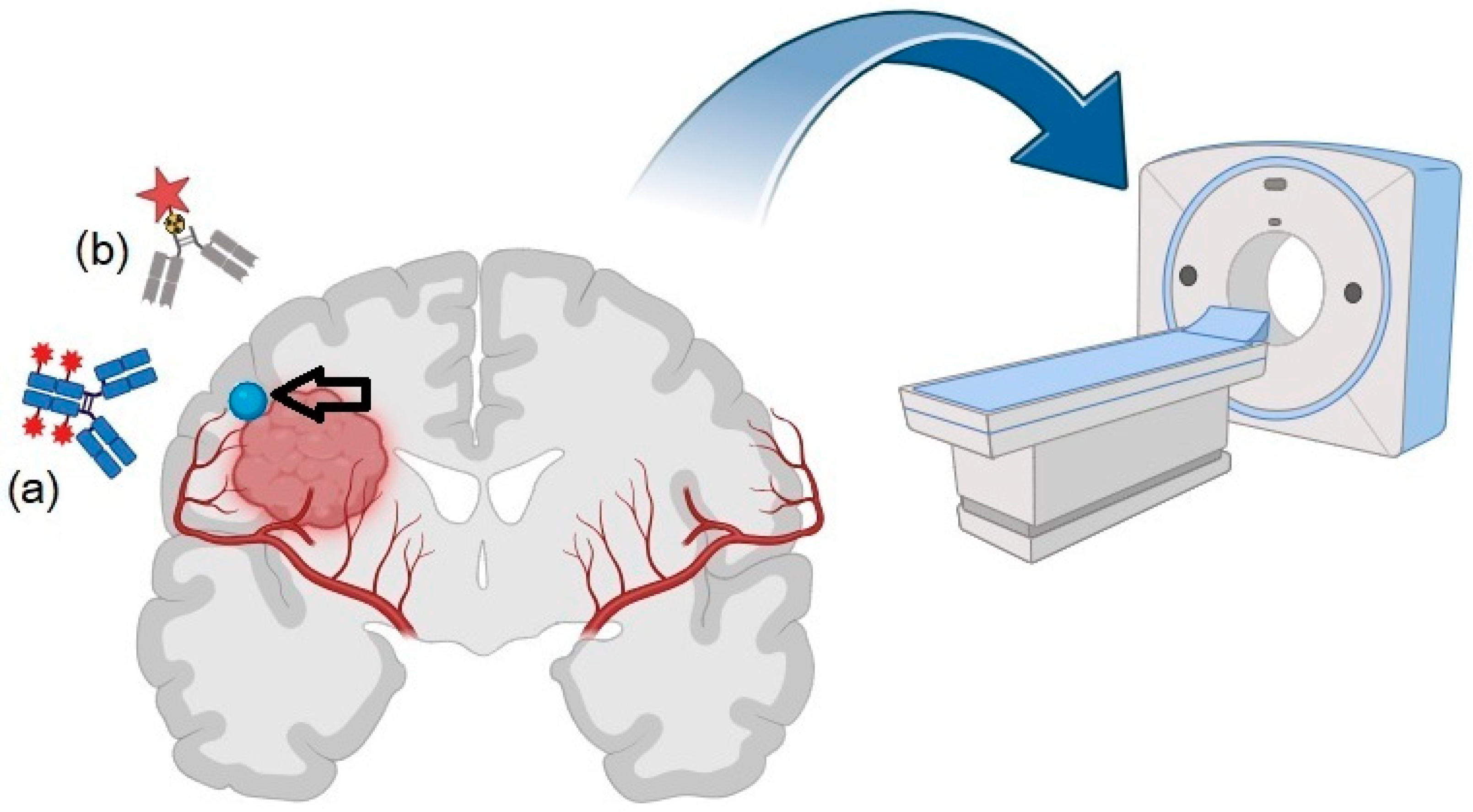
1. Introduction
2. Gliomas
2.1. Classification
2.2. Natural History
2.3. Cinical Management
2.3.1. Surgery
2.3.2. Radiotherapy
2.3.3. Chemotherapy
3. Immuno-PET: Basic Principles and Technical Features
4. Targets for Immuno-PET
4.1. EGFR
4.2. VEGF
4.3. Cell Differentiation Antigens
4.4. Prostate-Specific Membrane Antigen (PSMA)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Liang, J.; Lv, X.; Lu, C.; Ye, X.; Chen, X.; Fu, J.; Luo, C.; Zhao, Y. Prognostic Factors of Patients with Gliomas—An Analysis on 335 Patients with Glioblastoma and Other Forms of Gliomas. BMC Cancer 2020, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Frosina, G. Recapitulating the Key Advances in the Diagnosis and Prognosis of High-Grade Gliomas: Second Half of 2021 Update. Int. J. Mol. Sci. 2023, 24, 6375. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wee, C.W. Treatment of Adult Gliomas: A Current Update. Brain NeuroRehabil. 2022, 15, e24. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O. Immunotherapy for Malignant Gliomas. In Cancer Treatment and Research; Springer: Cham, Switzerland, 2015; Volume 163, pp. 143–158. [Google Scholar] [CrossRef]
- Rajesh, Y.; Pal, I.; Banik, P.; Chakraborty, S.; Borkar, S.A.; Dey, G.; Mukherjee, A.; Mandal, M. Insights into Molecular Therapy of Glioma: Current Challenges and next Generation Blueprint. Acta Pharmacol. Sin. 2017, 38, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Higuchi, T.; Pomper, M.G.; Rowe, S.P. Theranostics in Oncology-Thriving, Now More than Ever. Diagnostics 2021, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Brat, D.J.; Verhaak, R.G.W.; Aldape, K.D.; Yung, W.K.A.; Salama, S.R.; Cooper, L.A.D.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Opoku-Darko, M.; Eagles, M.E.; Cadieux, M.; Isaacs, A.M.; Kelly, J.J.P. Natural History and Growth Patterns of Incidentally Discovered Diffusely Infiltrating Low-Grade Gliomas: A Volumetric Study. World Neurosurg. 2019, 132, e133–e139. [Google Scholar] [CrossRef]
- Altieri, R.; Hirono, S.; Duffau, H.; Ducati, A.; Fontanella, M.M.; La Rocca, G.; Melcarne, A.; Panciani, P.P.; Spena, G.; Garbossa, D. Natural History of de Novo High Grade Glioma: First Description of Growth Parabola. J. Neurosurg. Sci. 2020, 64, 399–403. [Google Scholar] [CrossRef]
- Lombardi, G.; Barresi, V.; Castellano, A.; Tabouret, E.; Pasqualetti, F.; Salvalaggio, A.; Cerretti, G.; Caccese, M.; Padovan, M.; Zagonel, V.; et al. Clinical Management of Diffuse Low-Grade Gliomas. Cancers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for Potentials and Limitations of Brain Plasticity Using an Atlas of Functional Resectability of WHO Grade II Gliomas: Towards a “Minimal Common Brain”. Neuroimage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Arusell, R.; Scheithauer, B.; O’Fallon, J.; O’Neill, B.; Dinapoli, R.; Nelson, D.; Earle, J.; Jones, C.; Cascino, T.; et al. Prospective Randomized Trial of Low- versus High-Dose Radiation Therapy in Adults with Supratentorial Low-Grade Glioma: Initial Report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 2002, 20, 2267–2276. [Google Scholar] [CrossRef]
