Somatosensory Mismatch Response in Patients with Cerebral Palsy
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Procedure and Data Analysis
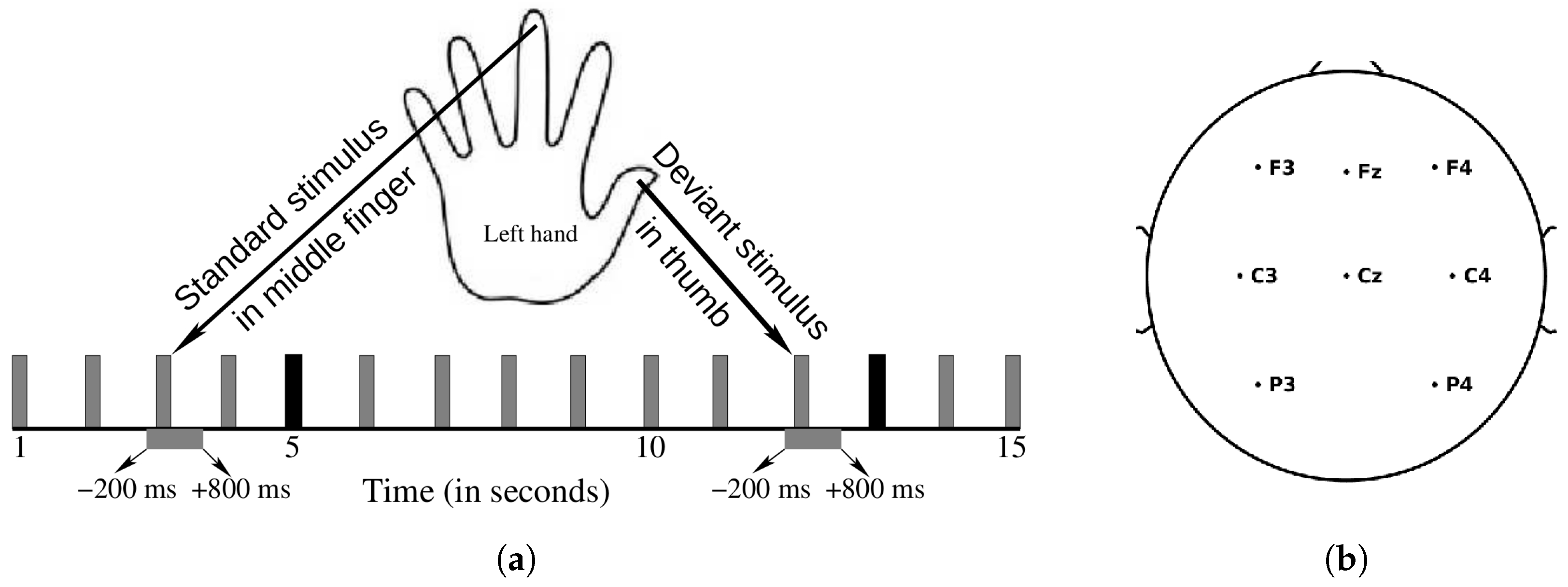
