The Effect of Different Ferrule Configurations and Preparation Designs on the Fatigue Performance of Endodontically Treated Maxillary Central Incisors: A 3D Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
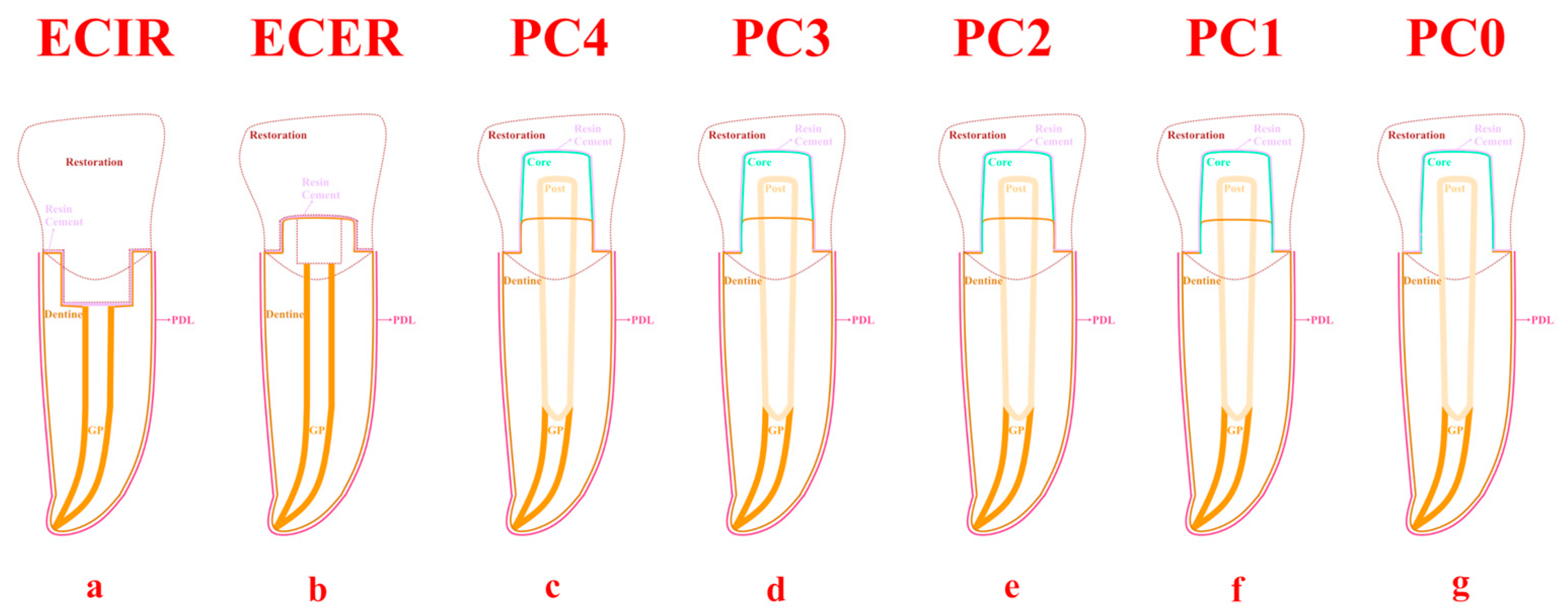
2.1. Preparation of Samples
2.2. Numerical Simulation
2.3. Calculation of Fatigue Performance
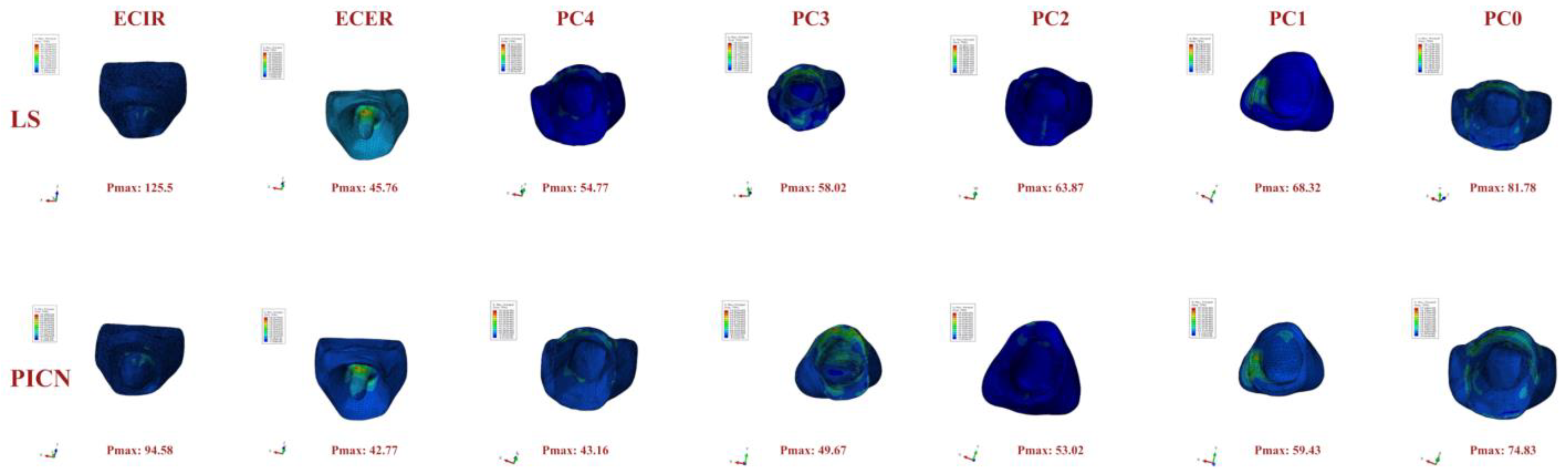
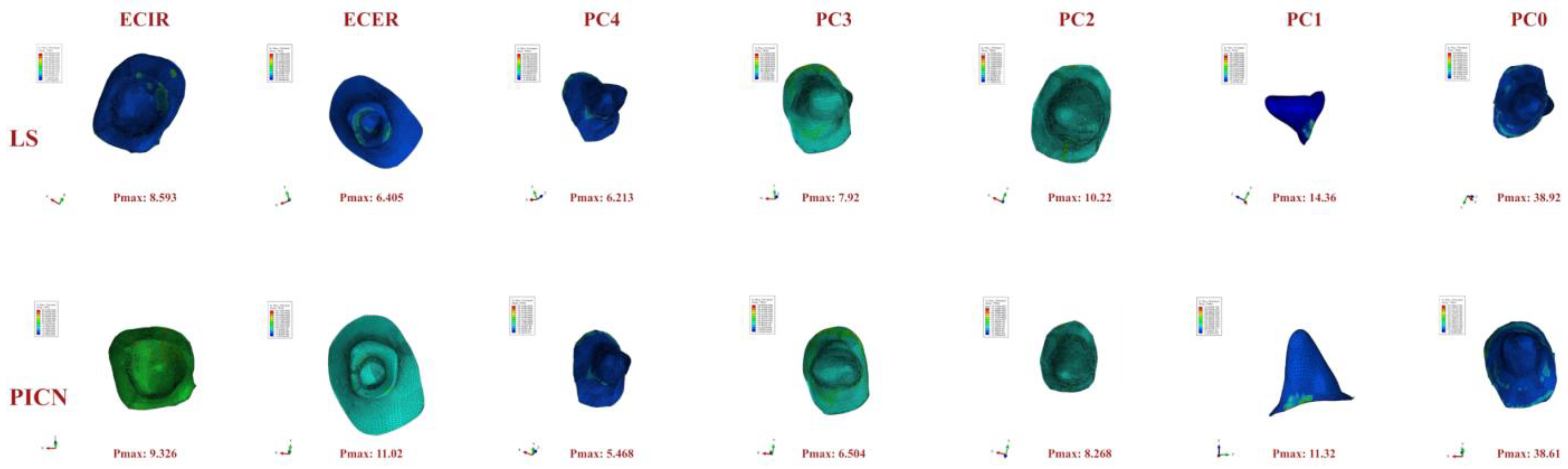
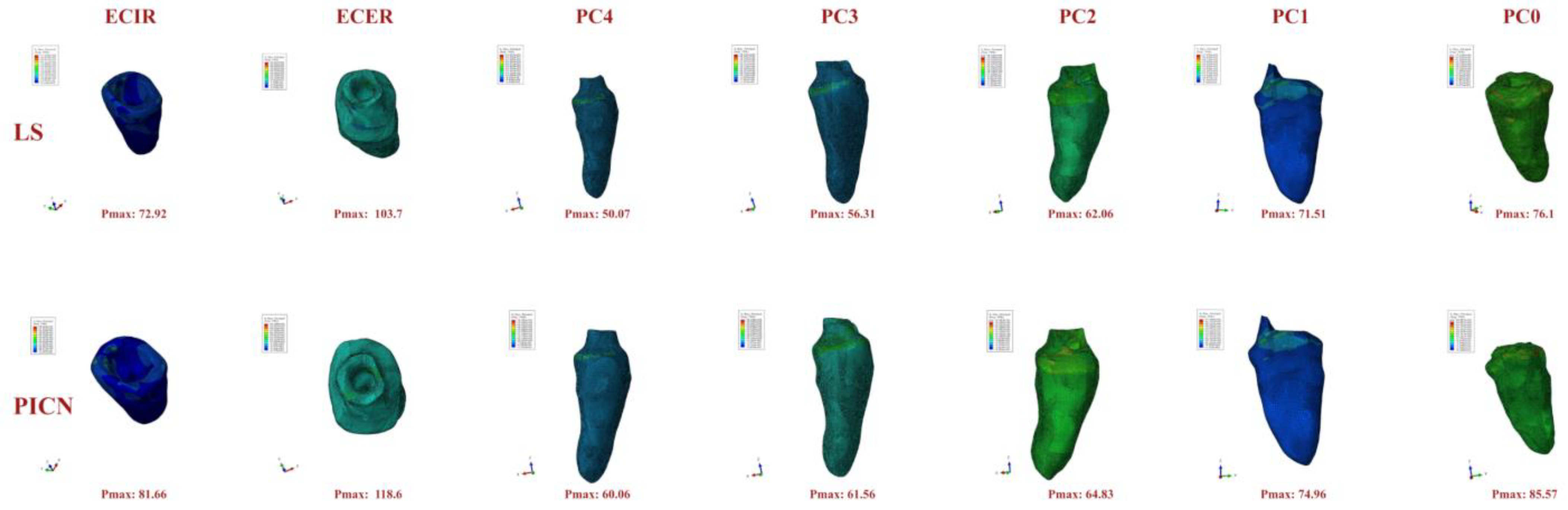
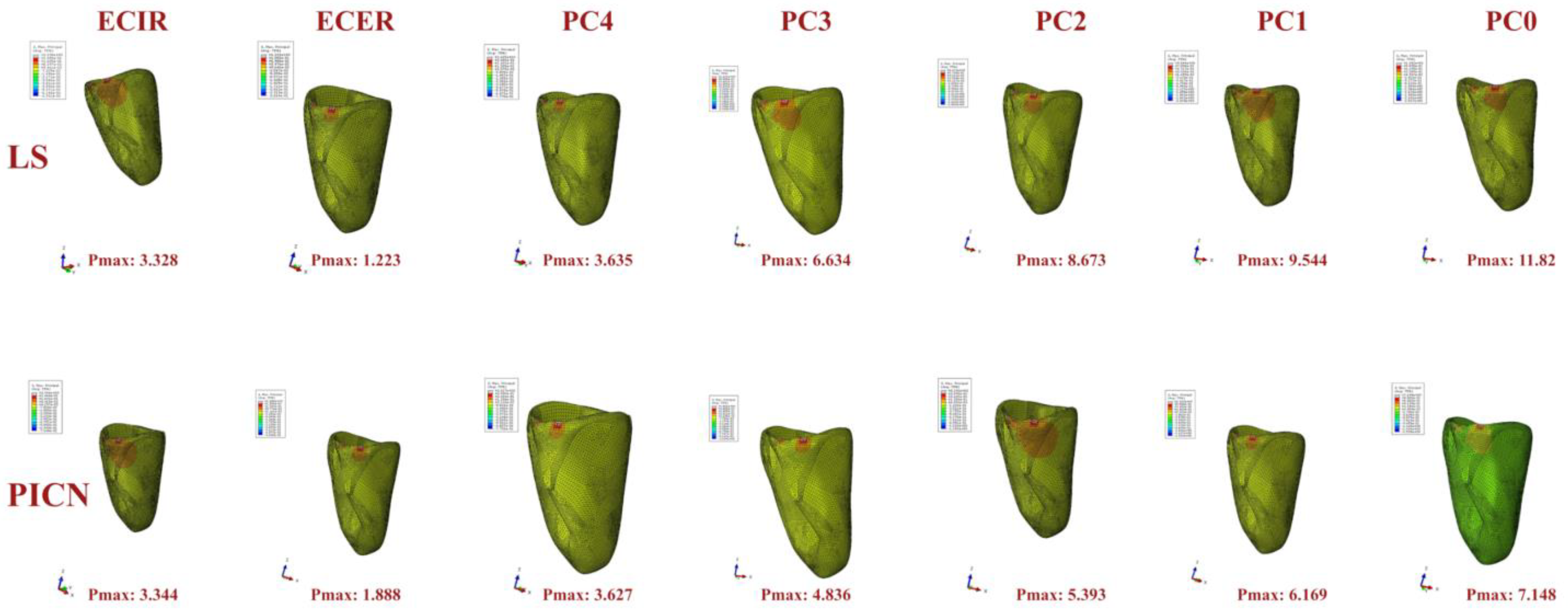
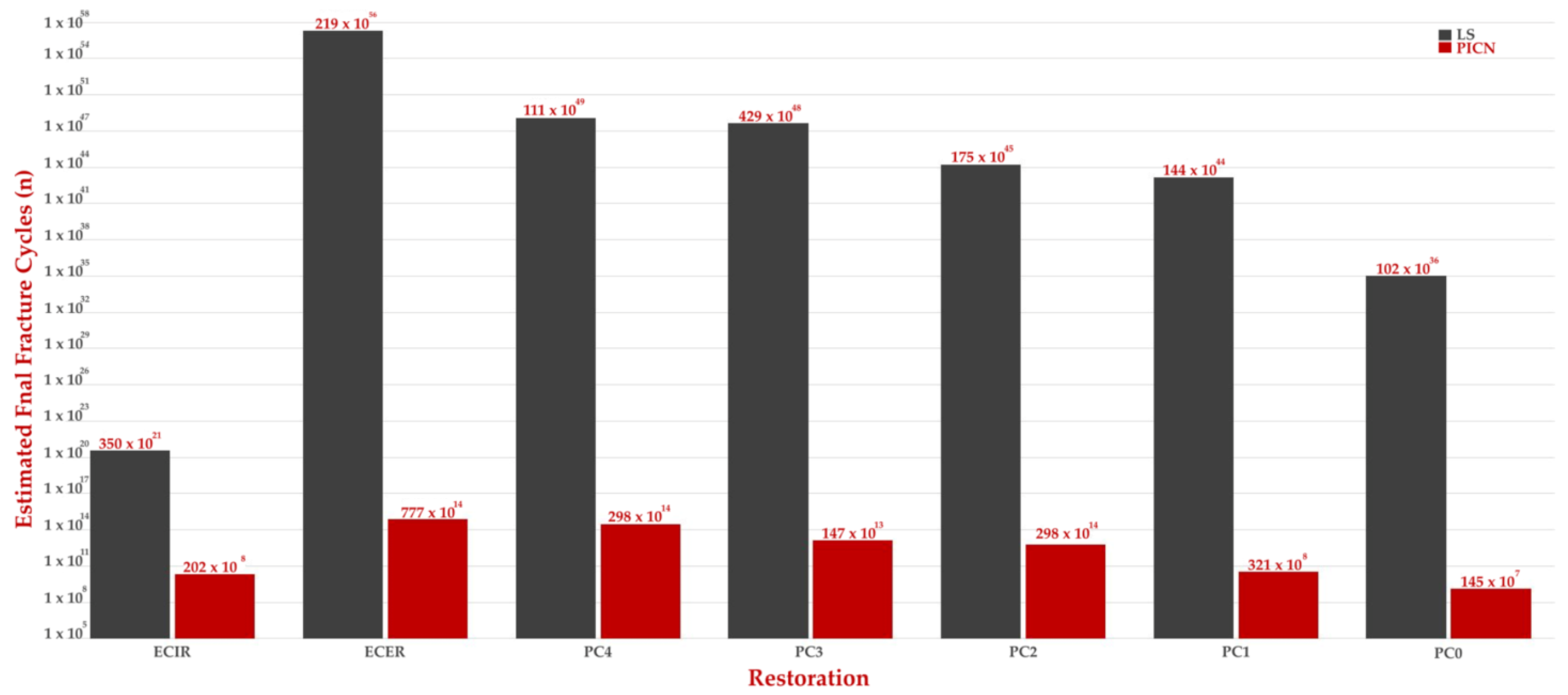
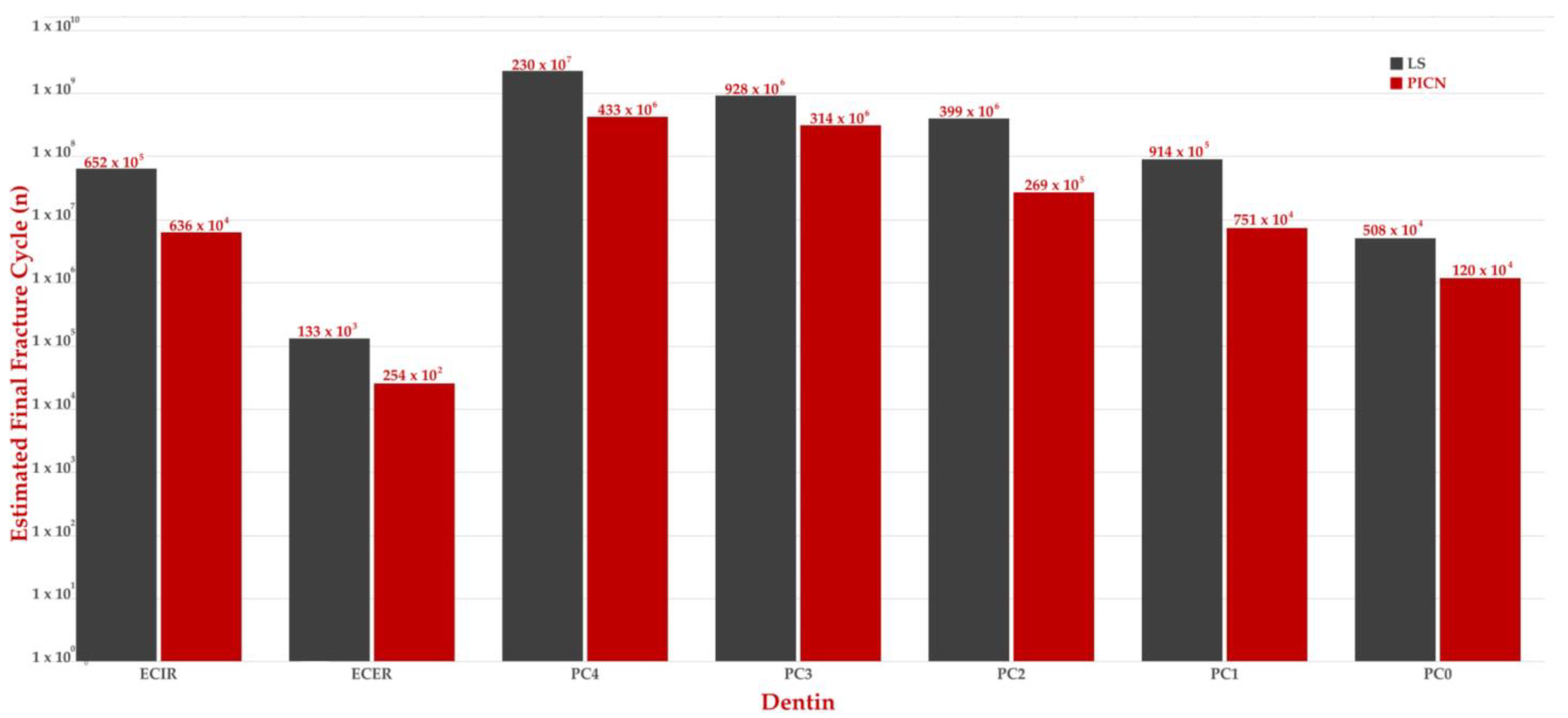
3. Results
4. Discussion
5. Conclusions
- The presence of a circumferential ferrule is critical in endodontically treated teeth with excessive coronal tissue loss. However, post–core restorations, which are applied in cases where at least two walls are intact, can be expected to be successful.
- Endocrowns for anterior teeth are not a good treatment alternative.
- If the remaining number of coronal walls is 1 or 0, an endocrown with internal retention may also be a good treatment alternative.
- The use of more rigid restorative materials, such as LS, may be recommended for endodontically treated teeth with excessive coronal tissue loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Peng, M.D.; Wang, Y.N.; Li, Q. The effects of ferrule configuration on the anti-fracture ability of fiber post-restored teeth. J. Dent. 2015, 43, 117–125. [Google Scholar] [CrossRef]
- Juloski, J.; Apicella, D.; Ferrari, M. The effect of ferrule height on stress distribution within a tooth restored with fibre posts and ceramic crown: A finite element analysis. Dent. Mater. 2014, 30, 1304–1315. [Google Scholar] [CrossRef]
- Eraslan, Ö.; Eraslan, O.; Eskitaşcıoğlu, G.; Belli, S. Conservative restoration of severely damaged endodontically treated premolar teeth: A FEM study. Clin. Oral Investig. 2011, 15, 403–408. [Google Scholar] [CrossRef]