- Wang, T.J.C.; Mehta, M.P. Low-Grade Glioma Radiotherapy Treatment and Trials. Neurosurg. Clin. N. Am. 2019, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Frosina, G. Radiotherapy of High-Grade Gliomas: Dealing with a Stalemate. Crit. Rev. Oncol. Hematol. 2023, 190, 104110. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, A.P. Chemotherapy for Malignant Gliomas. Oncology 1993, 7, 93–100; discussion 103+106. [Google Scholar] [PubMed]
- See, S.-J.; Gilbert, M.R. Chemotherapy in Adults with Gliomas. Ann. Acad. Med. Singap. 2007, 36, 364–366. [Google Scholar]
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging Using Radiolabelled Targeted Proteins: Radioimmunodetection and Beyond. EJNMMI Radiopharm. Chem. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Takasu, S.; Takahashi, T.; Okamoto, S.; Oriuchi, N.; Nakayashiki, N.; Okamoto, K.; Muramatsu, H.; Hayashi, T.; Nakahara, N.; Mizuno, M.; et al. Radioimmunoscintigraphy of Intracranial Glioma Xenograft with a Technetium-99m-Labeled Mouse Monoclonal Antibody Specifically Recognizing Type III Mutant Epidermal Growth Factor Receptor. J. Neurooncol. 2003, 63, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Schillaci, O.; Santoni, R.; Manni, C.; Danieli, R.; Simonetti, G. Usefulness of SPECT/CT with a Hybrid Camera for the Functional Anatomical Mapping of Primary Brain Tumors by [Tc99m] Tetrofosmin. Cancer Biother. Radiopharm. 2006, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Danieli, R.; Filippi, L.; Romano, P.; Cossu, E.; Manni, C.; Simonetti, G. Scintimammography with a Hybrid SPECT/CT Imaging System. Anticancer Res. 2007, 27, 557–562. [Google Scholar] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef] [PubMed]
- Lugat, A.; Bailly, C.; Chérel, M.; Rousseau, C.; Kraeber-Bodéré, F.; Bodet-Milin, C.; Bourgeois, M. Immuno-PET: Design Options and Clinical Proof-of-Concept. Front. Med. 2022, 9, 1026083. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-K.; Park, B.-N.; Ryu, E.-K.; An, Y.-S.; Lee, S.-J. Current Perspectives on 89Zr-PET Imaging. Int. J. Mol. Sci. 2020, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Levin Klausen, T.; Høgild Keller, S.; Vinter Olesen, O.; Aznar, M.; Andersen, F.L. Innovations in PET/CT. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 268–279. [Google Scholar]
- Meikle, S.R.; Sossi, V.; Roncali, E.; Cherry, S.R.; Banati, R.; Mankoff, D.; Jones, T.; James, M.; Sutcliffe, J.; Ouyang, J.; et al. Quantitative PET in the 2020s: A Roadmap. Phys. Med. Biol. 2021, 66, 06RM01. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, L.; Peng, R.; Yue, C.; Huang, L. Prognostic Value of 18F-FDG PET/CT in Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 1014063. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, B.; Liu, Q.; Xia, L.; Liu, S.; Zheng, C.; Liu, H.; Mo, Y.; Zhang, X.; Hu, Y.; et al. Patlak-Ki Derived from Ultra-High Sensitivity Dynamic Total Body [18F]FDG PET/CT Correlates with the Response to Induction Immuno-Chemotherapy in Locally Advanced Non-Small Cell Lung Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3400–3413. