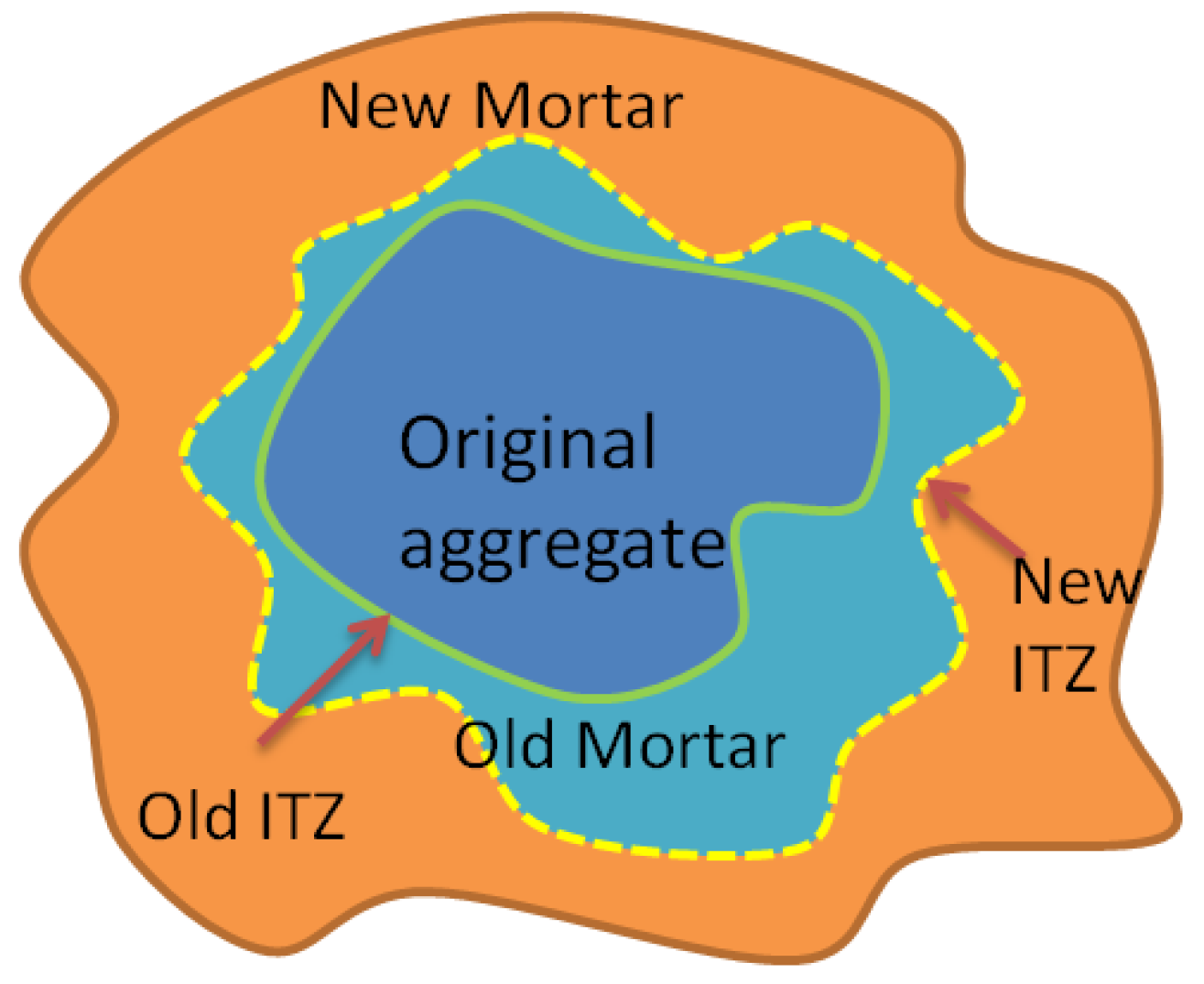
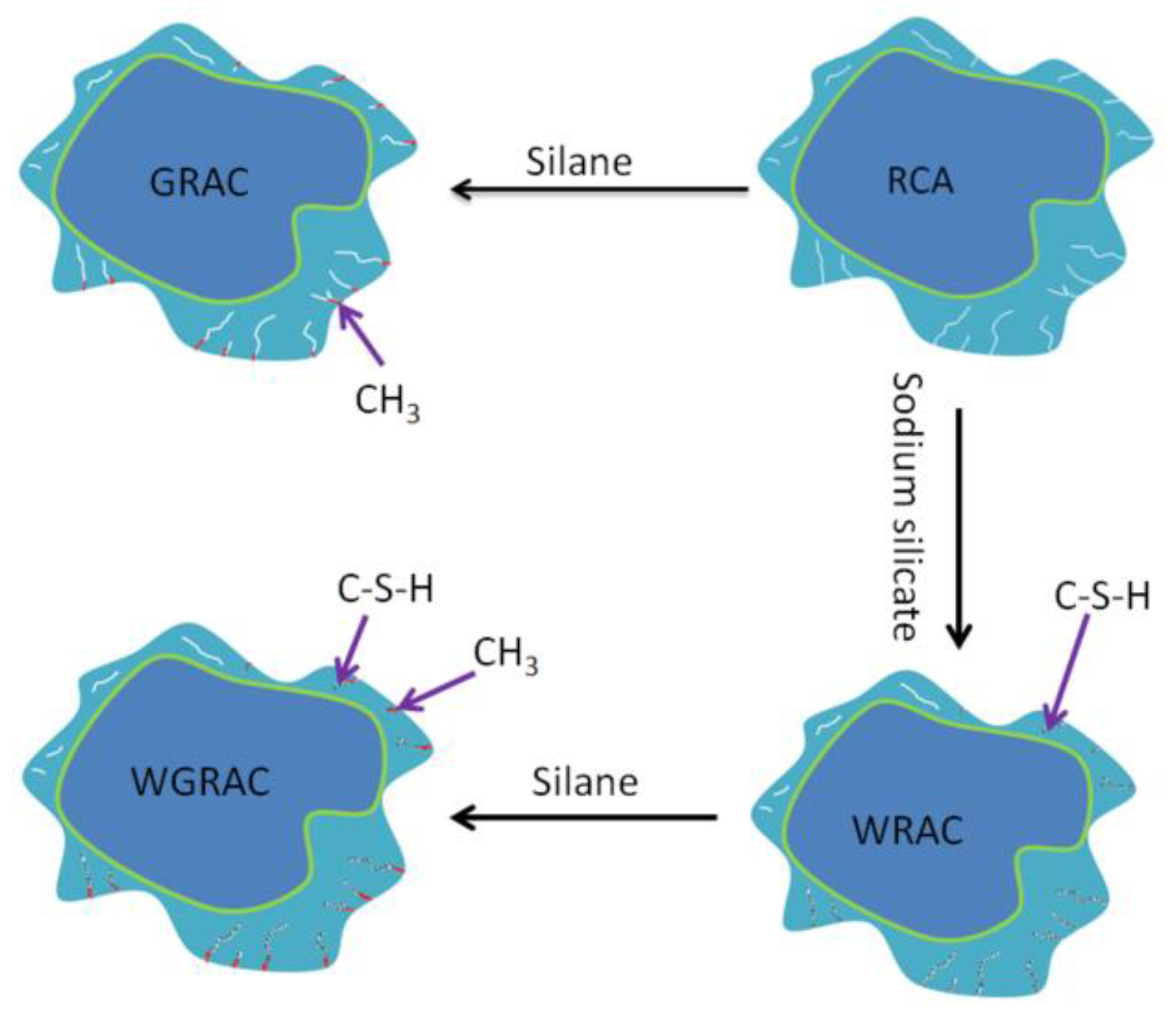
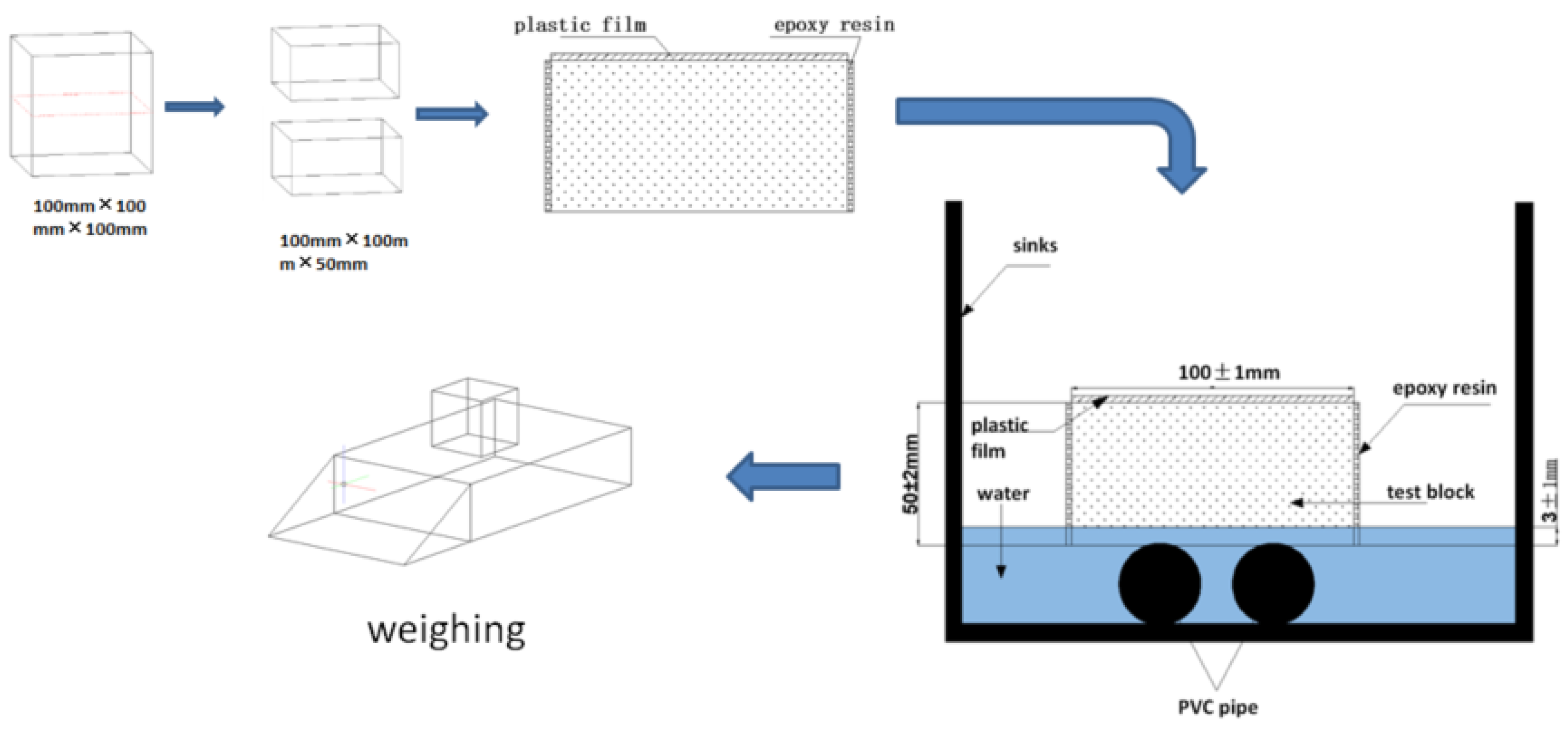