- Gresnigt, M.M.; Özcan, M.; van den Houten, M.L.; Schipper, L.; Cune, M.S. Fracture strength, failure type and Weibull characteristics of lithium disilicate and multiphase resin composite endocrowns under axial and lateral forces. Dent. Mater. 2016, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, J.; Zhang, X.; Yan, X. Effects of 3 different residual root treatments after post-and-core restoration: An in vitro fracture resistance experiment and finite element analysis. J. Prosthet. Dent. 2020, 124, 485.e1–485.e10. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Vichi, A.; Fadda, G.; Cagidiaco, M.; Tay, F.; Breschi, L.; Polimeni, A.; Goracci, C. A Randomized controlled trial of endodontically treated and restored premolars. J. Dent. Res. 2012, 91, S72–S78. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Alikhasi, M.; Siadat, H. Biomechanical behavior of endocrown restorations with different cavity design and CAD-CAM materials under a static and vertical load: A finite element analysis. J. Prosthet. Dent. 2022, 127, 600.e1–600.e8. [Google Scholar] [CrossRef]
- Pantaleón, D.S.; Valenzuela, F.M.; Morrow, B.R.; Pameijer, C.H.; García-Godoy, F. Effect of Ferrule Location with Varying Heights on Fracture Resistance and Failure Mode of Restored Endodontically Treated Maxillary Incisors. J. Prosthodont. 2019, 28, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.; Santos, R.; Silva, A.; Valdívia, A.; Oliveira-Neto, L.; Griza, S.; Soares, C.; Faria-E-Silva, A. Ferrule Design Does Not Affect the Biomechanical Behavior of Anterior Teeth Under Mechanical Fatigue: An In Vitro Evaluation. Oper. Dent. 2019, 44, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gillen, B.M.; Looney, S.W.; Gu, L.-S.; Loushine, B.A.; Weller, R.N.; Loushine, R.J.; Pashley, D.H.; Tay, F.R. Impact of the Quality of Coronal Restoration versus the Quality of Root Canal Fillings on Success of Root Canal Treatment: A Systematic Review and Meta-analysis. J. Endod. 2011, 37, 895–902. [Google Scholar] [CrossRef]
