Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile
Abstract
1. Introduction
2. Materials and Methods
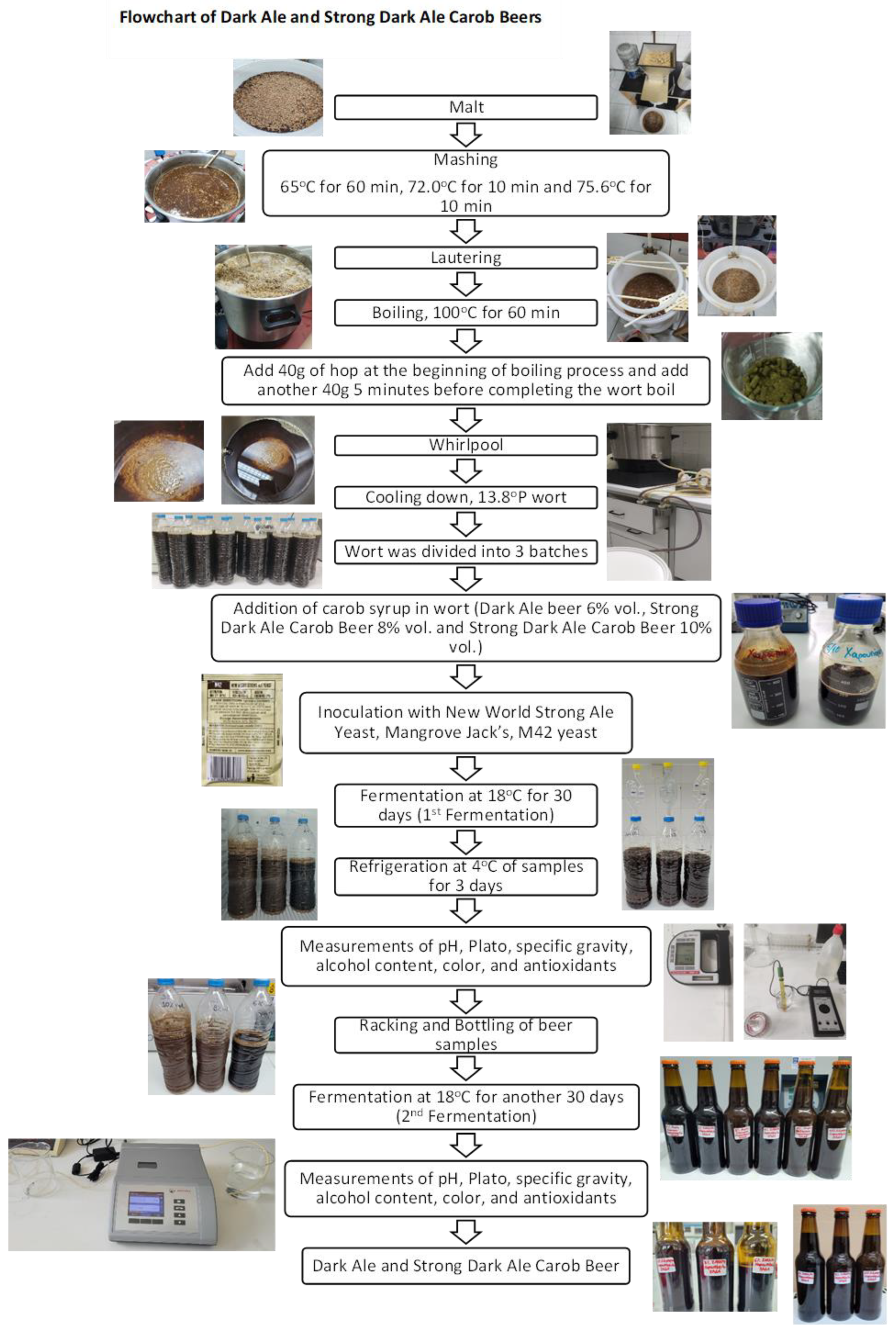
2.1. Production and Treatment of Beer Samples Prior to Analysis
2.1.1. Preparation of Carob Syrup
2.1.2. Preparation of Dark and Strong Dark Ale Carob Beers
2.1.3. Sample Preparation for Beer Analysis
2.2. Analysis of Beer and Carob Syrup Samples
2.2.1. Physicochemical Characteristics (pH, Brix, Plato, Specific Gravity, Alcohol Content, Color)
2.2.2. Spectrophotometric Assays
Determination of Total Phenolic Content (TPC)
Ferric Reducing/Antioxidant Power Assay (FRAP)
Scavenging Activity on 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic Acid) Radical (ABTS●+)
2.3. Phenolic Profile by LC-QToF-MS Analysis
2.3.1. Reagents and Materials
2.3.2. Mass Spectra Analysis
2.4. Statistical Analysis of Results
3. Results and Discussion
3.1. Carob Syrup
3.2. Characteristics of Dark and Strong Dark Ale Beer Samples
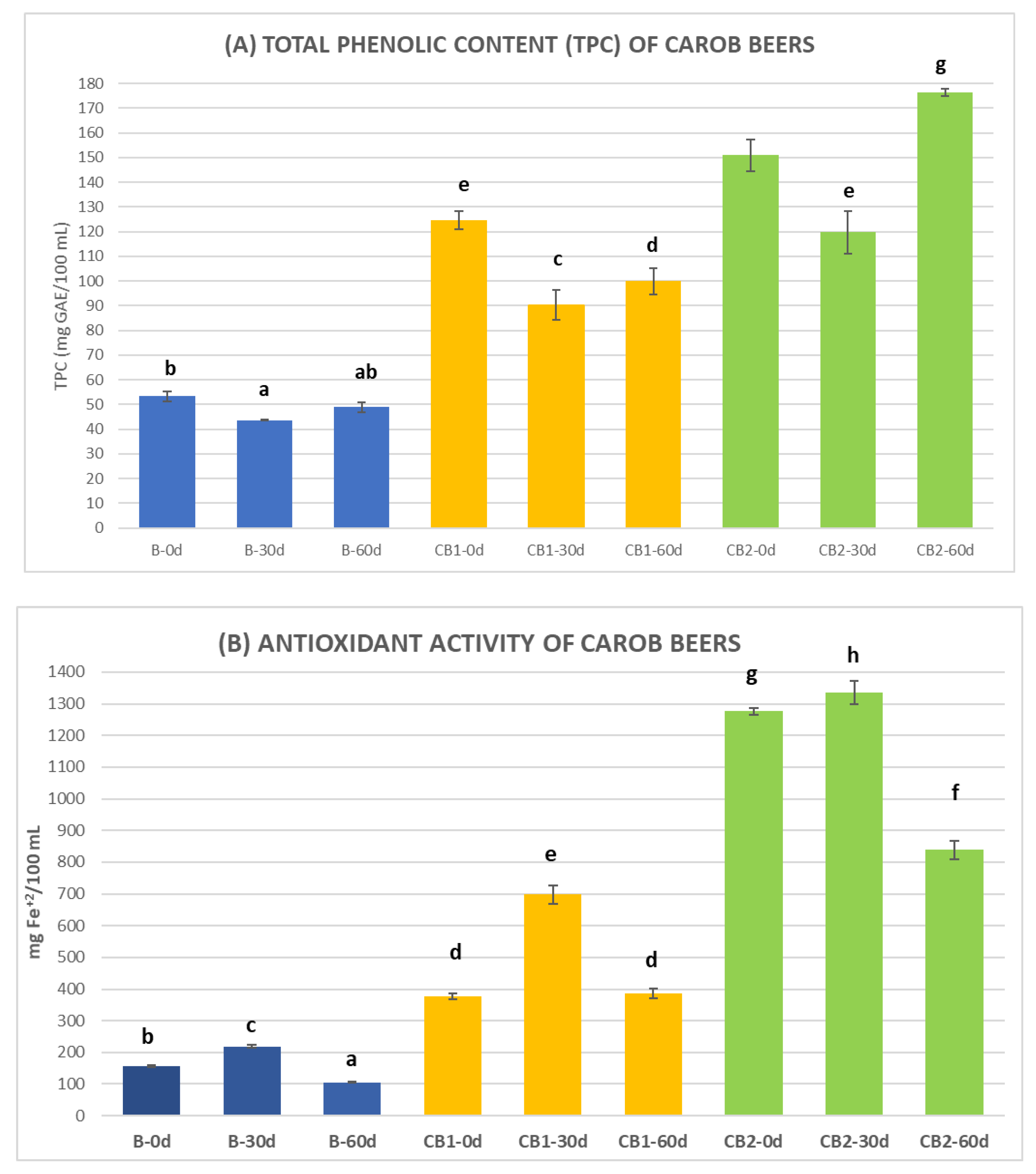
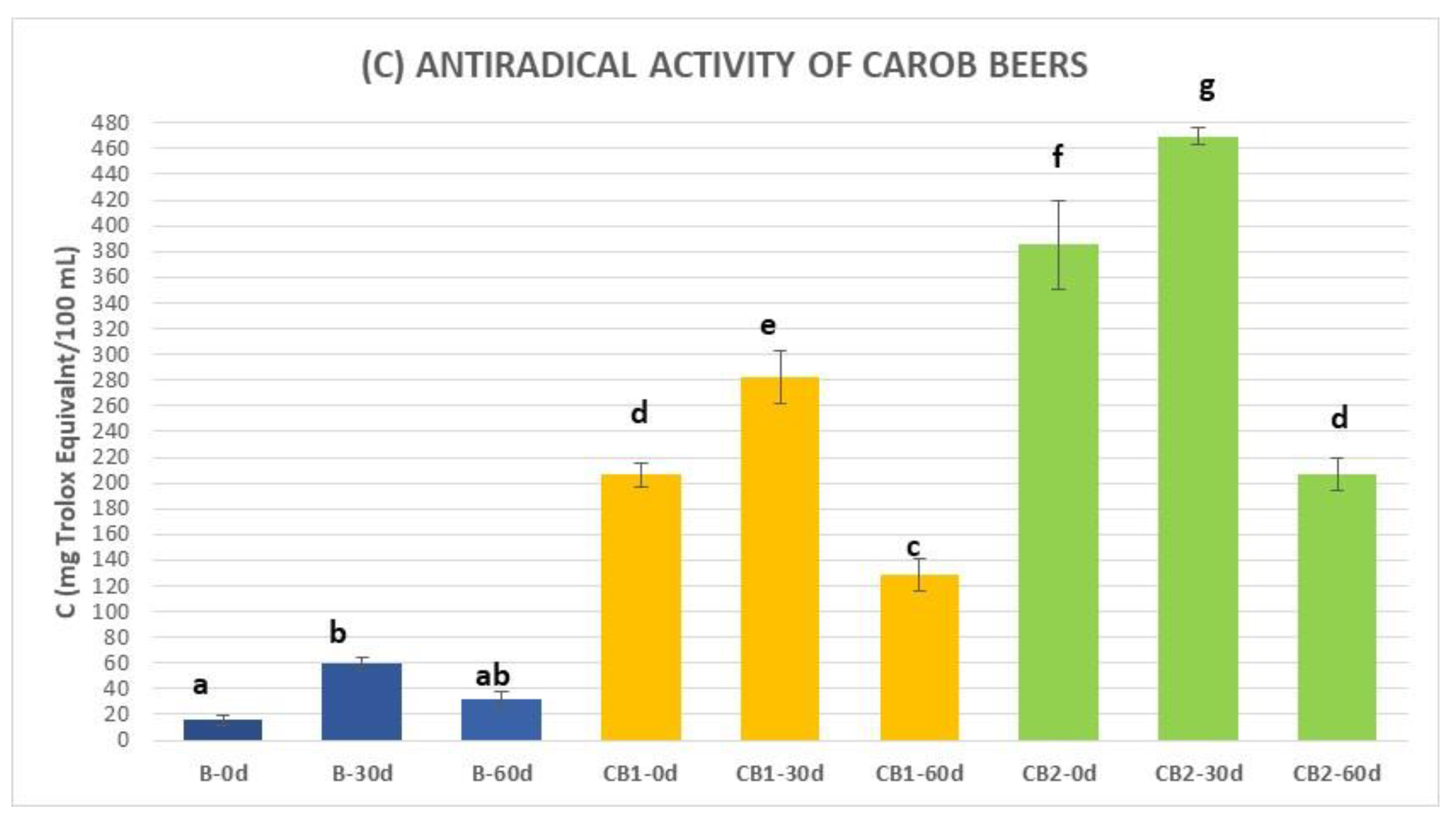
3.3. Total Phenolic Content (TPC), Antioxidant Activity, and Antiradical Activity
3.4. LC-QToF-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valamoti, S.M. Brewing beer in wine country? First archaeobotanical indications for beer making in Early and Middle Bronze Age Greece. Veg. Hist. Archaeobot. 2018, 27, 611–625. [Google Scholar] [CrossRef]
- Miller, S.R.; Sirrine, J.R.; McFarland, A.; Howard, P.H.; Malone, T. Craft beer as a means of economic development: An economic impact analysis of the Michigan value chain. Beverages 2019, 5, 35. [Google Scholar] [CrossRef]
- BarthHaas World Beer Production 2022–2023. Available online: https://www.barthhaas.com/resources/barthhaas-report#!beer-production (accessed on 17 November 2023).