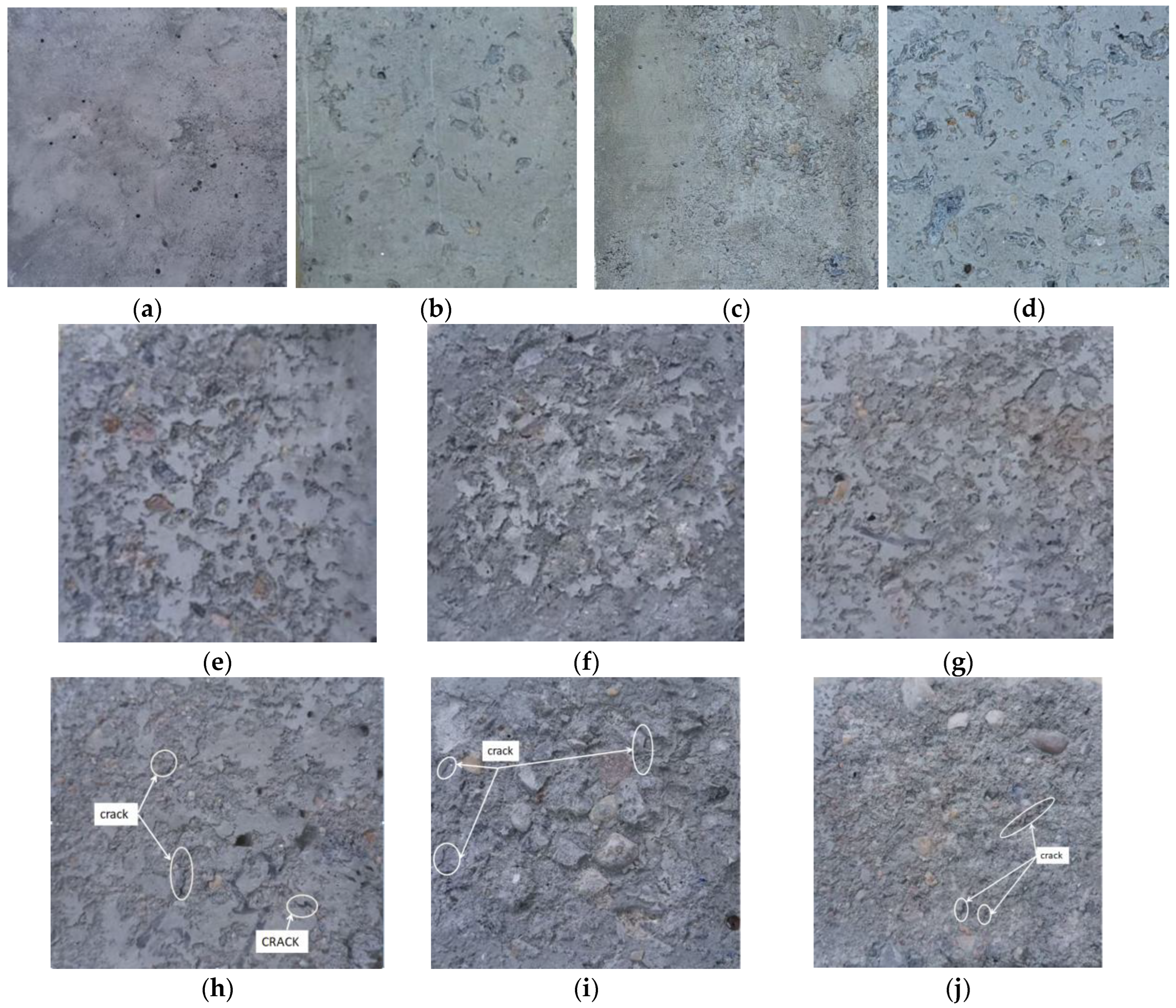
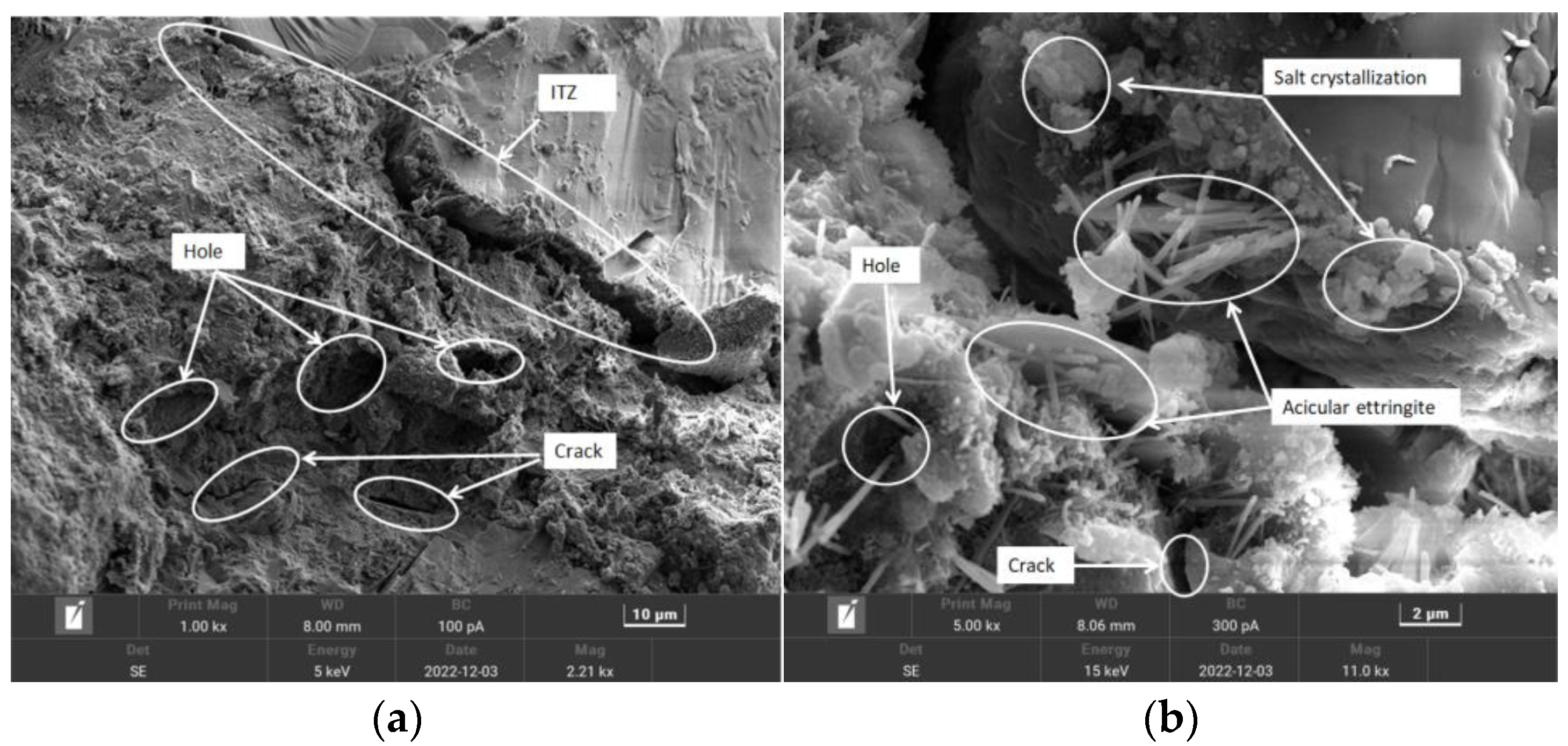
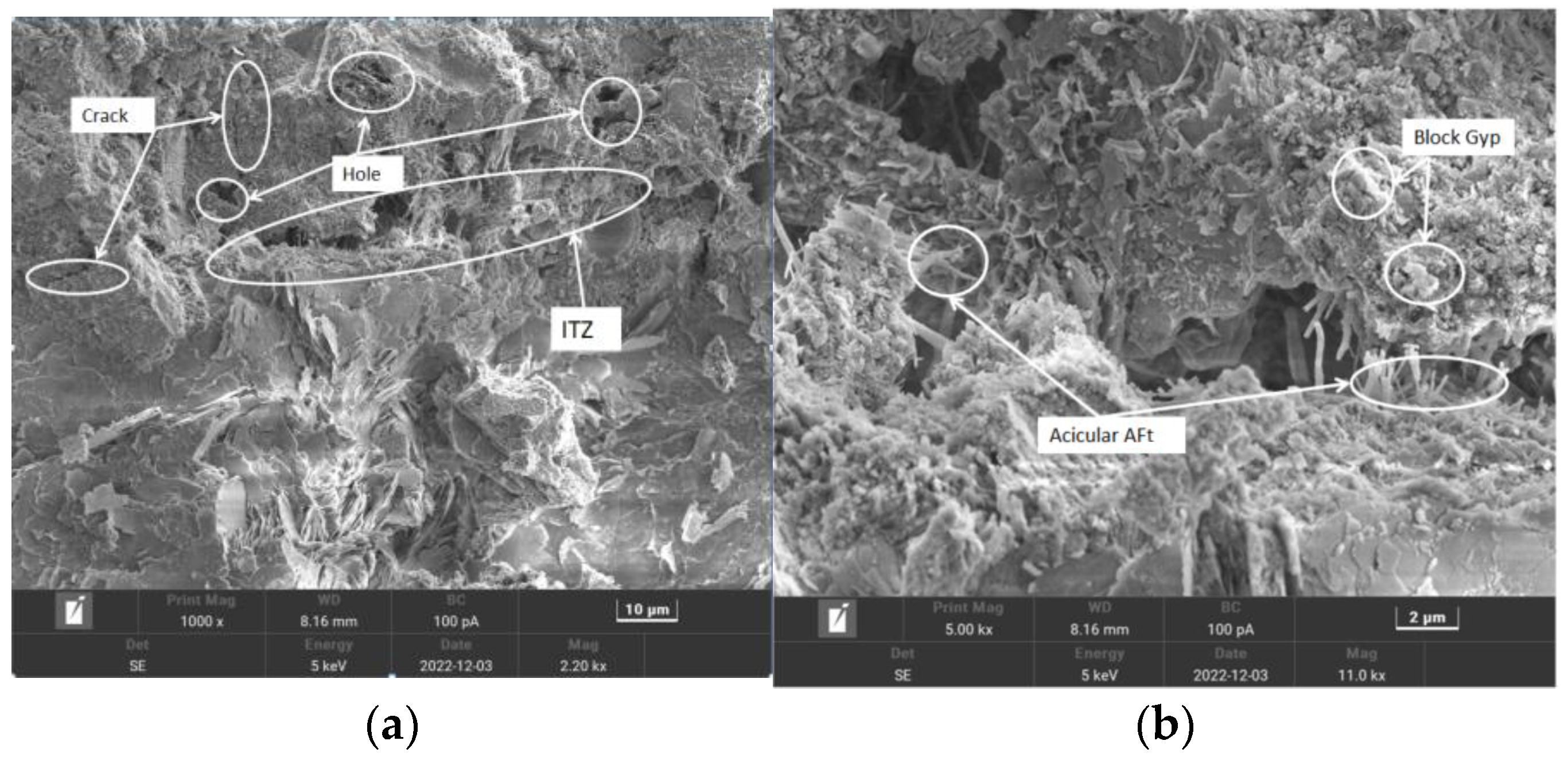
3.2.3. Analysis of Data Calculations
According to the experimental data, the water absorption rate can be divided into three stages, which are the pre-fast absorption stage (T1/2 = 0–84.8 s), the secondary slow absorption stage (T1/2 = 84.8–207.8 s), and the late gentle absorption stage (T1/2 = 207.8–777.7 s).