- Forberger, N.; Göhring, T.N. Influence of the type of post and core on in vitro marginal continuity, fracture resistance, and fracture mode of lithia disilicate-based all-ceramic crowns. J. Prosthet. Dent. 2008, 100, 264–273. [Google Scholar] [CrossRef]
- Silva-Sousa, A.C.; Moris, I.C.; Barbosa, A.F.S.; Silva-Sousa, Y.T.C.; Sousa-Neto, M.D.; Pires, C.R.F.; Gomes, E.A. Effect of restorative treatment with endocrown and ferrule on the mechanical behavior of anterior endodontically treated teeth: An in vitro analysis. J. Mech. Behav. Biomed. Mater. 2020, 112, 104019. [Google Scholar] [CrossRef]
- Biacchi, G.; Basting, R.; Basting, G.B.R.; Carvalho, A.; Bruzi, G.; Anderson, R.; Maia, H.; Giannini, M.; Magne, P.; El-Damanhoury, H.; et al. Comparison of fracture strength of endocrowns and glass fiber post-retained conventional crowns. Oper. Dent. 2012, 37, 130–136. [Google Scholar] [CrossRef]
- Li, X.; Kang, T.; Zhan, D.; Xie, J.; Guo, L. Biomechanical behavior of endocrowns vs fiber post-core-crown vs cast post-core-crown for the restoration of maxillary central incisors with 1 mm and 2 mm ferrule height: A 3D static linear finite element analysis. Medicine 2020, 99, e22648. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J. Emerging ceramic-based materials for dentistry. J. Dent. Res. 2014, 93, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Ikeda, H. Development of zirconia-based polymer-infiltrated ceramic network for dental restorative material. J. Mech. Behav. Biomed. Mater. 2024, 150, 106320. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.S.; Henriques, B.; de Oliveira, A.P.N.; Silva, F.; Gomes, J.R.; Souza, J.C.M. Micro-scale abrasion and sliding wear of zirconium-lithium silicate glass-ceramic and polymer-infiltrated ceramic network used in dentistry. Wear 2020, 448–449, 203214. [Google Scholar] [CrossRef]
- Homaei, E.; Jin, X.-Z.; Pow, E.H.N.; Matinlinna, J.P.; Tsoi, J.K.-H.; Farhangdoost, K. Numerical fatigue analysis of premolars restored by CAD/CAM ceramic crowns. Dent. Mater. 2018, 34, e149–e157. [Google Scholar] [CrossRef]