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- FHA-Food & Beverage Top 9 Beer Industry Trends in 2023. Available online: https://fhafnb.com/blog/beer-industry-trends/ (accessed on 17 November 2023).
- Nunes Filho, R.C.; Galvan, D.; Effting, L.; Terhaag, M.M.; Yamashita, F.; Benassi, M.d.T.; Spinosa, W.A. Effects of adding spices with antioxidants compounds in red ale style craft beer: A simplex-centroid mixture design approach. Food Chem. 2021, 365, 130478. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of volatiles and polyphenol content, physicochemical parameters and antioxidant activity in beers with dotted hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Kim, J.; Bashir, K.M.I.; Cho, M.G. Antioxidant Content of Aronia Infused Beer. Fermentation 2020, 6, 71. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, food processing and health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef]
- Chib, A.; Gupta, N.; Bhat, A.; Anjum, N.; Yadav, G. Role of antioxidants in food. Int. J. Chem. Stud. 2020, 8, 2354–2361. [Google Scholar] [CrossRef]
- Kouka, P.; Chatzieffraimidi, G.A.; Raftis, G.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xynos, N.; Skaltsounis, A.L.; Tsatsakis, A.M.; Kouretas, D. Antioxidant effects of an olive oil total polyphenolic fraction from a Greek Olea europaea variety in different cell cultures. Phytomedicine 2018, 47, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob pulp: A nutritional and functional by-product worldwide spread in the formulation of different food products and beverages. a review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Theophilou, I.C.; Neophytou, C.M.; Constantinou, A.I. Carob and its Components in the Management of Gastrointestinal Disorders. J. Hepatol. Gastroenterol. 2017, 1, 5. [Google Scholar]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Cavdarova, M.; Makris, D.P. Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass Valorization 2014, 5, 773–779. [Google Scholar] [CrossRef]