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Morris, O.; Fairclough, M.; Grigg, J.; Prenant, C.; McMahon, A. A Review of Approaches to 18F Radiolabelling Affinity Peptides and Proteins. J. Labelled Comp. Radiopharm. 2019, 62, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Song, I.H.; Shin, J., II; Park, Y.S.; Kim, J.Y.; Kim, K., II; Lee, Y.J.; Kang, J.H. PET Imaging Biomarkers of Anti-EGFR Immunotherapy in Esophageal Squamous Cell Carcinoma Models. Cells 2018, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with (44)Sc-Labeled Cetuximab Fab Fragment. Bioconjug. Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Köllermann, J.; Helpap, B. Expression of Vascular Endothelial Growth Factor (VEGF) and VEGF Receptor Flk-1 in Benign, Premalignant, and Malignant Prostate Tissue. Am. J. Clin. Pathol. 2001, 116, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.; Histed, S.; Xu, B.; Bhadrasetty, V.; Szajek, L.P.; Williams, M.R.; Wong, K.; Wu, H.; Lane, K.; Coble, V.; et al. Immuno-PET Imaging of Tumor Endothelial Marker 8 (TEM8). Mol. Pharm. 2014, 11, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes Expressed in Human Tumor Endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef]
- Jansen, M.H.A.; Lagerweij, T.; Sewing, A.C.P.; Vugts, D.J.; van Vuurden, D.G.; Molthoff, C.F.M.; Caretti, V.; Veringa, S.J.E.; Petersen, N.; Carcaboso, A.M.; et al. Bevacizumab Targeting Diffuse Intrinsic Pontine Glioma: Results of 89Zr-Bevacizumab PET Imaging in Brain Tumor Models. Mol. Cancer Ther. 2016, 15, 2166–2174. [Google Scholar] [CrossRef]
- Jansen, M.H.; Veldhuijzen van Zanten, S.E.M.; van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; van Dongen, G.A.; Kaspers, G.-J.L. Molecular Drug Imaging: 89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2017, 58, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Q.; Zhang, N.; Du, X.; Xu, G.; Yan, X. CD146, from a Melanoma Cell Adhesion Molecule to a Signaling Receptor. Signal Transduct. Target Ther. 2020, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hernandez, R.; Rao, J.; Yin, L.; Qu, Y.; Wu, J.; England, C.G.; Graves, S.A.; Lewis, C.M.; Wang, P.; et al. Targeting CD146 with a 64Cu-Labeled Antibody Enables in Vivo ImmunoPET Imaging of High-Grade Gliomas. Proc. Natl. Acad. Sci. USA 2015, 112, E6525–E6534. [Google Scholar] [CrossRef]
- Hernandez, R.; Sun, H.; England, C.G.; Valdovinos, H.F.; Barnhart, T.E.; Yang, Y.; Cai, W. ImmunoPET Imaging of CD146 Expression in Malignant Brain Tumors. Mol. Pharm. 2016, 13, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-Infiltrated Innate Immune Cells Resemble M0 Macrophage Phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef]
- Lapa, C.; Linsenmann, T.; Lückerath, K.; Samnick, S.; Herrmann, K.; Stoffer, C.; Ernestus, R.-I.; Buck, A.K.; Löhr, M.; Monoranu, C.-M. Tumor-Associated Macrophages in Glioblastoma Multiforme-a Suitable Target for Somatostatin Receptor-Based Imaging and Therapy? PLoS ONE 2015, 10, e0122269. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; McCarl, L.; Kumar, R.; Edinger, R.S.; Kurland, B.F.; Anderson, C.J.; Panigrahy, A.; Kohanbash, G.; Edwards, W.B. Preclinical ImmunoPET Imaging of Glioblastoma-Infiltrating Myeloid Cells Using Zirconium-89 Labeled Anti-CD11b Antibody. Mol. Imaging Biol. 2020, 22, 685–694. [Google Scholar] [CrossRef]