- Penteado, M.M.; de Andrade, G.S.; Araujo, R.M.; Borges, A.L.S.; Valandro, L.F.; Pereira, G.K.R.; da Silva, J.M.F. Fatigue survival of endodontically treated teeth restored with different fiber-reinforced composite resin post strategies versus universal 2-piece fiber post system: An in vitro study. J. Prosthet. Dent. 2023, 129, 456–463. [Google Scholar] [CrossRef]
- Gönder, H.Y.; Fidancıoğlu, Y.D.; Fidan, M.; Mohammadi, R.; Karabekiroğlu, S. Comparison of Resin Cement’s Different Thicknesses and Poisson’s Ratios on the Stress Distribution of Class II Amalgam Restoration Using Finite Element Analysis. Appl. Sci. 2023, 13, 4125. [Google Scholar] [CrossRef]
- Mutluay, M.M.; Yahyazadehfar, M.; Ryou, H.; Majd, H.; Do, D.; Arola, D. Fatigue of the resin–dentin interface: A new approach for evaluating the durability of dentin bonds. Dent. Mater. 2013, 29, 437–449. [Google Scholar] [CrossRef]
- Nalla, R.; Kinney, J.; Marshall, S.; Ritchie, R. On the in vitro fatigue behavior of human dentin: Effect of mean stress. J. Dent. Res. 2004, 83, 211–215. [Google Scholar] [CrossRef]
- Ausiello, P.; Franciosa, P.; Martorelli, M.; Watts, D.C. Numerical fatigue 3D-FE modeling of indirect composite-restored posterior teeth. Dent. Mater. 2011, 27, 423–430. [Google Scholar] [CrossRef]
- Naumann, M.; Schmitter, M.; Frankenberger, R.; Krastl, G. ‘Ferrule Comes First. Post Is Second!’ Fake News and Alternative Facts? A Systematic Review. J. Endod. 2018, 44, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Elavarasu, P.; Karumaran, C.S.; Indira, R.; Anilkumar, R.; Mani, R.; Natarajan, R. An In Vitro Evaluation of Fracture Resistance of Endodontically Treated Maxillary Central Incisor Restored with Custom-Made Cast Post and Core with Uniform and Nonuniform Core Ferrule Heights. J. Pharm. Bioallied Sci. 2019, 11, S407–S412. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.; Gupta, N.; Bhalla, V. Evaluating effect of ferrule height, configuration, and post and core approaches on stress distribution of flared root canal: A three-dimensional finite element analysis. Saudi Endod. J. 2021, 11, 308. [Google Scholar] [CrossRef]