- Eshghi, S.; Rostami, A.A.; Jamali, B. Carob Tree: A Suitable Species for the Future. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2018; Volume 1190, pp. 67–70. [Google Scholar]
- Tataridis, P.; Kanellis, A.; Logothetis, S.; Nerantzis, E. Use of non-saccharomyces Torulaspora delbrueckii yeast strains in winemaking and brewing. J. Nat. Sci. Matica. Srpska. Novi. Sad. 2013, 124, 415–426. [Google Scholar] [CrossRef]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.; Proestos, C. Comparison of the Antioxidant and Antiradical Activity of Pomegranate (Punica granatum L.) by Ultrasound-Assisted and Classical Extraction. Anal. Lett. 2016, 49, 969–978. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Belkhir, M.; Akinocho, I.; Raboudi, F.; Pamies, D.; Barrajón, E.; Estevan, C.; Fattouch, S. Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Toufeili, I.; Itani, M.; MonaZeidan, M.; Yamani, O.A.; Kharroubi, S. Nutritional and Functional Potential of Carob Syrup Versus Date and Maple Syrup. Food Technol. Biotechnol. 2022, 60, 266–278. [Google Scholar] [CrossRef]
- Castro, L.F.; Affonso, A.D.; Lehman, R.M. Impact of Specialty Malts on Wort and Beer Characteristics. Fermentation 2021, 7, 137. [Google Scholar] [CrossRef]
- Silva, S.; Oliveira, A.I.; Cruz, A.; Oliveira, R.F.; Almeida, R.; Pinho, C. Physicochemical Properties and Antioxidant Activity of Portuguese Craft Beers and Raw Materials. Molecules 2022, 27, 8007. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Konings, W.N. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Bishop, L.R. European Brewery Convention Tests of The E.B.C. Colour Discs for Wort and Beer. J. Inst. Brew. 1966, 72, 443–451. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and pulsed ultrasound-assisted extraction of carob’s antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef]
- Siqueira, P.B.; Maria, H.; Bolini, A.; Macedo, G.A. Polyphenols and antioxidant properties in forced and naturally aged Brazilian beer. J. Brew. Distill. 2011, 2, 45–50. [Google Scholar]
- Yang, D.; Gao, X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Antioxidant Capacity of Hops. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 467–474. [Google Scholar]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Gasinski, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gasior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Yang, H.; Zhou, H. Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation. Fermentation 2022, 8, 400. [Google Scholar] [CrossRef]
- Lazzari, A.; Barbosa, H.D.; Machado Filho, E.R.; Maldonado da Silva, L.H.; Anjo, F.A.; Sato, F.; Lourenzi Franco Rosa, C.I.; Matumoto Pintro, P.T. Effect on Bioactive Compounds and Antioxidant Activity in the Brewing Process for Beers Using Rubim and Mastruz as Hop Replacements. J. Am. Soc. Brew. Chem. 2023, 81, 265–275. [Google Scholar] [CrossRef]
- He, G.; Du, J.; Zhang, K.; Wei, G.; Wang, W. Antioxidant capability and potableness of fresh cloudy wheat beer stored at different temperatures. J. Inst. Brew. 2012, 118, 386–392. [Google Scholar] [CrossRef]