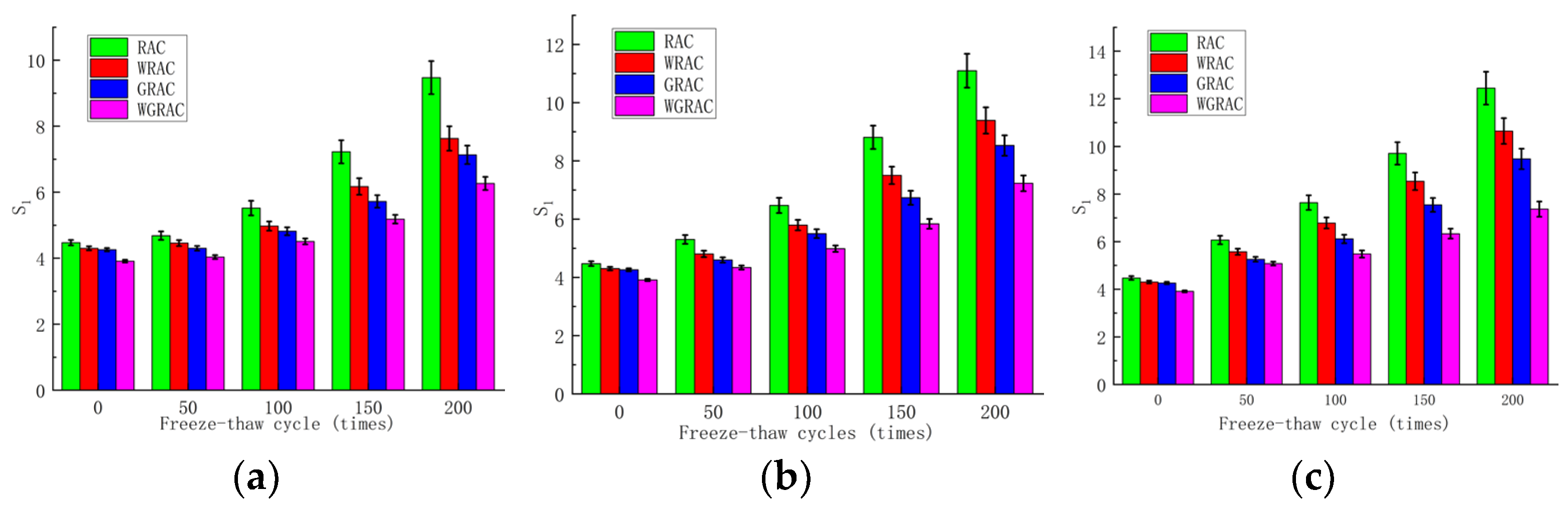
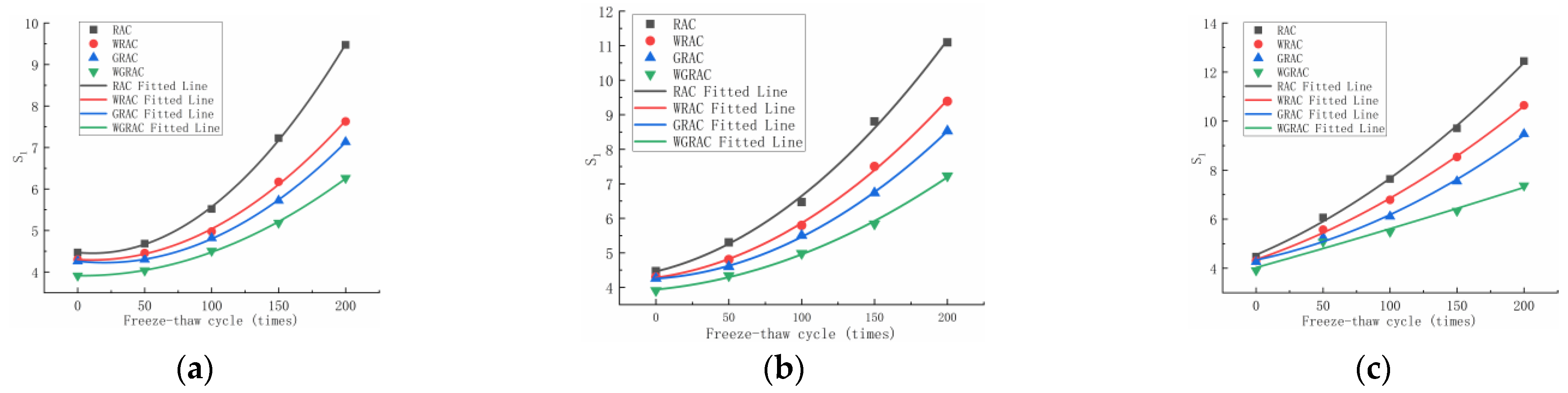
The initial water absorption rates of the RAC, WRAC, GRAC, and WGRAC groups can be obtained from the linear fit of the preliminary phase (T1/2 = 0–84.8 s) of the cumulative water absorption curve per unit time, and its relationship with the number of freeze–thaw cycles in different media is shown below.
As can be seen in
Figure 6a, at 50 freeze–thaw cycles, the water absorption of the WRAC, GRAC, and WGRAC groups decreased by 4.82%, 8.1%, and 13.8% compared to the RAC group. At 200 freeze–thaw cycles, the water absorption of the WRAC, GRAC, and WGRAC groups decreased by 19.4%, 24.6%, and 33.8% compared to the RAC group. As can be seen in
Figure 6b, at 50 freeze–thaw cycles, the capillary water absorption of the WRAC, GRAC, and WGRAC groups decreased by 9.3%, 13.2%, and 18.1%, which are lower than that of the RAC group. At 200 freeze–thaw cycles, the pre-absorption rates of the WRAC, GRAC, and WGRAC groups decreased by 15.38%, 23.14%, and 34.83%, respectively, compared with the RAC group. As can be seen in
Figure 6c, at 50 freeze–thaw cycles, the water absorption of the WRAC, GRAC, and WGRAC groups decreased by 8.1%, 13.3%, and 16.3% compared with that of the RAC group, and when 200 freeze–thaw cycles were performed, the water absorption of the WRAC, GRAC, and WGRAC groups decreased by 14.4%, 23.8%, and 38.6% compared with that of the RAC group, respectively.
