Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards
Abstract
1. Introduction
2. Wood and Its Pectocellulosic Cell Wall Organization
- Xyloglucans: backbone of β-(1-4)-linked glucoses, with branches of xylose, galactose, and fucose;
- Glucuronoxylans and glucuronoarabinoxylans: skeleton of xyloses linked by β-(1-4) bonds, with glucuronic acid and arabinose branches;
- Mannans and glucomannans: skeleton made up entirely of mannose (for mannans and galactomannans) or glucose and mannose (for mannans and galactoglucomannans), linked by β-(1-4) bonds;
- β-(1-3,1-4)-glucans: backbone of glucoses linked by β-(1-3) or β-(1-4).
- Homogalacturonans: linear chains of uronic acids linked by α-(1-4) bonds, which account for around 65% of pectin. The homogalacturonans are often methylated and sometimes acetylated.
- Rhamnogalacturonans I: homogalacturonan and rhamnose skeletons, with arabinose and galactose branches on the rhamnose units. The uronic acid units of the backbone can be methylated and sometimes acetylated. Rhamnogalacturonanes I account for between 20 and 35% of pectin.
- Rhamnogalacturonans II: branched homogalacturonan skeletons with more than 12 types of sugar and 20 different types of bonds. The rhamnogalacturonans II account for around 10% of pectin.
- Xylogalacturonans: homogalacturonan skeletons with some branches of β-(O-3)-xylose.
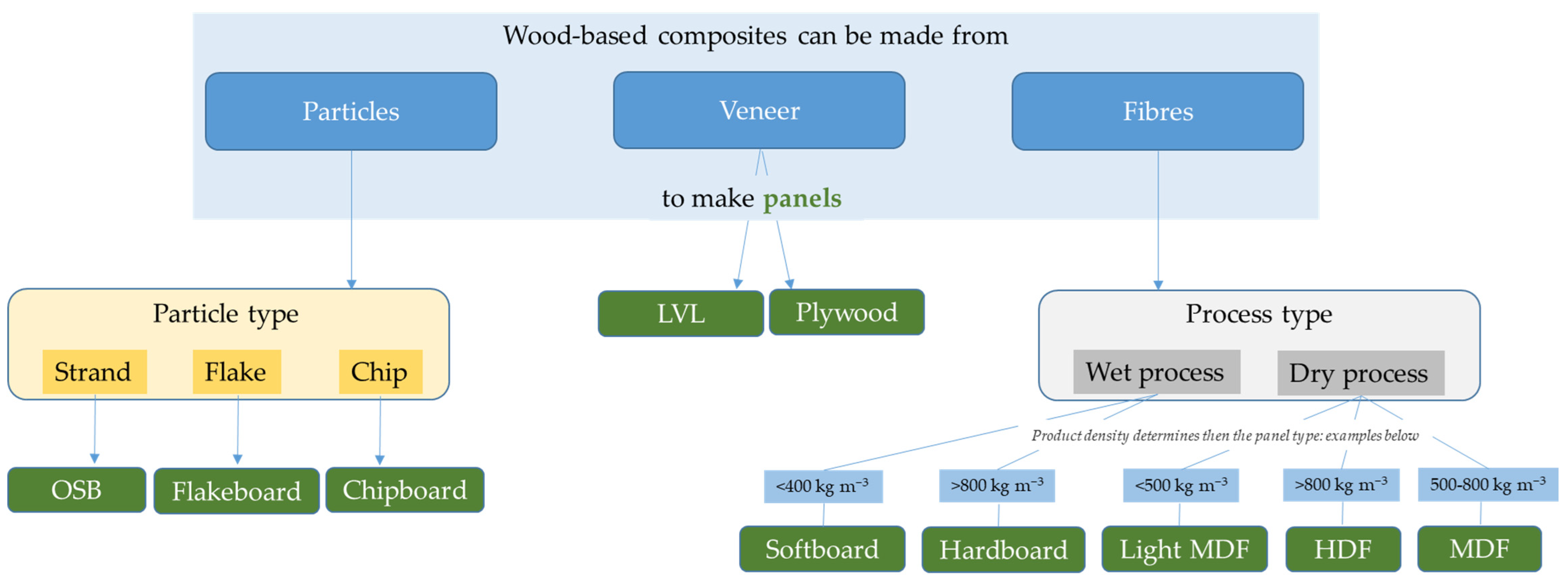
3. Wood-Based Composite Boards
3.1. Wood-Based Boards Containing a Binder
3.1.1. Different Types of Industrial Wood-Based Boards
3.1.2. Development of New Panels with Biobased Binders
3.2. Wood-Based Binderless Boards
3.2.1. Self-Adhesion Mecanisms for Wood-Based Binderless Boards
3.2.2. Some Examples of Wood-Based Binderless Boards
4. Why and How to Measure Volatile Organic Compound Emissions from a Material?
4.1. How to Sample and Analyse VOCs
4.1.1. VOC Sampling
- -
- a bag (made from the following materials: polyvinyl fluoride (tradename: Tedlar), polytetrafluoroethylene and fluorinated ethylene propylene copolymer (tradename: Teflon), polyethyleneterephtalate (tradename: Nalofan), polyvinylidene difluoride (tradename: Altef)…) offering a wide range of volumes (from a few mL to several dozen liters) and being low-cost but fragile;
- -
- a polished stainless-steel canister, reusable, with a range of volumes from a few mL to several dozen liters, but are expensive;
- -
- a glass vial or ampoule, infinitely reusable after cleaning.
- a solid-phase microextraction (SPME) fiber;
- a stir bar sorption extraction system (SBSE);
- a passive dosimeter containing a sorbent, as previously mentioned, with various ge-ometries (Radiello tube, badge G.A.B.I.E, 3M type 3500, PerkinElmer tube).