- Ditrych, M.; Kordialik-Bogacka, E.; Czyżowska, A. Antiradical and reducing potential of commercial beers. Czech J. Food Sci. 2015, 33, 261–266. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Christou, A.; Martinez-Piernas, A.B.; Stavrou, I.J.; Garcia-Reyes, J.F.; Kapnissi-Christodoulou, C.P. HPLC-ESI-HRMS and chemometric analysis of carobs polyphenols–Technological and geographical parameters affecting their phenolic composition. J. Food Compos. Anal. 2022, 114, 104744. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phyther. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Son, J.; Jang, J.H.; Choi, I.H.; Lim, C.G.; Jeon, E.J.; Bae Bang, H.; Jeong, K.J. Production of trans-cinnamic acid by whole-cell bioconversion from l-phenylalanine in engineered Corynebacterium glutamicum. Microb. Cell Fact. 2021, 20, 1–20. [Google Scholar] [CrossRef]
- Qi, S.; Feng, Z.; Li, Q.; Qi, Z.; Zhang, Y. Myricitrin Modulates NADPH Oxidase-Dependent ROS Production to Inhibit Endotoxin-Mediated Inflammation by Blocking the JAK/STAT1 and NOX2/p47 phox Pathways. Oxid. Med. Cell Longev. 2017, 2017, 9738745. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S. An insight into novel therapeutic potentials of taxifolin. Front. Pharmacol. 2023, 14, 1173855. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Introduction to Natural Products Analysis. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–15. [Google Scholar]
- Wong, C.P.; Morita, H. 1.08 Bacterial Type III Polyketide Synthases. Comprehensive Natural Products III, 250. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef]
- Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical characterization and evaluation of biological activities of egyptian carob pods (Ceratonia siliqua L.) aqueous extract: In vitro study. Plants 2021, 10, 2626. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Tavares, C.S.; Roseiro, J.C.; Rauter, A.P. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013, 44, 119–126. [Google Scholar] [CrossRef]