The water absorption of the specimens did not change much at the low number of freeze and thaw cycles in the clear water freezing and thawing environment. The reason for this is that in the early stage of freezing and thawing, the specimen is more complete and dense, and the damage caused by freezing and thawing is mainly concentrated in the surface layer, which has less effect on the interior of the concrete. With the increase in the number of freeze–thaw cycles, the freeze–thaw damage gradually increases, destroying the concrete surface mortar; the internal closed pores of the concrete are gradually destroyed and connected; the water infiltration into the internal channels of the concrete is gradually increased; the freeze–thaw damage is gradually diffused to the internal concrete; and therefore, the amplitude of the change in the capillary absorption rate of the concrete is gradually increased. At a low number of freeze–thaw cycles in a sulfate freezing and thawing environment, the capillary water absorption rate of each group of specimens in the early stage has a certain increase compared with that of clear water, but the growth rate is relatively slow because the hydration reaction between sulfate and concrete internally generates crystals of calcium alumina, gypsum, etc., which fill the pores and make the concrete relatively dense, allowing it to resist part of the freezing and thawing damage. With the increase in the number of freezing and thawing cycles, the specimen’s surface erosion spalling becomes more and more serious, and the sulfate solution is more likely to penetrate into the concrete interior and constantly crystallize and expand, under the pressures of crystallization and infiltration, to accelerate the specimen’s internal freezing and thawing damage, so that the specimen’s water absorption ability is accelerated. At a low number of freeze–thaw cycles in freeze–thaw environments with chloride salts, the pre-capillary water absorption ability of each group of specimens was higher than that of the other two because the chloride salts lowered the freezing point of the water, reduced the freezing time, and increased the time needed for the chloride ions to penetrate. Additionally, the chlorine ion’s own penetration ability is very strong due to the existence of the concentration difference; the chlorine ion with the water quickly