4.1.2. VOC Analysis
4.2. How to Measure VOC Emission Rates from Wood-Based Materials?
| Reference | Associated Standard | Volume of the Chamber (L) | Chamber Model (Material) | Emission Surface (m2) | Air Flow (L min−1) | Specific Air Flow Rate (m3 m−2 h−1) | Air Velocity (m s−1) | Loading Rate (m2 m−3) | Air Change Rate (h−1) | Temperature, Relative Humidity (°C, %) |
|---|---|---|---|---|---|---|---|---|---|---|
| [108] | ASTM D5116-06 [109] | 216 | (Polished stainless steel) | / | 1.8 | / | / | / | 0.5 | 25 °C, 50% |
| [110] | QB 1952.2–2011 [111] | 1000 | / | 0.4 | / | / | 0.2 | 0.4 | 1 | |
| [112] | / | 1000 | (Stainless steel) | 0.9 | / | / | 0.1–0.3 | 0.9; 1.8 and 3.6 | 1 | 23 °C, 45% |
| [113] | / | 203 | Model VCE 200, Vötsch Industrietechnik (polished stainless steel) | 26.6 × 103 | / | 3.83 | / | 0.131 | 0.502 | 25 °C, 50% |
| 24 | Designed for the study using a desiccator (glass) | 60 × 103 | / | 4.04 | / | 0.250 | 1.01 | |||
| 0.044 | µ-CTE, Markes (stainless steel) | 0.267 × 103 | / | 4.28–5.06 | / | 6.07 | 26.1–30.7 | |||
| [114] | EN 717-1 [115] | 225 | (Stainless steel) | 0.225 | / | / | 0.1–0.3 | 1 | 1 | 23 °C, 45% |
| ASTM D 6007-02 [116] | 1000 | (Aluminum) | 4.84 | / | / | 2–5 | 0.43 | 2 | 24 °C, 50% | |
| EN 717-2 [117] | 4 | (Glass) | 0.02 | 1 | / | / | / | 60 | 60 °C, ≤3% | |
| [118] | / | 30 | / | 0.588 | 15 × 103 or 30 × 103 | / | / | 19.6 | 0.5 or 1 | 23, 35, 50, or 60 °C, 50% |
| [119] | / | 60 × 10−3 | MOSEC (Glass) | 0.0017 | / | / | / | / | / | 23 °C |
| [120] | / | 8000 | (stainless steel) | / | / | / | / | / | / | |
| [121] | / | 0.044 | µ-CTE, Markes (stainless steel) | / | / | / | / | / | / | 40, 60, or 80 °C |
| [122] | ENV 13419-1 [123] | 1000 | Glass | / | / | 1 | 0.1–0.3 | 1.5; 1 and 1.35 | / | 23 °C, 50% |
| [124] | ISO 16000-9 [125] | 50.9 | CLIMPAQ, Climtech (glass, stainless steel, and aluminum) | 0.099 | / | 0.16 | / | 1.96 | 0.3 | |
| [126] | ISO 16000-10 [127] | 0.035 | FLEC, Chematech (stainless steel) | 0.0177 | / | 0.0106 | / | 506 | 514 |
| Emission Chamber | Chamber Volume(s) (Internal Dimensions) | T (°C) | RH (%) | d (L min−1) | ACR (h−1) | Examples of Standards |
|---|---|---|---|---|---|---|
| Model VCE 200 or 1000, Vötsch Industrietechnik | 200 L (61 cm × 61 cm × 56 cm) 1000 L (75 cm × 163 cm × 75 cm) | 20–130 | 5–95 | / | 0.1–1.8 | ISO 16000-1 [128] ENV 13419-1 [123] |
| µ-CTE, Markes | 44 mL 114 mL | 20–120 20–250 | / | 10–70 50–500 | / | ISO 16000-25 [129] ISO 12219-3 [130] ASTM D7706-17 [131] |
| CLIMPAQ, Climtech | 50 L (21 cm × 81 cm × 22 cm) | ambient | / | / | 0.02–140 | EN 717-1 [115] ISO 16000 [132] |
| FLEC, Chematech | 35 mL (Φ = 150 mm; hmax = 18 mm) | ambient | / | / | / | ISO 16000-10:2006 [127] |
5. Wood-Based Panel Emissions
5.1. Wood Panels Bonded with an Adhesive
5.1.1. VOC Emissions from Wood-Based Panels with a Petrochemical Binder
5.1.2. VOC Emissions from Wood-Based Panels with Bio-Based Adhesive
5.2. Wood Panels Bonded without Adhesive
5.2.1. VOC Emissions from Heat-Treated Softwoods
5.2.2. VOC Emissions from Heat-Treated Hardwoods
5.2.3. VOC Emissions from Binderless Boards
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Yearbook of Forest Products 2020; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Data. Available online: https://www.fao.org/faostat/en/#compare (accessed on 26 June 2023).