- de Carvalho, A.B.G.; de Andrade, G.S.; Tribst, J.P.M.; Grassi, E.D.A.; Ausiello, P.; Saavedra, G.d.S.F.A.; Bressane, A.; de Melo, R.M.; Borges, A.L.S. Mechanical Behavior of Different Restorative Materials and Onlay Preparation Designs in Endodontically Treated Molars. Materials 2021, 14, 1923. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Sato, T.; Hisanaga, R.; Nomoto, S.; Yotsuya, M.; Yoshinari, M.; Takemoto, S. Influence of one-wall remaining coronal tooth with resin abutment and fiber post on static and dynamic fracture resistance. Dent. Mater. J. 2022, 41, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Güngör, M.B.; Bal, B.T.; Yilmaz, H.; Aydin, C.; Nemli, S.K. Fracture strength of CAD/CAM fabricated lithium disilicate and resin nano ceramic restorations used for endodontically treated teeth. Dent. Mater. J. 2017, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dejak, B.; Młotkowski, A. Strength comparison of anterior teeth restored with ceramic endocrowns vs custom-made post and cores. J. Prosthodont. Res. 2018, 62, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Rocca, G.; Rizcalla, N.; Krejci, I. Fiber-reinforced resin coating for endocrown preparations: A technical report. Oper. Dent. 2013, 38, 242–248. [Google Scholar] [CrossRef][Green Version]
- Lin, C.; Chang, Y.; Chang, C.; Pai, C.; Huang, S. Finite element and Weibull analyses to estimate failure risks in the ceramic endocrown and classical crown for endodontically treated maxillary premolar. Eur. J. Oral Sci. 2010, 118, 87–93. [Google Scholar] [CrossRef]
- Rathi, A.; Chowdhry, P.; Kaushik, M.; Reddy, P.; Roshni, R.; Mehra, N. Effect of different periodontal ligament simulating materials on the incidence of dentinal cracks during root canal preparation. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 196–200. [Google Scholar] [CrossRef]