- Holzgreve, A.; Biczok, A.; Ruf, V.C.; Liesche-Starnecker, F.; Steiger, K.; Kirchner, M.A.; Unterrainer, M.; Mittlmeier, L.; Herms, J.; Schlegel, J.; et al. PSMA Expression in Glioblastoma as a Basis for Theranostic Approaches: A Retrospective, Correlational Panel Study Including Immunohistochemistry, Clinical Parameters and PET Imaging. Front. Oncol. 2021, 11, 646387. [Google Scholar] [CrossRef]
- Muoio, B.; Albano, D.; Dondi, F.; Bertagna, F.; Garibotto, V.; Kunikowska, J.; Piccardo, A.; Annunziata, S.; Espeli, V.; Migliorini, D.; et al. Diagnostic Accuracy of PET/CT or PET/MRI Using PSMA-Targeting Radiopharmaceuticals in High-Grade Gliomas: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics 2022, 12, 1665. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Durack, J.C.; Lyashchenko, S.K.; Cheal, S.M.; Beylergil, V.; Lefkowitz, R.A.; Carrasquillo, J.A.; Martinez, D.F.; Fung, A.M.; et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin. Cancer Res. 2015, 21, 5277–5285. [Google Scholar] [CrossRef]
- Krebs, S.; Grommes, C.; McDevitt, M.R.; Carlin, S.D.; O’Donoghue, J.A.; Graham, M.S.; Young, R.J.; Schöder, H.; Gutin, P.H.; Bander, N.H.; et al. [89Zr]Zr-HuJ591 Immuno-PET Targeting PSMA in IDH Mutant Anaplastic Oligodendroglioma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 783–785. [Google Scholar] [CrossRef]
- Lenffer, B.; Ruben, J.; Senthi, S.; Millar, J.; Ong, W.L. Management and Outcomes of Glioblastoma: 20-Year Experience in a Single Australian Institution. J. Med. Imaging Radiat. Oncol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Kim, N.; Borra, R.; Mouridsen, K.; Hansen, M.B.; Kim, Y.-H.; Hong, C.-K.; Kim, J.H. Prediction of Pseudoprogression in Post-Treatment Glioblastoma Using Dynamic Susceptibility Contrast-Derived Oxygenation and Microvascular Transit Time Heterogeneity Measures. Eur. Radiol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Muoio, B.; Espeli, V.; Treglia, G. Neuro-Oncology and Positron Emission Tomography: “Just Can’t Get Enough”. Cancers 2023, 15, 4739. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, B.; Buresta, T.; Nuvoli, S.; Spanu, A.; Schillaci, O.; Fravolini, M.L.; Palumbo, I. SPECT and PET Serve as Molecular Imaging Techniques and in Vivo Biomarkers for Brain Metastases. Int. J. Mol. Sci. 2014, 15, 9878–9893. [Google Scholar] [CrossRef]
- De Marco, R.; Pesaresi, A.; Bianconi, A.; Zotta, M.; Deandreis, D.; Morana, G.; Zeppa, P.; Melcarne, A.; Garbossa, D.; Cofano, F. A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective. Cancers 2022, 15, 90. [Google Scholar] [CrossRef]
- Mulgaonkar, A.; Udayakumar, D.; Yang, Y.; Harris, S.; Öz, O.K.; Ramakrishnan Geethakumari, P.; Sun, X. Current and Potential Roles of Immuno-PET/-SPECT in CAR T-Cell Therapy. Front. Med. 2023, 10, 1199146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandey, M.K. Radiometals in Imaging and Therapy: Highlighting Two Decades of Research. Pharmaceuticals 2023, 16, 1460. [Google Scholar] [CrossRef]