- Sari, B.; Ayrilmis, N.; Baharoglu, M.; Gümüskaya, E.; Bardak, S. Effects of chemical composition of wood and resintype on properties of particleboard. Lignocellulose 2012, 1, 174–184. [Google Scholar]
- Astari, L.; Prasetivo, W.; Suryanegara, L. Properties of Particleboard Made from Wood Waste with Various Size. IOP Conf. Ser. Earth Environ. Sci. 2018, 166, 012004. [Google Scholar] [CrossRef]
- Baharoğlu, M.; Nemli, G.; Sarı, B.; Birtürk, T.; Bardak, S. Effects of anatomical and chemical properties of wood on the quality of particleboard. Compos. Part B Eng. 2013, 52, 282–285. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Pánek, M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers 2020, 12, 2289. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, W.; Huang, S.; Zhang, Y. Association between the Emission Rate and Temperature for Chemical Pollutants in Building Materials: General Correlation and Understanding. Environ. Sci. Technol. 2013, 47, 8540–8547. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xiong, J.; Cai, C.; Xu, W.; Zhang, Y. Influence of humidity on the initial emittable concentration of formaldehyde and hexaldehyde in building materials: Experimental observation and correlation. Sci. Rep. 2016, 6, 23388. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lv, M.; Yang, X. The combined effects of temperature and humidity on initial emittable formaldehyde concentration of a medium-density fiberboard. Build. Environ. 2016, 98, 80–88. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Tao, J.; Zhang, Z.; Shi, S.Q. Research Progress on Formaldehyde Emission of Wood-Based Panel. Int. J. Polym. Sci. 2018, 2018, 9349721. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Tan, Y.; Liu, J.; Wang, K.; Ji, W.; Sun, L.; Yu, X.; Zhao, J.; Xu, B.; et al. Measurement of the key parameters of VOC emissions from wooden furniture, and the impact of temperature. Atmos. Environ. 2021, 259, 118510. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, W.; Little, J.C. Predicting emissions of volatile and semivolatile organic compounds from building material: A review. Build. Environ. 2013, 64, 7–25. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006; (Consolidated Version: 01/12/2023); Official Journal of the European Union L (353): 1–1355. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1272 (accessed on 31 January 2024).
- Hussin, M.H.; Abd Latif, N.H.; Hamidon, T.S.; Idris, N.N.; Hashim, R.; Appaturi, J.N.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Fatriasari, W.; et al. Latest advancements in high-performance bio-based wood adhesives: A critical review. J. Mater. Res. Technol. 2022, 21, 3909–3946. [Google Scholar] [CrossRef]
- Arias, A.; González-Rodríguez, S.; Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M.; Moreira, M.T. Recent developments in bio-based adhesives from renewable natural resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Iswanto, A.H.; Lubis, M.A.R.; Sutiawan, J.; Al-Edrus, S.S.O.; Lee, S.H.; Antov, P.; Kristak, L.; Reh, R.; Mardawati, E.; Santoso, A.; et al. Latest Advancements in the Development of High-Performance Lignin- and Tannin-Based Non-Isocyanate Polyurethane Adhesive for Wood Composites. Polymers 2023, 15, 3864. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Md Tahir, P.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Valchev, I.; Yordanov, Y.; Savov, V.; Antov, P. Optimization of the Hot-Pressing Regime in the Production of Eco-Friendly Fibreboards Bonded with Hydrolysis Lignin. Period. Polytech. Chem. Eng. 2021, 66, 125–134. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Labonne, L.; Merah, O.; Talou, T.; Ballas, S.; Véronèse, T.; Evon, P. Optimization of thermopressing conditions for the production of binderless boards from a coriander twin-screw extrusion cake. J. Appl. Polym. Sci. 2017, 134, 44650. [Google Scholar] [CrossRef]
- Gonçalves, D.; Bordado, J.M.; Marques, A.C.; Galhano dos Santos, R. Non-Formaldehyde, Bio-Based Adhesives for Use in Wood-Based Panel Manufacturing Industry-A Review. Polymers 2021, 13, 4086. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Mofication: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Higuchi, T. Biochemistry and Molecular Biology of Wood; Timell, T.E., Ed.; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Hon, D.N.S.; Shiraishi, N. Wood and Cellulosic Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Le Floch, A.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Brett, C.T. Cellulose microfibrils in plants: Biosynthesis, deposition, and integration into the cell wall. Int. Rev. Cytol. 2000, 199, 161–199. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. Cellulose I microfibril assembly: Computational molecular mechanics energy analysis favours bonding by van der Waals forces as the initial step in crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, I.S. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and its Potential Industrial Applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as Natural Antioxidant Capacity. In Lignin—Trends and Applications; Poletto, M., Ed.; InTech: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Thoemen, H.; Irle, M.; Sernek, M. (Eds.) Wood-Based Panels: An Introduction for Specialists; Brunel Univ. Press: London, UK, 2010. [Google Scholar]
- Arias, A.; González-García, S.; Feijoo, G.; Moreira, M.T. Tannin-based bio-adhesives for the wood panel industry as sustainable alternatives to petrochemical resins. J. Ind. Ecol. 2022, 26, 627–642. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Lignin in straw and its applications as an adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Zhou, X.; Lu, B.; He, M.; Sun, S.; Ling, X. Improving Water Resistance of Soy-Protein Wood Adhesive by Using Hydrophilic Additives. BioResources 2014, 10, 41–54. [Google Scholar] [CrossRef]