- Su, M.-Z.; Chang, H.-H.; Chiang, Y.-C.; Cheng, J.-H.; Fuh, L.-J.; Wang, C.-Y.; Lin, C.-P. Modeling viscoelastic behavior of periodontal ligament with nonlinear finite element analysis. J. Dent. Sci. 2013, 8, 121–128. [Google Scholar] [CrossRef]
- Sieper, K.; Wille, S.; Kern, M. Fracture strength of lithium disilicate crowns compared to polymer-infiltrated ceramic-network and zirconia reinforced lithium silicate crowns. J. Mech. Behav. Biomed. Mater. 2017, 74, 342–348. [Google Scholar] [CrossRef]
- Yazigi, C.; Kern, M.; Chaar, M.S.; Libecki, W.; Elsayed, A. The influence of the restorative material on the mechanical behavior of screw-retained hybrid-abutment-crowns. J. Mech. Behav. Biomed. Mater. 2020, 111, 103988. [Google Scholar] [CrossRef]
- Zhu, J.; Rong, Q.; Wang, X.; Gao, X. Influence of remaining tooth structure and restorative material type on stress distribution in endodontically treated maxillary premolars: A finite element analysis. J. Prosthet. Dent. 2017, 117, 646–655. [Google Scholar] [CrossRef]
- Lise, D.P.; Van Ende, A.; De Munck, J.; Suzuki, T.Y.U.; Vieira, L.C.C.; Van Meerbeek, B. Biomechanical behavior of endodontically treated premolars using different preparation designs and CAD/CAM materials. J. Dent. 2017, 59, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ural, Ç.; Çağlayan, E. A 3-dimensional finite element and in vitro analysis of endocrown restorations fabricated with different preparation designs and various restorative materials. J. Prosthet. Dent. 2021, 126, 586.e1–586.e9. [Google Scholar] [CrossRef] [PubMed]
- Adanir, N.; Belli, S. Evaluation of Different Post Lengths’ Effect on Fracture Resistance of a Glass Fiber Post System. Eur. J. Dent. 2008, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.D.; McLaren, C.I.; Yaman, P.; Bin-Shuwaish, M.S.; Dennison, J.D.; McDonald, N.J. The effect of post type and length on the fracture resistance of endodontically treated teeth. J. Prosthet. Dent. 2009, 101, 174–182. [Google Scholar] [CrossRef]