- Tsatsaragkou, K.; Yiannopoulos, S.; Kontogiorgi, A.; Poulli, E.; Krokida, M.; Mandala, I. Effect of Carob Flour Addition on the Rheological Properties of Gluten-Free Breads. Food Bioprocess Technol. 2014, 7, 868–876. [Google Scholar] [CrossRef]

| (A) Ingredients | Type | # | %/IBU |
|---|---|---|---|
| Attica municipal water | Water | 1 | - |
| Pale Malt (2 Row) Vergina (5.5 EBC) | Grain | 2 | 75.0% |
| Vienna Malt (Weyermann) (9.0 EBC) | Grain | 3 | 6.7% |
| Caramunich II (Weyermann) (110.0 EBC) | Grain | 4 | 5.0% |
| Chocolate Malt (Simpsons) (1000.0 EBC) | Grain | 5 | 4.7% |
| Special W (Weyermann) (300.0 EBC) | Grain | 6 | 3.3% |
| Carafa Special I (Weyermann) (1000.0 EBC) | Grain | 7 | 2.8% |
| Wheat Malt, Pale (Weyermann) (3.9 EBC) | Grain | 8 | 2.4% |
| Northern Brewer [10.00%]—Boil 60.0 min | Hop | 9 | 48.4 IBUs |
| Irish Moss (Boil 10.0 min) | Fining | 10 | - |
| Cascade [7.80%]—Steep/Whirlpool 5.0 min | Hop | 11 | 3.4 IBUs |
| New World Strong Ale (Mangrove Jack’s #M42) | Yeast | 12 | - |
| (B) Mash Steps | |||
| Name | Description | Step Temperature | Step Time |
| Mash In | Add water at 69 °C | 65.0 °C | 60 min |
| Mash Step | heat to 72.0 °C over 8 min | 72.0 °C | 10 min |
| Mash Out | Heat to 75.6 °C | 75.6 °C | 10 min |
| Sample | pH | Brix |
|---|---|---|
| Carob syrup A (Uniwa) | 5.08 | 67.80 |
| Carob syrup B (Uniwa) | 4.91 | 64.30 |
| Carob syrup C (Uniwa) | 5.08 | 71.35 |
| Carob syrup D (Crete) | 5.41 | 74.43 |
| Commercial Carob syrup | 5.31 | 72.61 |