- Cheng, H.N.; Dowd, M.K.; He, Z. Investigation of modified cottonseed protein adhesives for wood composites. Ind. Crop. Prod. 2013, 46, 399–403. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sain, M. Protein Extraction from Secondary Sludge of Paper Mill Wastewater and its Utilization as a Wood Adhesive. BioResources 2011, 6, 961–970. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding—A review. J. Adhes. Sci. Technol. 2017, 31, 910–931. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, P.; Musa, S.; Mai, C.; Zhang, K. Dialdehyde Cellulose as a Bio-Based Robust Adhesive for Wood Bonding. ACS Sustain. Chem. Eng. 2019, 7, 10452–10459. [Google Scholar] [CrossRef]
- Jia, L.; Chu, J.; Li, J.; Ren, J.; Huang, P.; Li, D. Formaldehyde and VOC emissions from plywood panels bonded with bio-oil phenolic resins. Environ. Pollut. 2020, 264, 114819. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Dhawale, P.V.; Vineeth, S.K.; Gadhave, R.V.; Gadhave, R.V.; Jabeen Fatima, M.J.; Supekar, M.V.; Thakur, V.K.; Raghavan, P. Tannin as a renewable raw material for adhesive applications: A review. Mater. Adv. 2022, 3, 3365–3388. [Google Scholar] [CrossRef]
- Nasir, M.; Khali, D.P.; Jawaid, M.; Tahir, P.M.; Siakeng, R.; Asim, M.; Khan, T.A. Recent development in binderless fiber-board fabrication from agricultural residues: A review. Constr. Build. Mater. 2019, 211, 502–516. [Google Scholar] [CrossRef]
- Pintiaux, T.; Viet, D.; Vandenbossche, V.; Rigal, L.; Rouilly, A. Binderless Materials Obtained by Thermo-Compressive Processing of Lignocellulosic Fibers: A Comprehensive Review. BioResources 2015, 10, 1915–1963. [Google Scholar] [CrossRef]
- Tajuddin, M.; Ahmad, Z.; Ismail, H. A Review of Natural Fibers and Processing Operations for the Production of Binderless Boards. BioResources 2016, 12, 5600–5617. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, A.; Xue, L. A review of preparation of binderless fiberboards and its self-bonding mechanism. Wood Sci. Technol. 2015, 49, 661–679. [Google Scholar] [CrossRef]
- Vitrone, F.; Ramos, D.; Ferrando, F.; Salvadó, J. Binderless fiberboards for sustainable construction. Materials, production methods and applications. J. Build. Eng. 2021, 44, 102625. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, S.; Wu, J.; Xu, J. Study on preparation of high-performance binderless board from Broussonetia papyrifera. J. Wood Sci. 2023, 69, 17. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; McSweeny, J.D.; Rowell, R.M. Heat treatment of wet wood fiber: A study of the effect of reaction conditions on the formation of furfurals. Wood Mater. 2012, 7, 202–208. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood Modification by Heat Treatment: A Review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Bouajila, J.; Limare, A.; Joly, C.; Dole, P. Lignin plasticization to improve binderless fiberboard mechanical properties. Polym. Eng. Sci. 2005, 45, 809–816. [Google Scholar] [CrossRef]
- Bouajila, J.; Dole, P.; Joly, C.; Limare, A. Some laws of a lignin plasticization. J. Appl. Polym. Sci. 2006, 102, 1445–1451. [Google Scholar] [CrossRef]
- Van Dam, J.E.G.; van den Oever, M.; Teunissen, W.; Keijsers, E.; Peralta, A. Process for production of high density/high performance binderless boards from whole coconut husk. Part 1: Lignin as intrinsic thermosetting binder resin. Ind. Crop. Prod. 2004, 19, 207–216. [Google Scholar] [CrossRef]
- Okuda, N.; Sato, M. Manufacture and mechanical properties of binderless boards from kenaf core. J. Wood Sci. 2004, 50, 53–61. [Google Scholar] [CrossRef]
- Okuda, N.; Hori, K.; Sato, M. Chemical changes of kenaf core binderless boards during hot pressing (I): Influence of the pressing temperature condition. J. Wood Sci. 2006, 52, 244–248. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Lin, Z.; Peng, W.-X.; Yuan, T.-Q.; Xu, F.; Wu, Y.-Q.; Yang, J.; Wang, Y.-S.; Sun, R.-C. Chemical Changes of Raw Materials and Manufactured Binderless Boards during Hot Pressing: Lignin Isolation and Characterization. BioResources 2014, 9, 1055–1071. [Google Scholar] [CrossRef]
- Baskaran, M.; Hashim, R.; Sulaiman, O.; Hiziroglu, S.; Sato, M.; Sugimoto, T. Optimization of press temperature and time for binderless particleboard manufactured from oil palm trunk biomass at different thickness levels. Mater. Today Commun. 2015, 3, 87–95. [Google Scholar] [CrossRef]
- Evon, P.; Vinet, J.; Labonne, L.; Rigal, L. Influence of thermo-pressing conditions on the mechanical properties of biodegradable fiberboards made from a deoiled sunflower cake. Ind. Crop. Prod. 2015, 65, 117–126. [Google Scholar] [CrossRef]
- Boon, J.G.; Hashim, R.; Sulaiman, O.; Hiziroglu, S.; Sugimoto, T.; Sato, M. Influence of processing parameters on some properties of oil palm trunk binderless particleboard. Eur. J. Wood Wood Prod. 2013, 71, 583–589. [Google Scholar] [CrossRef]
- Okuda, N.; Sato, M. Manufacture and mechanical properties of binderless boards from kenaf core. J. Wood Sci. 2004, 50, 53–61. [Google Scholar] [CrossRef]
- Hashim, R.; Saari, N.; Sulaiman, O.; Sugimoto, T.; Hiziroglu, S.; Sato, M.; Tanaka, R. Effect of particle geometry on the properties of binderless particleboard manufactured from oil palm trunk. Mater. Des. 2010, 31, 4251–4257. [Google Scholar] [CrossRef]
- Hidayat, H.; Keijsers, E.R.P.; Prijanto, U.; van Dam, J.E.G.; Heeres, H.J. Preparation and properties of binderless boards from Jatropha curcas L. seed cake. Ind. Crop. Prod. 2014, 52, 245–254. [Google Scholar] [CrossRef]