2.2.1. Stimuli and Procedure
2.2.2. EEG Acquisition
2.2.3. Data Processing
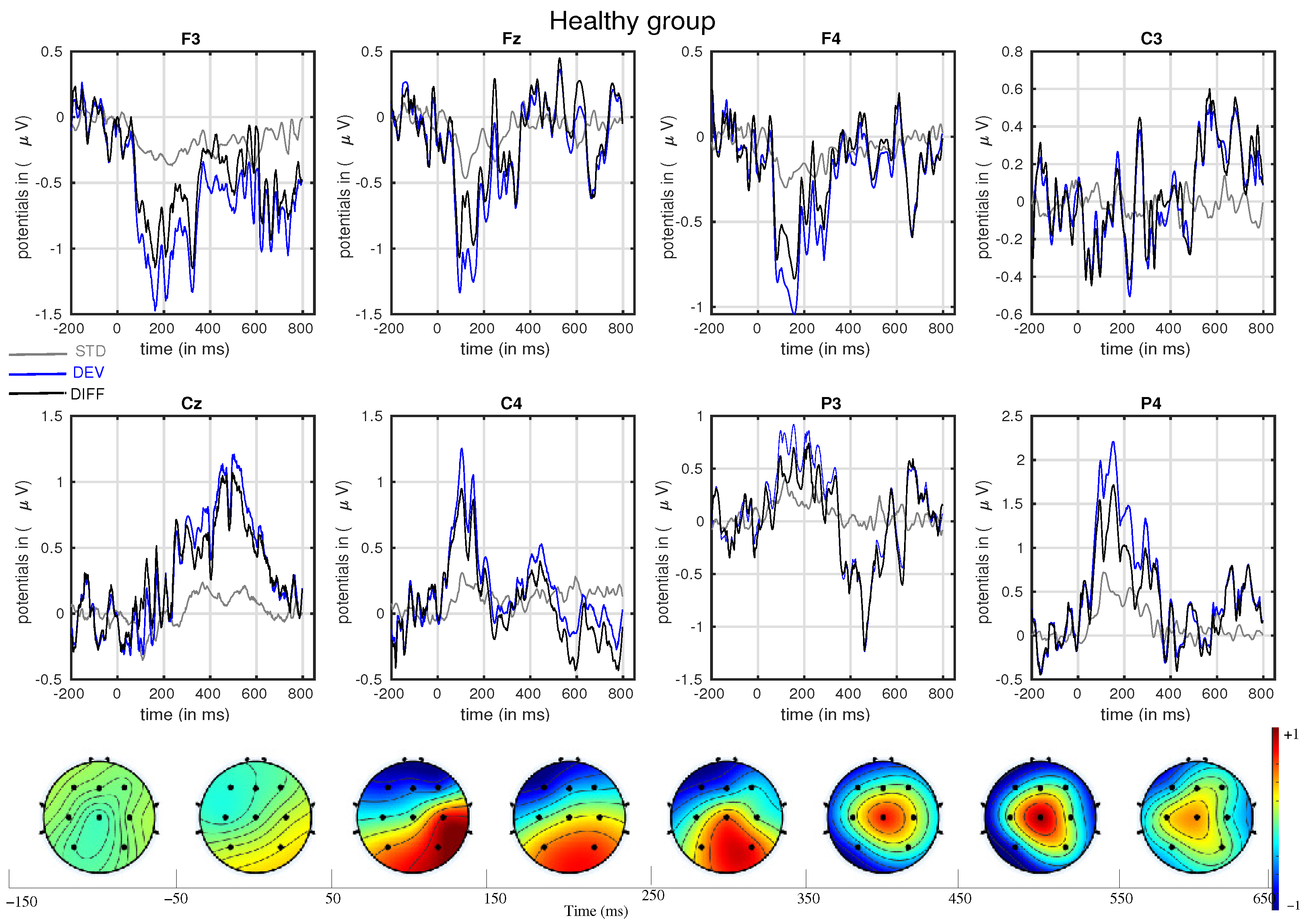
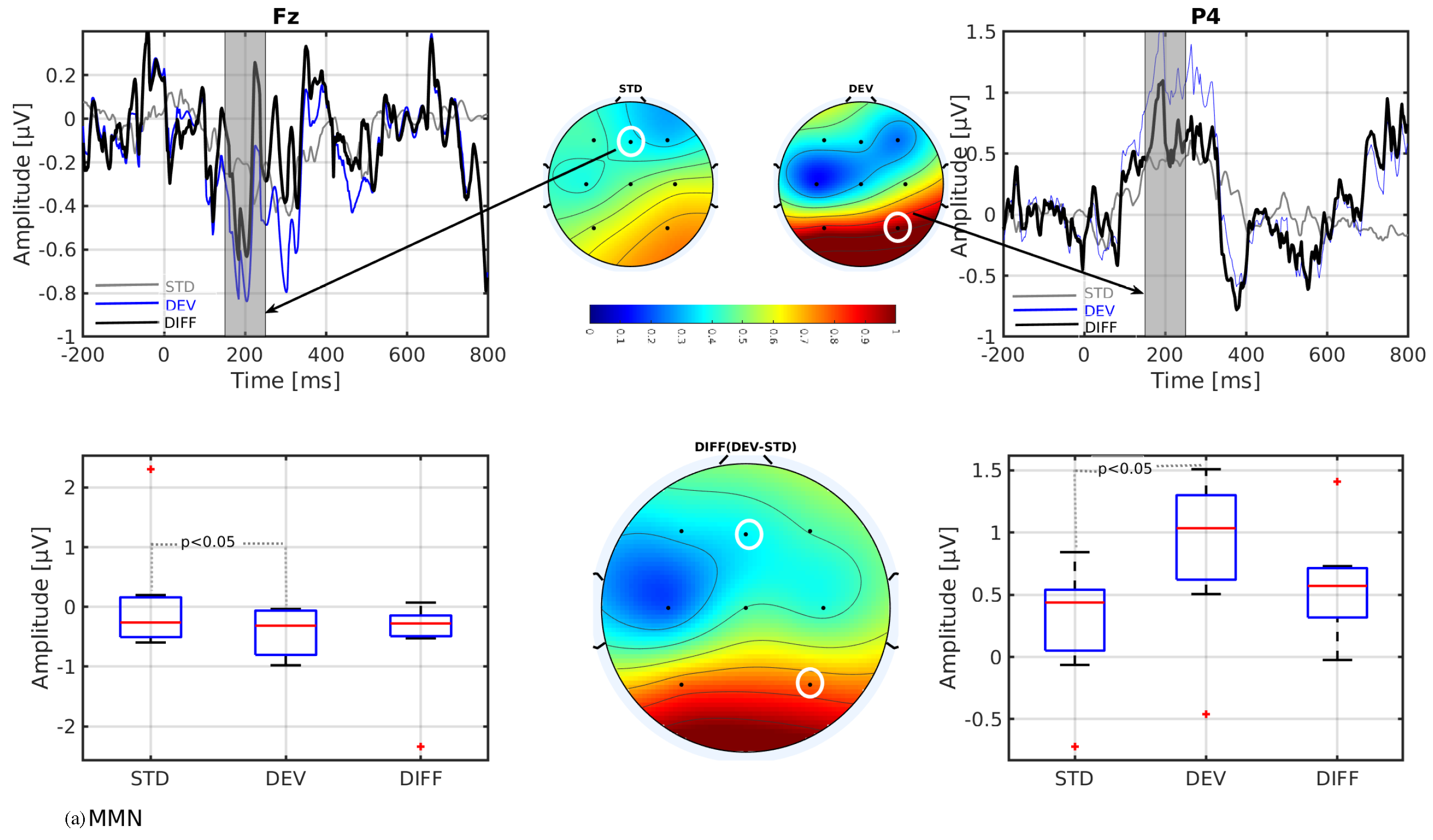