- Tokumaru, A.M.; Saito, Y.; Murayama, S.; Kazutomi, K.; Sakiyama, Y.; Toyoda, M.; Yamakawa, M.; Terada, H. Imaging-Pathologic Correlation in Corticobasal Degeneration. Am. J. Neuroradiol. 2009, 30, 1884–1892. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Thurber, G.M.; Keliher, E.J.; Marinelli, B.; Weissleder, R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. USA 2012, 109, 4762–4767. [Google Scholar] [CrossRef]
- Triumbari, E.K.A.; Morland, D.; Laudicella, R.; Bauckneht, M.; Albano, D.; Annunziata, S. Clinical Applications of Immuno-PET in Lymphoma: A Systematic Review. Cancers 2022, 14, 3488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, N.; An, Z. Engineering antibody and protein therapeutics to cross the blood-brain barrier. Antib. Ther. 2022, 5, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.H.; Snyder, D.J.; Spasojevic, I.; Gedeon, P.C.; Sanchez-Perez, L.; Sampson, J.H. First in human dose calculation of a single-chain bispecific antibody targeting glioma using the MABEL approach. J. Immunother. Cancer 2020, 8, e000213. [Google Scholar] [CrossRef]
- Cavaco, M.; Gaspar, D.; Arb Castanho, M.; Neves, V. Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Pharmaceutics 2020, 12, 62. [Google Scholar] [CrossRef]
- Lepareur, N.; Ramée, B.; Mougin-Degraef, M.; Bourgeois, M. Clinical Advances and Perspectives in Targeted Radionuclide Therapy. Pharmaceutics 2023, 15, 1733. [Google Scholar] [CrossRef]
- Singh, A.; Patel, A.; Chaudhary, H.; Yadav, K.; Minocha, N. Nanotheranostics: The Fabrication of Theranostics with Nanoparticles and Their Application to Treat the Neurological Disorders. Recent Pat. Nanotechnol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Filippi, L.; Frantellizzi, V.; De Vincentis, G.; Schillaci, O.; Evangelista, L. Clinical Applications of TSPO PET for Glioma Imaging: Current Evidence and Future Perspective-A Systematic Review. Diagnostics 2023, 13, 1813. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.L.; Shih, Y.H.; Chiang, P.F.; Chen, C.T.; Chang, M.C. Multifunctional Cyanine-Based Theranostic Probe for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2021, 22, 12214. [Google Scholar] [CrossRef]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Luwor, R.B.; Stylli, S.S.; Kaye, A.H. Using bioluminescence imaging in glioma research. J. Clin. Neurosci. 2015, 22, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, K.; Jiang, S.; Liu, Y.; Ai, T.; Tian, J. Bioluminescence Tomography Based on Gaussian Weighted Laplace Prior Regularization for In Vivo Morphological Imaging of Glioma. IEEE Trans. Med. Imaging 2017, 36, 2343–2354, Erratum in IEEE Trans. Med. Imaging 2018, 37, 2161. [Google Scholar] [CrossRef] [PubMed]
| Reference | Location/Year | Study | Isotope | Half-Life | Immuno-PET Ligand | Target | Animal/Human Study and Tracer Administration Modality | Comparison with Other Imaging Modalities | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Chakravarty et al. [37] | USA/2014 | Pre-clinical | 44Sc | 3.97 h | 44Sc- Cetuximab-Fab | EGFR | human glioblastoma (U87MG) tumor-bearing mice, i.v. | N.A. | 44Sc-Cetuximab-Fab in the tumor, with a peak uptake of approximately 12% ID/g observed at 4 h post-injection, potentially suitable for 1 day protocol |