- Mobarak, F.; Fahmy, Y.; Augustin, H. Binderless Lignocellulose Composite from Bagasse and Mechanism of Self-Bonding. Holzforschung 1982, 36, 131–136. [Google Scholar] [CrossRef]
- Nonaka, S.; Umemura, K.; Kawai, S. Characterization of bagasse binderless particleboard manufactured in high-temperature range. J. Wood Sci. 2013, 59, 50–56. [Google Scholar] [CrossRef]
- Xu, J.; Han, G.; Wong, E.D.; Kawai, S. Development of binderless particleboard from kenaf core using steam-injection pressing. J. Wood Sci. 2003, 49, 327–332. [Google Scholar] [CrossRef]
- Panyakaew, S.; Fotios, S. New thermal insulation boards made from coconut husk and bagasse. Energy Build. 2011, 43, 1732–1739. [Google Scholar] [CrossRef]
- Angles, M.N.; Reguant, J.; Montane, D.; Ferrando, F.; Farriol, X.; Salvado, J. Binderless composites from pretreated residual softwood. J. Appl. Polym. Sci. 1999, 73, 2485–2491. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.-M.; Wan, H.; Brunette, G. Binderless panels made with black spruce bark. BioResources 2011, 6, 3960–3972. [Google Scholar] [CrossRef]
- Hashim, R.; Said, N.; Lamaming, J.; Baskaran, M.; Sulaiman, O.; Sato, M.; Hiziroglu, S.; Sugimoto, T. Influence of press temperature on the properties of binderless particleboard made from oil palm trunk. Mater. Des. 2011, 32, 2520–2525. [Google Scholar] [CrossRef]
- Riquelme-Valdés, J.; Ramírez, E.; Contreras, D.; Freer, J.; Rodríguez, J. Fiberboard manufactured without resin using the Fenton reaction. J. Chil. Chem. Soc. 2008, 53, 1722–1725. [Google Scholar] [CrossRef][Green Version]
- Rowell, R.M.; McSweeny, J.D. Heat Treatments of Wood Fibers for Self-Bonding and Stabilized Fiberboards. Mol. Cryst. Liq. Cryst. 2008, 483, 307–325. [Google Scholar] [CrossRef]
- Widsten, P.; Qvintus-Leino, P.; Tuominen, S.; Laine, J.E. Manufacture of Fiberboard from Wood Fibers Activated with Fentons Reagent (H2O2/FeSO4). Holzforschung 2003, 57, 447–452. [Google Scholar] [CrossRef]
- Xie, L.; Liu, J.; Du, A. Effect of hot-pressing factors on binderless fiberboard properties. In Proceedings of the 2012 International Conference on Biobase Material Science and Engineering, Changsha, China, 21–23 October 2012; pp. 8–11. [Google Scholar] [CrossRef]
- World Health Organization. Indoor Air Quality: Organic Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 1989; p. 70. Available online: http://whqlibdoc.who.int/cgi-bin/repository.pl?url=/euro/r&s/EURO_R&S_111.pdf (accessed on 15 November 2023).
- ADEME. Les Composés Organiques Volatils. Réduction des Émissions de COV dans L’industrie; Editions Dunod; Collection Technique et Ingénierie: Paris, France, 2013; p. 264. [Google Scholar]
- EPA. Report to Congress on Indoor Air Quality: Volume II—Assessment and Control of Indoor Air Pollution; Technical Report EPA/400/1-89/001C; United States Environmental Protection Agency: Washington, DC, USA, 1989; p. 250. [Google Scholar]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; Taylor & Francis: Abingdon, UK, 2016. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 25 November 2023).
- Ras, M.R.; Borrull, F.; Marcé, R.M. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. Trends Anal. Chem. 2009, 28, 347–361. [Google Scholar] [CrossRef]
- Król, S.; Zabiegała, B.; Namieśnik, J. Monitoring VOCs in atmospheric air II. Sample collection and preparation. Trends Anal. Chem. 2010, 29, 1101–1112. [Google Scholar] [CrossRef]
- Even, M.; Juritsch, E.; Richter, M. Measurement of very volatile organic compounds (VVOCs) in indoor air by sorbent-based active sampling: Identifying the gaps towards standardisation. Trends Anal. Chem. 2021, 140, 116265. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; Kim, K.-H. Derivatization techniques for determination of carbonyls in air. Trends Anal. Chem. 2015, 64, 29–41. [Google Scholar] [CrossRef]
- Palluau, F.; Mirabel, P.; Millet, M. Influence of Relative Humidity and Ozone on the Sampling of Volatile Organic Compounds on Carbotrap/Carbosieve Adsorbents. Environ. Monit. Assess. 2007, 127, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K.; Engewald, W. Ambient air analysis of volatile organic compounds using adsorptive enrichment. Chromatographia 2003, 57 (Suppl. S1), S339–S347. [Google Scholar] [CrossRef]
- Palluau, F.; Mirabel, P.; Millet, M. Influence of ozone on the sampling and storage of volatile organic compounds in canisters. Environ. Chem. Lett. 2007, 5, 51–55. [Google Scholar] [CrossRef]
- Murat, P.; Harohalli Puttaswamy, S.; Ferret, P.-J.; Coslédan, S.; Simon, V. Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis- Gas Chromatography-Mass Spectrometry. Molecules 2020, 25, 2115. [Google Scholar] [CrossRef] [PubMed]
- Bertheau, E.; Simon, V.; Delgado Raynaud, C. Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions. Molecules 2022, 27, 8260. [Google Scholar] [CrossRef] [PubMed]
- Baerenzung dit Baron, T.; Yobrégat, O.; Jacques, A.; Simon, V.; Geffroy, O. A novel approach to discriminate the volatilome of Vitis vinifera berries by selected ion flow tube mass Spectrometry analysis and chemometrics. Food Res. Int. 2022, 157, 111434. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef]
- Vitola Pasetto, L.; Simon, V.; Richard, R.; Pic, J.-S.; Violleau, F.; Manero, M.-H. Aldehydes gas ozonation monitoring: Interest of SIFT/MS versus GC/FID. Chemosphere 2019, 235, 1107–1115. [Google Scholar] [CrossRef]
- ISO 16000-6; Indoor Air—Part 6: Determination of VolatileOrganic Compounds in Indoor and Test Chamber Air by Active Sampling on Tenax TA Sorbent, Thermal Desorption and Gas Chromatography Using MS or MS-FID. International Organization for Standardization: Geneva, Switzerland, 2011.