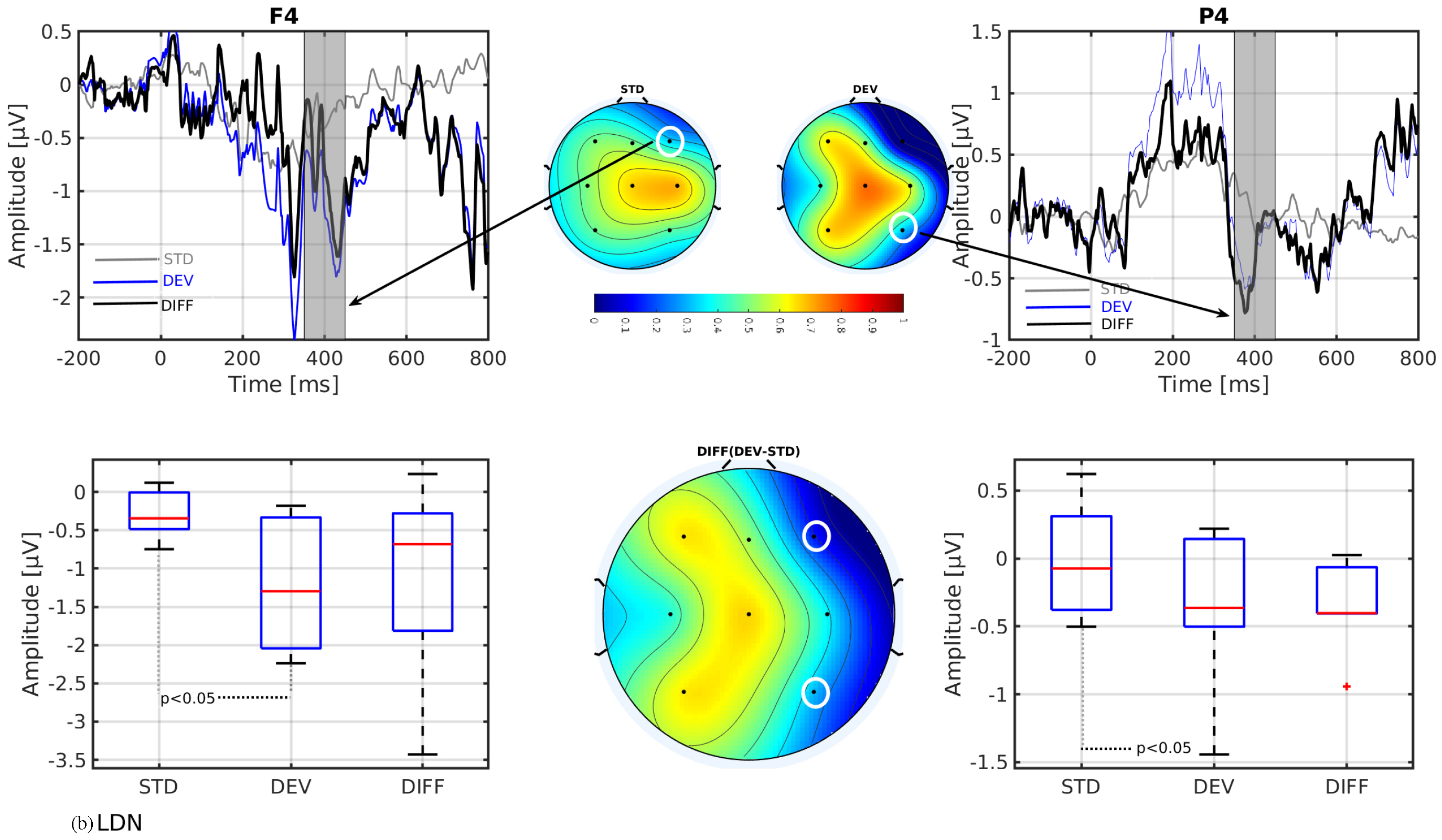
3. Results
4. Discussion
- The mismatch-related somatosensory responses can be observed in patients with cerebral palsy.