| t = 0 Days | 1st Fermentation (t = 30 Days) | 2nd Fermentation (t = 60 Days) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B: Dark Ale Beer (6% ABV) | CB1: Strong Dark Ale Carob Beer (8% ABV) | CB2: Strong Dark Ale Carob Beer (10% ABV) | B: Dark Ale Beer (6% ABV) | CB1: Strong Dark Ale Carob Beer (8% ABV) | CB2: Strong Dark Ale Carob Beer (10% ABV) | B: Dark Ale Beer (6% ABV) | CB1: Strong Dark Ale Carob Beer (8% ABV) | CB2: Strong Dark Ale Carob Beer (10% ABV) | |
| pH | 5.24 | 5.27 | 5.26 | 4.56 | 4.93 | 5.16 | 4.43 | 4.79 | 5.07 |
| °plato | 13.8 | 18.8 | 23.8 | 3.8 | 4.8 | 6.3 | 3.1 | 4.3 | 6.5 |
| Alcohol (% v/v) | 0% | 0% | 0% | 5.42 | 7.97 | 10.31 | 5.57 | 7.97 | 9.97 |
| Degree of fermentation % | n/a | n/a | n/a | 72.46 | 74.46 | 73.52 | |||
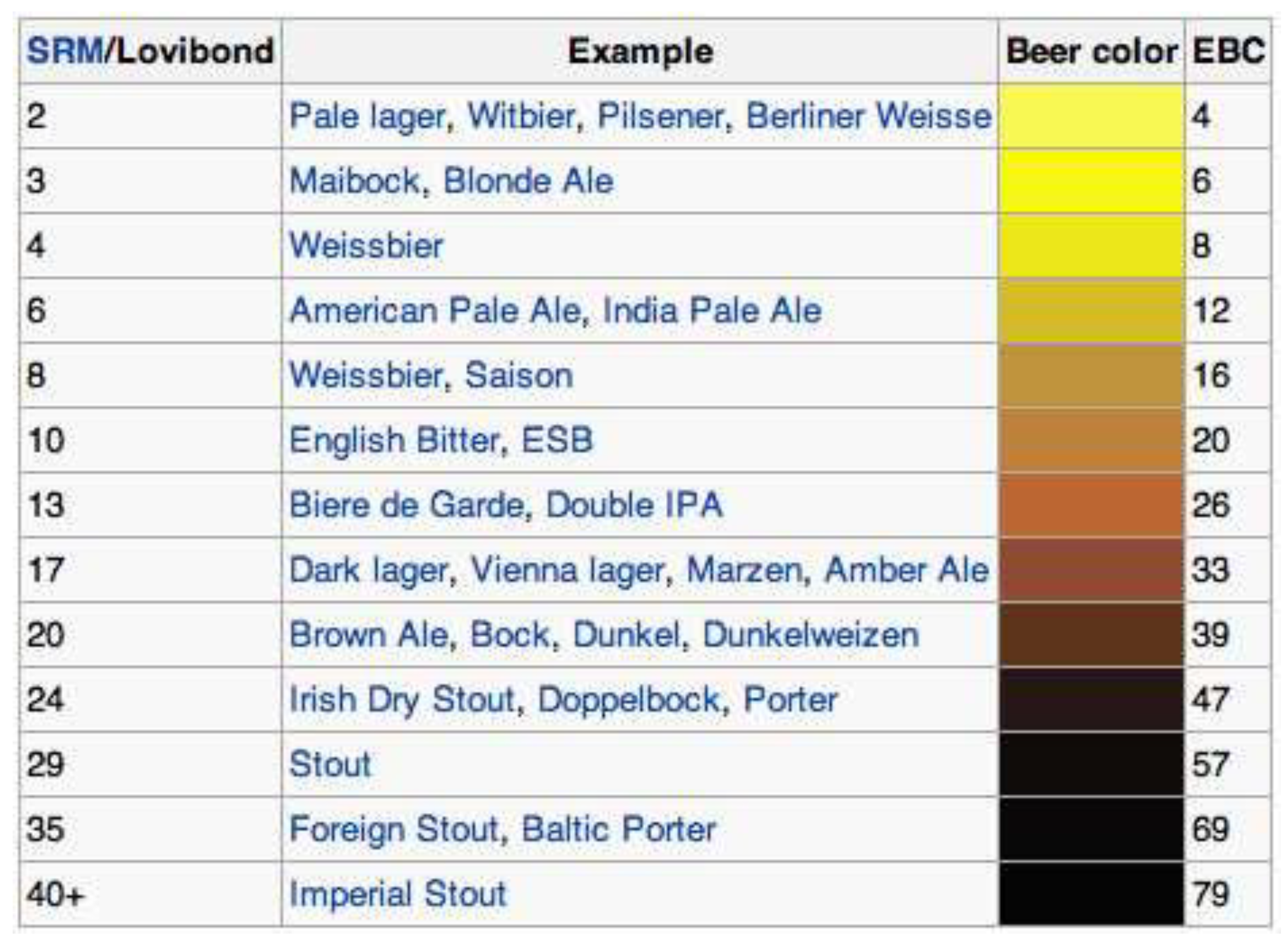
| EBC | 127.69 | 133.87 | 92.75 | 119.35 | 129.94 | 120.13 | 88.75 | 95.25 | 101.69 |
| SRM | 64.86 | 68.01 | 47.12 | 60.63 | 66.01 | 61.02 | 45.08 | 48.39 | 51.66 |
| Compound | Sample | 1 tR (min) | Molecular Formula | Theoretical m/z [M-H]− | Experimental m/z [M-H]− | Mass Error |
|---|---|---|---|---|---|---|
| Phloroglucinol | 2 CB1, 3 CB2, 4 C | 1.62 | C6H6O3 | 125.0244 | 125.0243 | 0.94 |
| Gallic acid | CB1, CB2, C | 1.64 | C7H6O5 | 169.0142 | 169.0137 | 3.24 |
| Protocatechuic acid | 5 Β, CB1, CB2, C | 2.39 | C7H6O4 | 153.0193 | 153.0198 | −3.05 |
| Gentisic acid | CΒ1, CΒ2, C | 3.26 | C7H6O4 | 153.0193 | 153.0188 | 3.50 |
| (-)-catechin | Β, CΒ1, CΒ2, C | 3.47 | C15H14O6 | 289.0718 | 289.0707 | 3.67 |
| 4-hydroxybenzoic acid | Β, CΒ1, CΒ2, C | 3.54 | C7H6O3 | 137.0244 | 137.0240 | 3.05 |
| Vanillic acid | Β, CΒ1, CΒ2, C | 4.08 | C8H8O4 | 167.0350 | 167.0350 | 0.00 |
| Epicatechin | Β, CΒ1, CΒ2, C | 4.32 | C15H14O6 | 289.0718 | 289.0714 | 1.25 |
| Syringic acid | Β, CΒ1, CΒ2, C | 4.39 | C9H10O5 | 197.0455 | 197.0457 | −0.77 |
| p-coumaric acid | Β, CΒ1, CΒ2, C | 5.54 | C9H8O3 | 163.0400 | 163.0392 | 4.97 |
| Ferulic acid | Β, CΒ1, CΒ2, C | 6.31 | C10H10O4 | 193.0506 | 193.0510 | −1.92 |