- Zhang, L.Z.; Niu, J.L. Modeling VOCs emissions in a room with a single-zone multi-component multi-layer technique. Build. Environ. 2004, 39, 523–531. [Google Scholar] [CrossRef]
- Deng, B.; Kim, C.N. An analytical model for VOCs emission from dry building materials. Atmos. Environ. 2004, 38, 1173–1180. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Lin, C.-C.; Hsu, S.-C. Comparison of conventional and green building materials in respect of VOC emissions and ozone impact on secondary carbonyl emissions. Build. Environ. 2015, 87, 274–282. [Google Scholar] [CrossRef]
- ASTM D5116-06; Standard Guide for Small Scale Environmental Chamber Determinations of Organic Emissions from Indoor Materials/Products. ASTM International: West Conshohocken, PA, USA, 2006.
- Cao, S.; Wen, Y.; Xi, C.; Li, X.; Zhang, L.; Wang, G.; Shang, J. Development of a method based on thermal desorption-gas chromatography/mass spectrometry for the determination of 103 volatile organic compounds in mattresses. Int. J. Environ. Anal. Chem. 2019, 100, 1044–1065. [Google Scholar] [CrossRef]
- QB/T 1952.2-2011; Upholstered Furniture-Spring Mattress. Standardization Administration of China: Beijing, China, 2011.
- Yan, M.; Zhai, Y.; Shi, P.; Hu, Y.; Yang, H.; Zhao, H. Emission of volatile organic compounds from new furniture products and its impact on human health. Hum. Ecol. Risk Assess. 2019, 25, 1886–1906. [Google Scholar] [CrossRef]
- Even, M.; Hutzler, C.; Wilke, O.; Luch, A. Emissions of volatile organic compounds from polymer-based consumer products: Comparison of three emission chamber sizes. Indoor Air 2020, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; Böhm, M.; Srba, J.; Beránková, J. Evaluation of formaldehyde emission from different types of wood-based panels and flooring materials using different standard test methods. Build. Environ. 2012, 49, 86–96. [Google Scholar] [CrossRef]
- EN 717-1; Wood-Based Panels—Determination of Formaldehyde Release—Part 1: Formaldehyde Emission by the Chamber Method. European Standard Norm: Geneva, Switzerland, 2004.
- ASTM D 6007-02; Standard Test Method for Determining Formaldehyde Concentration in Air from Wood Products Using a Small Scale Chamber. ASTM International: West Conshohocken, PA, USA, 2008.
- EN 717-2; Wood-Based Panels Determination of Formaldehyde Release. Part 2: Formaldehyde Release by the Gas Analysis Method. European Standard Norm: Geneva, Switzerland, 1994.
- Jiang, C.; Li, D.; Zhang, P.; Li, J.; Wang, J.; Yu, J. Formaldehyde and volatile organic compound (VOC) emissions from particleboard: Identification of odorous compounds and effects of heat treatment. Build. Environ. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Souillier, A.; Plaisance, H.; Desauziers, V. New SPME-based method for on-site measurement of gas-phase concentration of phthalates and alternatives at the surface of PVC floorings. Green Anal. Chem. 2022, 1, 100012. [Google Scholar] [CrossRef]
- He, J.; Zou, Z.; Yang, X. Measuring whole-body volatile organic compound emission by humans: A pilot study using an air-tight environmental chamber. Build. Environ. 2019, 153, 101–109. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Yin, H.; Deng, Y.; Jiang, Y.; Yuan, H.; Dong, C.; Li, J.; Hua, J.; Wang, J. Rapid profiling of volatile compounds in green teas using Micro-Chamber/Thermal Extractor combined with thermal desorption coupled to gas chromatography-mass spectrometry followed by multivariate statistical analysis. LWT 2018, 96, 42–50. [Google Scholar] [CrossRef]
- Massold, E.; Bähr, C.; Salthammer, T.; Brown, S.K. Determination of VOC and TVOC in Air Using Thermal Desorption GC-MS- Practical Implications for Test Chamber Experiments. Chromatographia 2005, 62, 75–85. [Google Scholar] [CrossRef]
- ENV 13419-1; Produits de Construction—Détermination des Emissions de Composés Organiques Volatils—Partie 1: Méthode de la Chambre D’essai D’émission (Norme X 43-520-1). AFNOR: Paris, France, 2000.
- Rizk, M.; Verriele, M.; Mendez, M.; Blond, N.; Dusanter, S.; Schoemaecker, C.; Blondeau, P.; Le Calvé, S.; Locoge, N. Fast sorption measurements of VOCs on building materials: Part 2—Comparison between FLEC and CLIMPAQ methods. Build. Environ. 2016, 99, 239–251. [Google Scholar] [CrossRef]
- ISO 16000-9; Indoor Air-Part 9: Determination of the Emission of Volatile Organic Compounds from Building Products and Furnishing. Emission Test Chamber Method. International Organization for Standardization: Geneva, Switzerland, 2006.
- Rizk, M.; Verriele, M.; Dusanter, S.; Schoemaecker, C.; Le Calve, S.; Locoge, N. Fast sorption measurements of volatile organic compounds on building materials: Part 1—Methodology developed for field applications. Build. Environ. 2016, 99, 200–209. [Google Scholar] [CrossRef]
- ISO 16000-10; Indoor Air. Part 10: Determination of the Emission of Volatile Organic Compounds from Building Products and Furnishing. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 16000-1; Indoor Air. Part 1: General Aspects of Sampling Strategy. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 16000-25; Determination of the Emission of Semi-Volatile Organic Compounds by Building Products—Microchamber Method. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 12219-3; Interior Air of Road Vehicles—Part 3: Screening Method for the Determination of the Emissions of Volatile Organic Compounds from Vehicle Interior Parts and Materials—Micro-Scale Chamber Method. International Organization for Standardization: Geneva, Switzerland, 2012.