- Jindal, S.; Jindal, R.; Mahajan, S.; Dua, R.; Jain, N.; Sharma, S. In vitro evaluation of the effect of post system and length on the fracture resistance of endodontically treated human anterior teeth. Clin. Oral Investig. 2012, 16, 1627–1633. [Google Scholar] [CrossRef]
- Gluskin, A.H.; Radke, R.A.; Frost, S.L.; Watanabe, L.G. The mandibular incisor: Rethinking guidelines for post and core design. J. Endod. 1995, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Savychuk, A.; Manda, M.; Galanis, C.; Provatidis, C.; Koidis, P. Stress generation in mandibular anterior teeth restored with different types of post-and-core at various levels of ferrule. J. Prosthet. Dent. 2018, 119, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Sevimli, G.; Cengiz, S.; Oruc, M.S. Endocrowns: Review. J. Istanbul Univ. Fac. Dent. 2015, 49, 57. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Rodrigues MD, P.; Faria-e-Silva, A.L.; Santos-Filho PC, F.; Veríssimo, C.; Kim, H.C.; Versluis, A. How biomechanics can affect the endodontic treated teeth and their restorative procedures? Braz. Oral Res. 2018, 32, 169–183. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Li, X.; Sun, C.; Gao, E.; Li, H. A comparison of the fracture resistances of endodontically treated mandibular premolars restored with endocrowns and glass fiber post-core retained conventional crowns. J. Adv. Prosthodont. 2016, 8, 489–493. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; Martorelli, M.; Lanzotti, A.; Zarone, F.; Watts, D.C.; Gloria, A. Mechanical behavior of endodontically restored canine teeth: Effects of ferrule, post material and shape. Dent. Mater. 2017, 33, 1466–1472. [Google Scholar] [CrossRef]


| Material | Young’s Modulus (MPa) | Poisson’s Ratio | Compressive Strength (MPa) | Flexural Strength (MPa) | Shear Strength (MPa) | Fracture Toughness (MPa m1/2) | Microhardness (HV) | Reference |
|---|---|---|---|---|---|---|---|---|