| Myricitrin | CΒ1, CΒ2, C | 6.48 | C21H20O12 | 463.0882 | 463.0863 | 4.10 |
| Taxifolin | CΒ1, CΒ2, C | 6.57 | C15H12O7 | 303.0510 | 303.0515 | −1.65 |
| Quercetin-3-glucoside | Β, CΒ1, CΒ2, C | 6.74 | C21H20O12 | 463.0882 | 463.0867 | 3.24 |
| Absisic acid | CΒ1, CΒ2, C | 9.05 | C15H20O4 | 263.1289 | 263.1294 | −1.81 |
| trans-cinnamic acid | CΒ1, CΒ2, C | 9.79 | C9H8O2 | 147.0451 | 147.0449 | 1.36 |
| Quercetin | CΒ1, CΒ2, C | 10.25 | C15H10O7 | 301.0354 | 301.0346 | 2.66 |
| Luteolin | CΒ1, CΒ2, C | 10.33 | C15H10O6 | 285.0405 | 285.0397 | 2.67 |
| Naringenin | CΒ1, CΒ2, C | 11.44 | C15H12O5 | 271.0612 | 271.0613 | −0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrovolou, K.; Tataridis, P.; Revelou, P.-K.; Strati, I.F.; Konteles, S.J.; Tarantilis, P.A.; Houhoula, D.; Batrinou, A. Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile. Appl. Sci. 2024, 14, 1199. https://doi.org/10.3390/app14031199
Pyrovolou K, Tataridis P, Revelou P-K, Strati IF, Konteles SJ, Tarantilis PA, Houhoula D, Batrinou A. Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile. Applied Sciences. 2024; 14(3):1199. https://doi.org/10.3390/app14031199
Chicago/Turabian StylePyrovolou, Katerina, Panagiotis Tataridis, Panagiota-Kyriaki Revelou, Irini F. Strati, Spyros J. Konteles, Petros A. Tarantilis, Dimitra Houhoula, and Anthimia Batrinou. 2024. "Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile" Applied Sciences 14, no. 3: 1199. https://doi.org/10.3390/app14031199
APA StylePyrovolou, K., Tataridis, P., Revelou, P.-K., Strati, I. F., Konteles, S. J., Tarantilis, P. A., Houhoula, D., & Batrinou, A. (2024). Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile. Applied Sciences, 14(3), 1199. https://doi.org/10.3390/app14031199