- ASTM D7706-17; Standard Practice for Rapid Screening of VOC Emissions from Products Using Micro-Scale Chambers. ASTM International: West Conshohocken, PA, USA, 2023.
- ISO 16000; Indoor Air. International Organization for Standardization: Geneva, Switzerland, 2023.
- Bourdin, D.; Mocho, P.; Desauziers, V.; Plaisance, H. Formaldehyde emission behavior of building materials: On-site measurements and modeling approach to predict indoor air pollution. J. Hazard. Mater. 2014, 280, 164–173. [Google Scholar] [CrossRef]
- Jensen, L.K.; Larsen, A.; Mølhave, L.; Hansen, M.K.; Knudsen, B. Health Evaluation of Volatile Organic Compound (VOC) Emissions from Wood and Wood-Based Materials. Arch. Environ. Health 2001, 56, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Mocho, P.; Plaisance, H.; Cantau, C.; Kinadjian, N.; Yrieix, C.; Desauziers, V. Assessment of VOCs material/air exchanges of building products using the DOSEC®-SPME method. Energy Procedia 2017, 122, 367–372. [Google Scholar] [CrossRef]
- Böhm, M.; Salem, M.Z.M.; Srba, J. Formaldehyde emission monitoring from a variety of solid wood, plywood, blockboard and flooring products manufactured for building and furnishing materials. J. Hazard. Mater. 2012, 221–222, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Risholm-Sundman, M.; Larsen, A.; Vestin, E.; Weibull, A. Formaldehyde emission—Comparison of different standard methods. Atmos. Environ. 2007, 41, 3193–3202. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Liang, W.; Yang, S.; Yang, X. Long-Term Formaldehyde Emissions from Medium-Density Fiberboard in a Full-Scale Experimental Room: Emission Characteristics and the Effects of Temperature and Humidity. Environ. Sci. Technol. 2015, 49, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.; Ohlmeyer, M.; Meier, D. Long-term development of VOC emissions from OSB after hot-pressing. Holzforschung 2005, 59, 519–523. [Google Scholar] [CrossRef]
- Roffael, E. Volatile organic compounds and formaldehyde in nature, wood and wood based panels. Holz Roh Werkst. 2006, 64, 144–149. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, S.Q.; Ingram, L. Chemical emissions from adhesive-bonded wood products at elevated temperatures. Wood Sci. Technol. 2011, 45, 627–644. [Google Scholar] [CrossRef]
- Tupciauskas, R.; Meile, K.; Godina, D.; Rizhikovs, J.; Syrpas, M.; Venskutonis, P.R. Qualitative Differences and Emission Persistence of Volatile Organic Compounds from Bio-Based Particleboards. Materials 2022, 15, 5278. [Google Scholar] [CrossRef] [PubMed]
- Mayes, D.; Oksanen, O. ThermoWood Handbook; Finnish Thermowood Association: Kolho, Finland, 2003. [Google Scholar]
- Sivrikaya, H.; Tesařová, D.; Jeřábková, E.; Can, A. Color change and emission of volatile organic compounds from Scots pine exposed to heat and vacuum-heat treatment. J. Build. Eng. 2019, 26, 100918. [Google Scholar] [CrossRef]
- Elaieb, M.; Candelier, K.; Pétrissans, A.; Dumarçay, S.; Gérardin, P.; Pétrissans, M. Heat treatment of Tunisian soft wood species: Effect on the durability, chemical modifications and mechanical properties. Maderas Cienc. Tecnol. 2015, 7, 699–710. [Google Scholar] [CrossRef]
- Hyttinen, M.; Masalin-Weijo, M.; Kalliokoski, P.; Pasanen, P. Comparison of VOC emissions between air-dried and heat-treated Norway spruce (Picea abies), Scots pine (Pinus sylvesteris) and European aspen (Populus tremula) wood. Atmos. Environ. 2010, 44, 5028–5033. [Google Scholar] [CrossRef]
- Peters, J.; Fischer, K.; Fischer, S. Characterization of emissions from thermally modified wood and their reduction by chemical treatment. Bioresources 2008, 3, 491–502. [Google Scholar] [CrossRef]
- Čech, P.; Tesařová, D. Comparison of VOC emissions from natural (untreated) Poplar wood and heat treated wood. Wood Sci. Technol. 2015, 90, 23–28. [Google Scholar]
- Xue, L.; Zhao, Z.; Zhang, Y.; Chu, D.; Mu, J. Analysis of Gas Chromatography-Mass Spectrometry Coupled with Dynamic Headspace Sampling on Volatile Organic Compounds of Heat-Treated Poplar at High Temperatures. BioResources 2016, 11, 3550–3560. [Google Scholar] [CrossRef]
- Simon, V.; Uitterhaegen, E.; Robillard, A.; Ballas, S.; Véronèse, T.; Vilarem, G.; Merah, O.; Talou, T.; Evon, P. VOC and carbonyl compound emissions of a fiberboard resulting from a coriander biorefinery: Comparison with two commercial wood-based building materials. Environ. Sci. Pollut. Res. 2020, 27, 16121–16133. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Burianová, K.; Ballas, S.; Véronèse, T.; Merah, O.; Talou, T.; Stevens, C.V.; Evon, P.; Simon, V. Characterization of volatile organic compound emissions from self-bonded boards resulting from a coriander biorefinery. Ind. Crop. Prod. 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Bertheau, E. Etude des Émissions de Composés Organiques Volatils de Panneaux de Bois de Chêne (Quercus robur L.) Sans Liant Issus de Procédés de Cuisson-Compression: Impact de L’eau. Ph.D. Thesis, University of Toulouse, Toulouse, France, 2023. [Google Scholar]