penetrates into the concrete interior; the concrete’s internal erosion, along with the increase in the number of freeze–thaw cycles, causes the surface concrete spalling to become more and more serious; and the internal freeze–thaw damage becomes more and more serious, which accelerates the freeze–thaw damage, and the growth rate of capillary water absorption gradually accelerates.
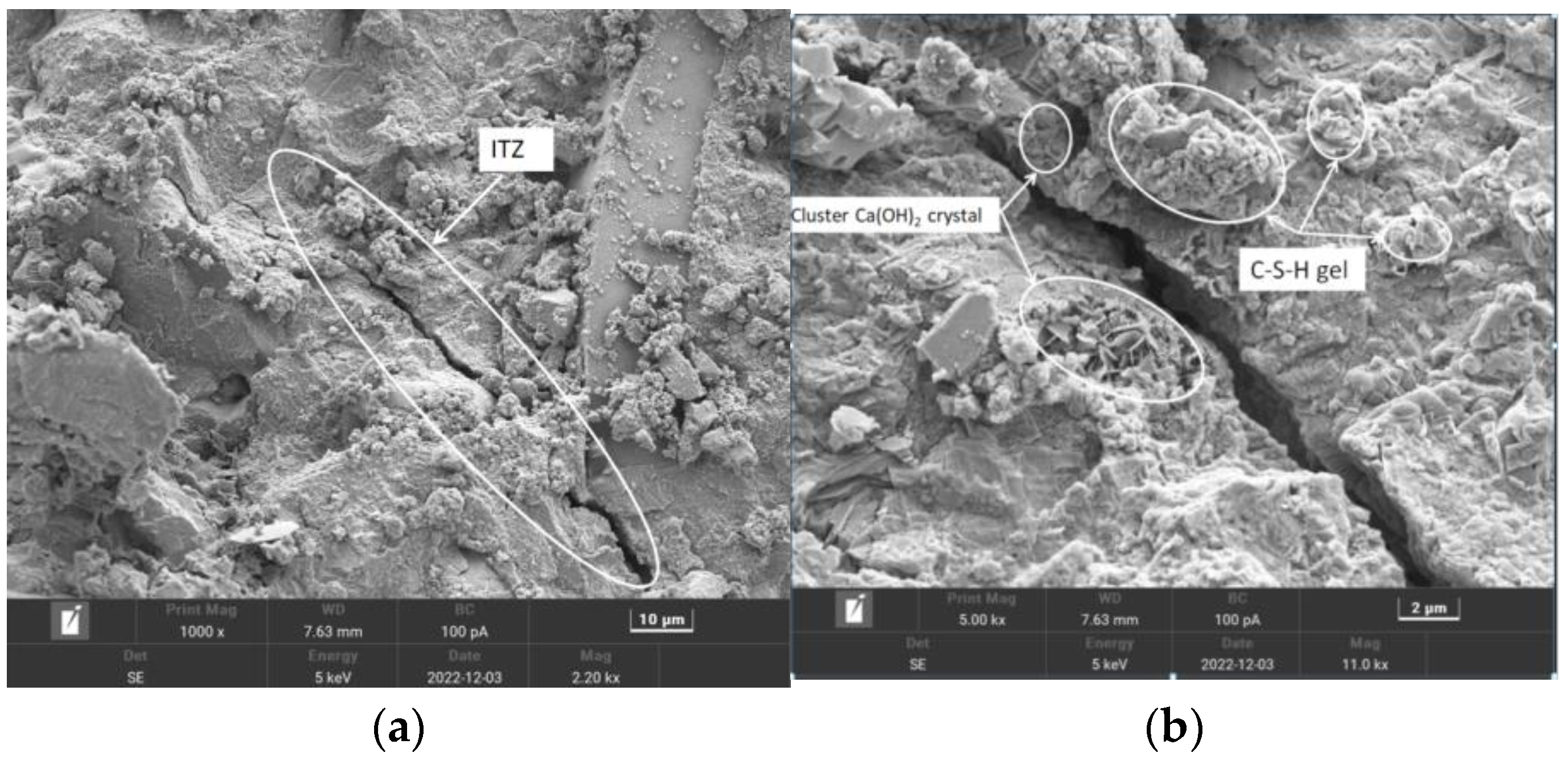
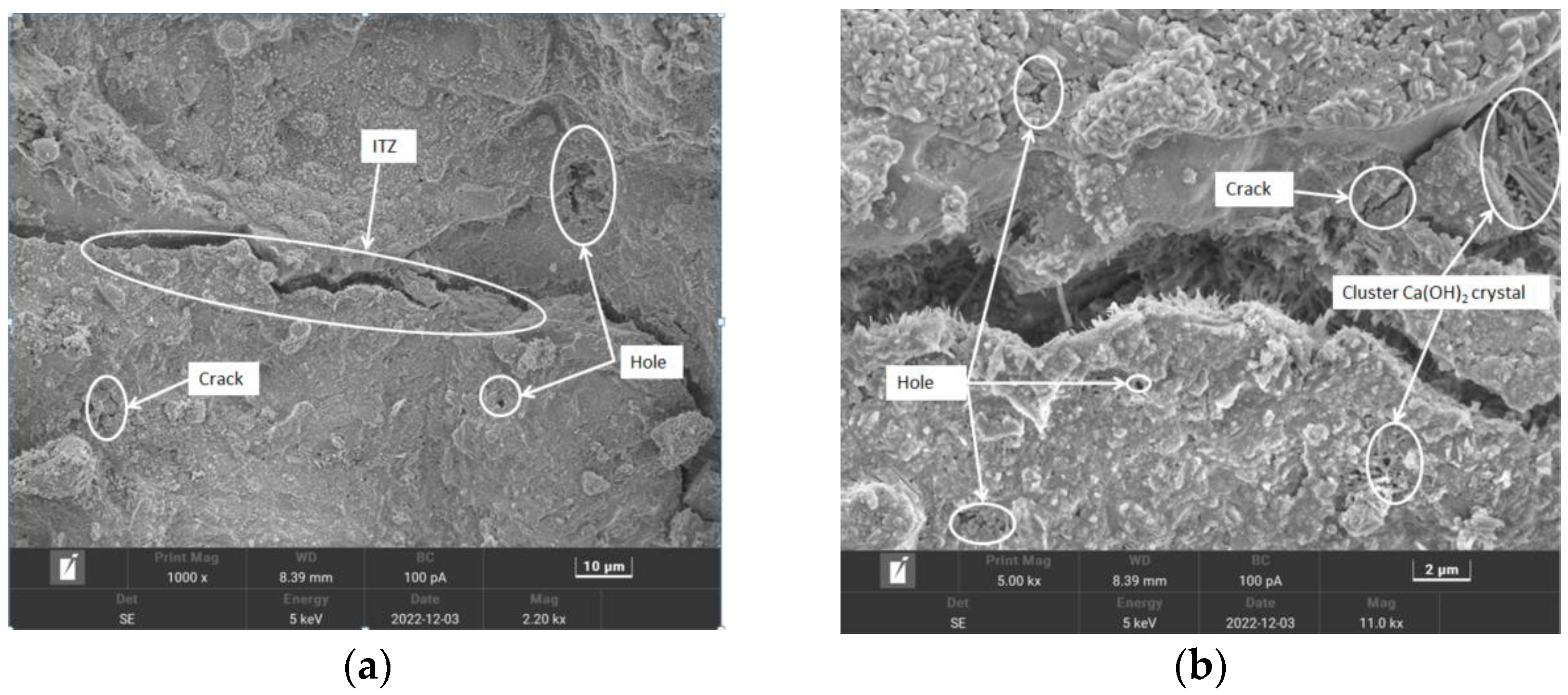
From the overall observation of the above figure, it can be seen that with the increase in the number of freezing and thawing cycles, the specimen’s pre-capillary water absorption ability gradually increased, the specimen under the chlorine salt medium freezing and thawing showed the fastest growth in capillary water absorption, with the slowest growth being observed for the water medium, and it can be seen that salt freezing of the concrete caused by the damage was greater than that caused by the water freezing, with the largest freeze–thawing damage being caused by chlorine salts. The water absorption of the pretreated specimens was lower than that of the unmodified pretreatment, and the composite-modified pretreatment was the most effective. Regarding the water glass solution and silane solution for recycled aggregate immersion modification treatment, the solution can enter the aggregate through penetration and react with the unhydrated material to generate silicate gel (C-S-H) and other substances to fill the internal pores of the aggregate; thus, the recycled coarse aggregate’s internal structure of densification has been improved, and the recycled concrete’s internal pore space is relatively small. The recycled concrete and silane hydrolysis attached to the surface of the recycled coarse aggregate, forming a hydrophobic barrier and preventing water molecules from penetrating into the concrete interior; the overall water absorption capacity of recycled concrete was reduced, and the water absorption rate decreased.
3.2.4. Secondary Water Absorption Rate
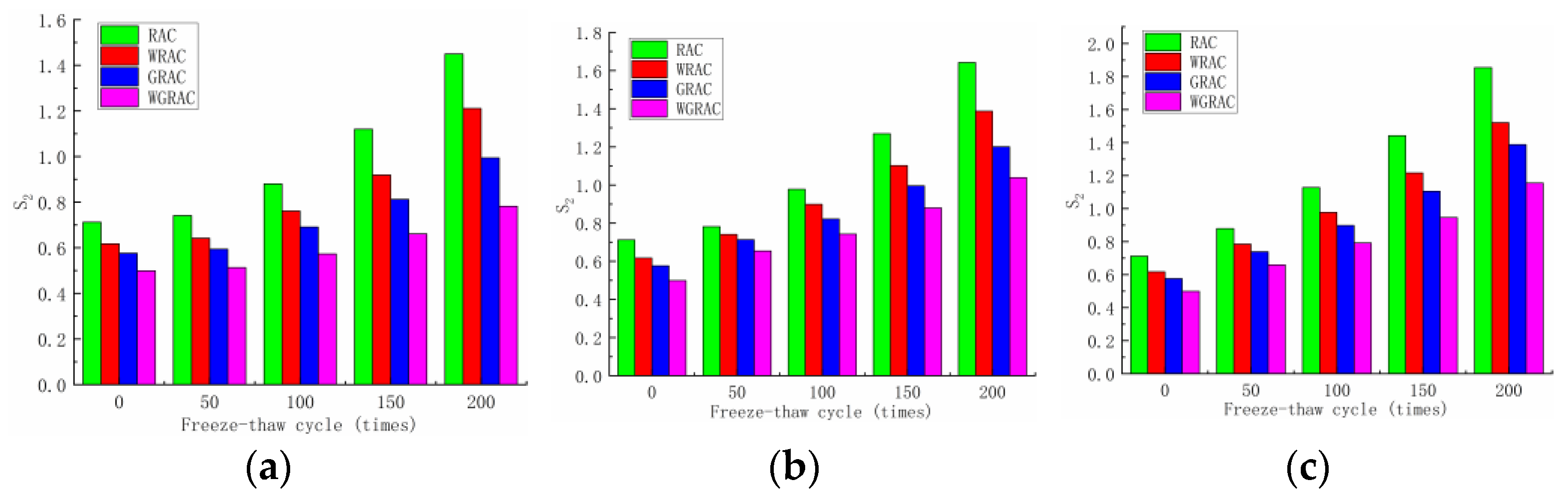
From the linear fit of the mid-term phase (T
1/2 = 84.8–207.8 s) of the unit’s cumulative water uptake curve, the secondary water uptake rates of the RAC, WRAC, GRAC, and WGRAC groups can be obtained, and their relationships with the number of freeze–thaw cycles in different media are shown in
Figure 7.