| LS | 102,700 | 0.22 | - | 356.7 | - | 2.8 | 676.7 | [18] |
| PICN | 30,100 | 0.23 | - | 135.8 | - | 1.4 | 261.7 | |
| Dentin | 18,600 | 0.31 | 297 | 105.5 | 12–138 | |||
| Cement | 7700 | 0.3 | 262 | 98 | 40 | |||
| Dental resin composite | 12,000 | 0.30 | ||||||
| Pulp | 2 | 0.45 | ||||||
| PDL | 68,900 | 0.45 | ||||||
| Gutta-percha | 140,000 | 0.45 | ||||||
| Cortical bone | 13,700 | 0.30 | ||||||
| Spongy (cancellous) bone | 1370 | 0.3 |
| Material | Young’s Modulus (MPa) | Poisson’s Ratio | Shear Modulus (MPa) | Tensile Failure Limit (MPa) | Compressive Failure Limit (MPa) | Reference |
|---|---|---|---|---|---|---|
| Glass fiber-reinforced post | X, 37.0 | XY, 0.27 | XY, 3.1 | 180.1–215.8 | 118.8–151.5 | [19] |
| Y, 9.5 | XZ, 0.34 | XZ, 3.5 | ||||
| Z, 9.5 | YZ, 0.27 | YZ, 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, M.G.; Mohammadi, R. The Effect of Different Ferrule Configurations and Preparation Designs on the Fatigue Performance of Endodontically Treated Maxillary Central Incisors: A 3D Finite Element Analysis. Appl. Sci. 2024, 14, 1355. https://doi.org/10.3390/app14041355
Demirel MG, Mohammadi R. The Effect of Different Ferrule Configurations and Preparation Designs on the Fatigue Performance of Endodontically Treated Maxillary Central Incisors: A 3D Finite Element Analysis. Applied Sciences. 2024; 14(4):1355. https://doi.org/10.3390/app14041355
Chicago/Turabian StyleDemirel, Mehmet Gökberkkaan, and Reza Mohammadi. 2024. "The Effect of Different Ferrule Configurations and Preparation Designs on the Fatigue Performance of Endodontically Treated Maxillary Central Incisors: A 3D Finite Element Analysis" Applied Sciences 14, no. 4: 1355. https://doi.org/10.3390/app14041355
APA StyleDemirel, M. G., & Mohammadi, R. (2024). The Effect of Different Ferrule Configurations and Preparation Designs on the Fatigue Performance of Endodontically Treated Maxillary Central Incisors: A 3D Finite Element Analysis. Applied Sciences, 14(4), 1355. https://doi.org/10.3390/app14041355