| Categories | Resin Type | Typical Adhesive System |
|---|---|---|
| Thermosetting | Amino | Urea-formaldehyde (UF) |
| Melamine-formaldehyde (MF) | ||
| Melamine-urea-formaldehyde (MUF) | ||
| Phenolic | Phenol-formaldehyde (PF) | |
| Resorcinol-formaldehyde (RF) | ||
| Phenol-resorcinol-formaldehyde (PRF) | ||
| Isocyanate | Diphenylmethane-4,4′-diisocyanate (pMDI) | |
| Thermoplastic | Vinyl | Polyvinyl acetate (PVAC) |
| Polyvinyl alcohol (PVA) |
| Wood Species | Pretreatment | References |
|---|---|---|
| Abies alba and Pinus insignis | Steam explosion | [80] |
| Oil palm trunk | / | [70] |
| Oil palm trunk | / | [72] |
| Picea mariana bark | Refining | [81] |
| Oil palm trunk | / | [82] |
| Pinus radiata | Fenton reaction | [83] |
| Aspen | Steam stabilization | [84] |
| Picea abies and Fagus sylvatica | Fenton reaction | [85] |
| Populus euramevicana | / | [86] |
| Compound | CAS | MW (g mol−1) | Bp (°C) [90,91] | CMR Classification [14] | |
|---|---|---|---|---|---|
| C1 | Formaldehyde | 50-00-0 | 30 | −19 | Carc. 2 |
| C2 | Acetaldehyde | 75-07-0 | 44 | 21 | Carc. 2 |
| Acetic acid | 64-19-7 | 60 | 118 | Carc. 3 | |
| C3 | Acetone | 67-64-1 | 58 | 56 | Carc. 3 |
| Acrolein | 107-02-8 | 56 | 52 | Carc. 3 | |
| Propanal | 123-38-6 | 58 | 48 | Carc. 3 | |
| Tetrachloroethylene | 127-18-4 | 166 | 121 | Carc. 2 | |
| C4 | Butanal | 204-646-6 | 72 | 75 | Carc. 3 |
| 2-Butenal | 4170-30-3 | 70 | 102 | Muta. 2 | |
| C5 | Furfural | 98-01-1 | 96 | 162 | Carc. 2 |
| C6 | Benzene | 71-43-2 | 78 | 80 | Carc.1, Muta. 2 |
| 2-Butoxyethanol | 111-76-2 | 118 | 170 | Carc. 3 | |
| 1,4-Dichlorobenzene | 106-46-7 | 147 | 173 | Carc. 2 | |
| n-Hexane | 110-54-3 | 86 | 69 | Repr. 2 | |
| C7 | Benzaldehyde | 100-52-7 | 106 | 179 | Carc. 3 |
| Toluene | 108-88-3 | 92 | 111 | Repr. 2 | |
| C8 | Ethylbenzene | 100-41-4 | 106 | 136 | Carc. 3 |
| Styrene | 100-42-5 | 104 | 145 | Carc. 3 | |
| Vanillin | 121-33-5 | 152 | 285 | Carc. 3 | |
| m-Xylene | 108-38-3 | 106 | 139 | Carc. 3 | |
| o-Xylene | 95-47-6 | 106 | 144 | Carc. 3 | |
| p-Xylene | 106-42-3 | 106 | 138 | Carc. 3 | |
| C9 | Syringaldehyde | 134-96-3 | 182 | 192 | Carc. 3 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 120 | 169 | Carc. 3 | |
| C10 | Naphtalene | 91-20-3 | 128 | 218 | Carc. 2 |
| Sabinene | 3387-41-5 | 136 | 163 | Carc. 3 | |
| C11 | Undecane | 1120-21-4 | 156 | 196 | Carc. 3 |
| Type of Wood Panel | VOCs Identified in Emissions | References |
|---|---|---|
| MDF | Formaldehyde * | [133] |
| Formaldehyde, acetaldehyde, hexanal | [134] | |
| Acetic acid, tetrahydrofuran, α-pinene, styrene, hexane, camphene, p-xylene, dichloromethane, naphthalene | [138] | |
| Particleboards | Formaldehyde, acetaldehyde, propanal, hexanal, pentanal, benzaldehyde, acrolein, acetone | [134] |
| Formaldehyde, n-hexane, 3-methylheptane, 2,2-dimethylhexane, undecane, dodecane, toluene, benzene, xylenes, 1,4-dichlorobenzene, naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, 2-methylfuran, 2,3-dimethylnaphthalene, 1,7-dimethylnaphthalene, pentanal, hexanal, nonanal, decanal, 2-pentanone, 4-methyl-2-pentanone, 2-ethylhexanol, isobutanol, n-butanol, ethyl acetate, n-butyl acetate, isoocyl acetate, dibutyl phthalate, dimethyl carbonate, trichloromethane, phthalic anhydride, TVOCs | [118] | |
| OSB | Formaldehyde, hexanal, and α-pinene, among others | [135] |
| Formaldehyde, acetaldehyde, propanal, pentanal, hexanal, benzaldehyde, furfural, acrolein | [134] | |
| Formaldehyde * | [133] | |
| Plywood | Formaldehyde * | [114,134,136,137] |
| Formaldehyde acetaldehyde, propanal, hexanal, pentanal | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertheau, E.; Simon, V.; Delgado Raynaud, C. Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards. Appl. Sci. 2024, 14, 1266. https://doi.org/10.3390/app14031266
Bertheau E, Simon V, Delgado Raynaud C. Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards. Applied Sciences. 2024; 14(3):1266. https://doi.org/10.3390/app14031266
Chicago/Turabian StyleBertheau, Elise, Valérie Simon, and Christine Delgado Raynaud. 2024. "Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards" Applied Sciences 14, no. 3: 1266. https://doi.org/10.3390/app14031266
APA StyleBertheau, E., Simon, V., & Delgado Raynaud, C. (2024). Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards. Applied Sciences, 14(3), 1266. https://doi.org/10.3390/app14031266