As can be seen in
Figure 7, the water absorption of the RAC, WRAC, GRAC, and WGRAC groups increased with the increase in the number of freeze–thaw cycles under the three media freeze–thaw cycles, but in comparison to the pre-absorption phase, the secondary water absorption showed a substantial decrease.
This is because, in the early stage of water absorption, unsaturated concrete comes into contact with water, and the concrete surface’s capillary pores undergo pressure under the action of rapid water absorption, leading to the absorption of more water and causing the capillary pores to be gradually saturated, resulting in the middle stage; the capillary pores undergo a gradual decrease in pressure. At this time, the water outside the test piece is mainly diffused into the concrete’s interior, and the rate of water absorption gradually slows down, so the secondary water absorption rate of the test piece is much smaller than the initial water absorption rate. By comparison, the chloride salt freeze–thaw specimens have the largest water absorption rates, followed by sulfate, and the water freeze–thaw specimens have the smallest rates. After comparison, it can be seen that the water absorption rate of the RAC group is greater than that of the WRAC group, and the water absorption rate of the GRAC group is greater than that of the WGRAC group, which indicates that the effect of composite-modified recycled coarse aggregate on the improvement of the internal pore space of the concrete is more obvious, the overall water absorption of the concrete decreases significantly, and the decrease in the water absorption rate is the largest.
3.2.5. Late Water Absorption
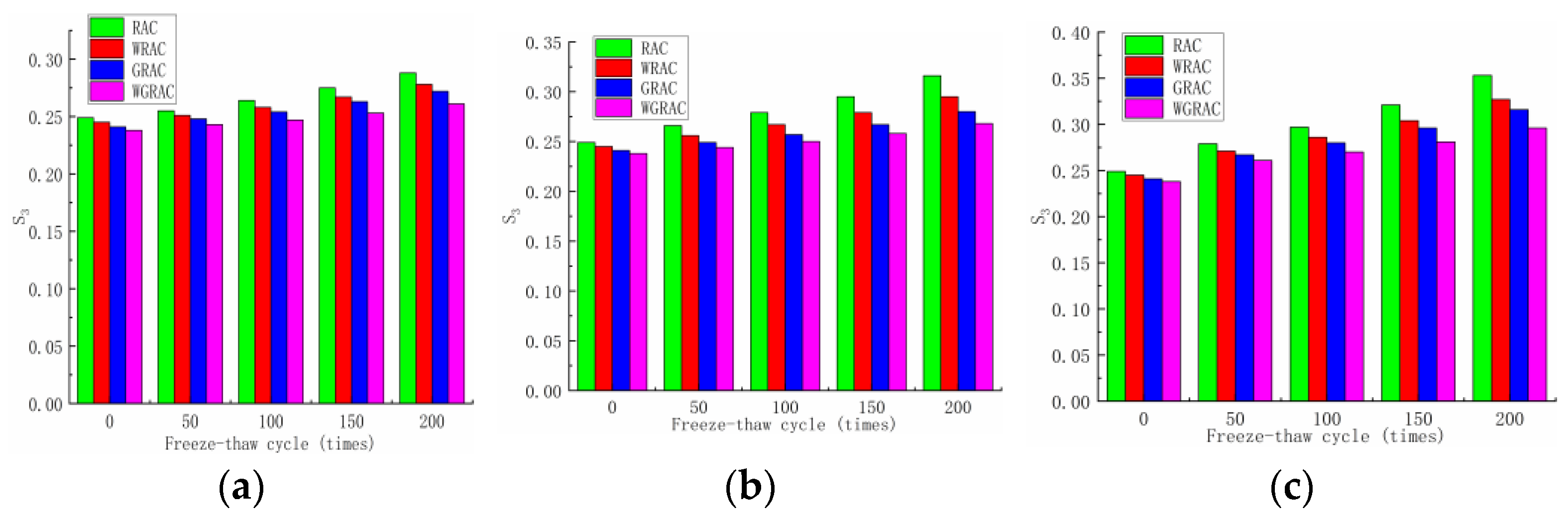
From the late period phase (T1/2 = 207.8–777.7 s) of the unit’s cumulative water uptake curve, a linear fit was made to obtain the late-period water uptake rates for the RAC, WRAC, GRAC, and WGRAC groups as a function of the number of freeze–thaw cycles for different media, as shown in the figure below.
According to
Figure 8, it can be seen that the water absorption rate of the specimen under three different media used for freezing and thawing is very small, and the change is very small, because in the late stage of freezing and thawing, the growth of internal cracks in the specimen gradually slows down, and the water absorption rate in the late stage also gradually tends to be leveled off.
Through the above data analysis and comparison, it can be seen that the initial water absorption rate of the concrete changes, S1, is large; the secondary water absorption rate, S2, does not change much; and the later water absorption rate, S3, has an even more minimal effect on the capillary water absorption of concrete. It can be found that the capillary water absorption capacity of concrete is mainly determined by the initial water absorption rate, S1, so the initial water absorption rate, S1, is mainly used in the actual project to measure the capillary water absorption capacity of concrete to judge the durability performance of concrete.