| Jansen et al. [41] | The Netherlands/2016 | Pre-clinical | 89Zr | 78.4 h | 89Zr-bevacizumab | VEGF | DIPG mouse model (Human E98-FM, U251-FM glioma cells, and HSJD-DIPG-007-FLUC primary DIPG cells), i.v. | N.A. | 89Zr-bevacizumab immuno-PET might help identify patients affected by DIPG suitable for bevacizumab therapy |
| Jansen et al. [42] | The Netherlands/2017 | Pilot study (fist-in-humans) | 89Zr | 78.4 h | 89Zr-bevacizumab | VEGF | DIPG patients, i.v. | Differently from MRI, 89Zr-bevacizumab PET imaging is helpful in candidates’ selection for bevacizumab treatment | 89Zr-bevacizumab immuno-PET may be feasible in children affected by DIPG, potentially useful to assess heterogeneity in VEGF expression in tumors |
| Yang et al. [44] | USA/2015 | Pre-clinical | 64Cu | 12.7 h | 64Cu-NOTA-YY146 | CD146 | mice bearing U87MG and U251 xenografts, i.v. | N.A. | In animal models, immuno-PET targeting CD146 showed potential for use in GBM detection and targeted therapy |
| Hernandez et al. [45] | USA/2016 | Pre-clinical | 89Zr | 78.4 h | 89Zr-Df-YY146 | CD146 | Mice bearing U87MG and U251 xenografts, i.v. | N.A. | Immuno-PET showed 89Zr-Df-YY146 in GBM xenografts, peaking at 48 h p.i. |
| Nigam et al. [48] | USA/2020 | Pre-clinical | 89Zr | 78.4 h | 89Zr-anti-CD11b Ab | CD11b | Mice bearing established orthotopic syngeneic GL261 gliomas, i.v. | MRI is useful for tumor volume assessment, but it is not capable of quantifying immune cell population status of tumor microenvironment | Immuno-PET showed promising results when used to visualize tumor-associated myeloid cells (TAMCs) in GBM |
| Krebs et al. [52] | USA/2022 | Case report | 89Zr | 78.4 h | 89Zr-huJ591 | PSMA | Human study (grade II oligodendroglioma, 1p/19q co-deleted, IDH mutant), i.v. | MRI used for fusion imaging | PSMA-targeted immuno-PET was able to detect oligodendroglioma-associated neovasculature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feo, M.S.; Granese, G.M.; Conte, M.; Palumbo, B.; Panareo, S.; Frantellizzi, V.; De Vincentis, G.; Filippi, L. Immuno-PET for Glioma Imaging: An Update. Appl. Sci. 2024, 14, 1391. https://doi.org/10.3390/app14041391
De Feo MS, Granese GM, Conte M, Palumbo B, Panareo S, Frantellizzi V, De Vincentis G, Filippi L. Immuno-PET for Glioma Imaging: An Update. Applied Sciences. 2024; 14(4):1391. https://doi.org/10.3390/app14041391
Chicago/Turabian StyleDe Feo, Maria Silvia, Giorgia Maria Granese, Miriam Conte, Barbara Palumbo, Stefano Panareo, Viviana Frantellizzi, Giuseppe De Vincentis, and Luca Filippi. 2024. "Immuno-PET for Glioma Imaging: An Update" Applied Sciences 14, no. 4: 1391. https://doi.org/10.3390/app14041391
APA StyleDe Feo, M. S., Granese, G. M., Conte, M., Palumbo, B., Panareo, S., Frantellizzi, V., De Vincentis, G., & Filippi, L. (2024). Immuno-PET for Glioma Imaging: An Update. Applied Sciences, 14(4), 1391. https://doi.org/10.3390/app14041391