- In line with our assumption, the observed mismatch-related ERP components from frontal and parietal channels were statistically significant at two predefined latency ranges: and channels at 150–250 ms and and channels at 350–450 ms after the stimulus onset. The observed response in the time range of 150–250 ms is considered as sMMN, and the response in the time range of 350–450 ms is considered as LDN. In terms of time window and channel location, these findings are qualitatively in good agreement with the studies involving healthy adults [21,25].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Näätänen, R.; Jacobsen, T.; Winkler, I. Memory-based or afferent processes in mismatch negativity (MMN): A review of the evidence. Psychophysiology 2005, 42, 25–32. [Google Scholar] [CrossRef]
- Schröger, E. Measurement and interpretation of the mismatch negativity. Behav. Res. Methods Instrum. Comput. 1998, 30, 131–145. [Google Scholar] [CrossRef]
- Näätänen, R.; Gaillard, A.; Mäntysalo, S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978, 42, 313–329. [Google Scholar] [CrossRef]
- Tales, A.; Troscianko, T.; Wilcock, G.K.; Newton, P.; Butler, S.R. Agerelated changes in the preattentional detection of visual change. Neuroreport 2002, 13, 969–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorenzo-Lopez, L.; Amenedo, E.; Pazo-Alvarez, P.; Cadaveira, F. Preattentive detection of motion direction changes in normal aging. Neuro Rep. 2004, 15, 2633–2636. [Google Scholar] [CrossRef]
- Strömmer, J.M.; Tarkka, I.M.; Astikainen, P. Somatosensory mismatch response in young and elderly adults. Front. Aging Neurosci. 2014, 6, 293. [Google Scholar] [CrossRef]
- Akatsuka, K.; Wasaka, T.; Nakata, H.; Inui, K.; Hoshiyama, M.; Kakigi, R. Mismatch responses related to temporal discrimination of somatosensory stimulation. Clin. Neurophysiol. 2005, 116, 1930–1937. [Google Scholar] [CrossRef]
- Astikainen, P.; Ruusuvirta, T.; Korhonen, T. Somatosensory event-related potentials in the rabbit cerebral and cerebellar cortices: A correspondence with mismatch responses in humans. Neurosci. Lett. 2001, 298, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Czigler, I.; Balázs, L.; Winkler, I. Memory-based detection of task-irrelevant visual changes. Psychophysiology 2002, 39, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Sun, G.; Gao, L.; Zhao, L. Visual mismatch negativity elicited by facial expressions: New evidence from the equiprobable paradigm. Behav. Brain Funct. 2012, 8, 7. [Google Scholar] [CrossRef]
- Kekoni, J.; Hämäläinen, H.; Saarinen, M.; Gröhn, J.; Reinikainen, K.; Lehtokoski, A.; Näätänen, R. Rate effect and mismatch responses in the somatosensory system: ERP-recordings in humans. Biol. Psychol. 1997, 46, 125–142. [Google Scholar] [CrossRef]
- Spackman, L.; Boyd, S.; Towell, A. Effects of stimulus frequency and duration on the somatosensory mismatch negativity. Clin. Neurophysiol. 2007, 118, e175. [Google Scholar] [CrossRef]
- Novitski, N.; Tervaniemi, M.; Huotilainen, M.; Näätänen, R. Frequency discrimination at different frequency levels as indexed by electrophysiological and behavioral measures. Cogn. Brain Res. 2004, 20, 26–36. [Google Scholar] [CrossRef]
- Shafer, V.; Yu, Y.; Garrido-Nag, K. Neural mismatch indices of vowel discrimination in monolingually and bilingually exposed infants: Does attention matter? Neurosci. Lett. 2012, 526, 10–14. [Google Scholar] [CrossRef][Green Version]
- Rinker, T.; Kohls, G.; Richter, C.; Maas, V.; Schulz, E.; Schecker, M. Abnormal frequency discrimination in children with SLI as indexed by mismatch negativity (MMN). Neurosci. Lett. 2007, 413, 99–104. [Google Scholar] [CrossRef]
- Stothart, G.; Tales, A.; Kazanina, N. Evoked potentials reveal age-related compensatory mechanisms in early visual processing. Neurobiol. Aging 2013, 34, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Tales, A.; Haworth, J.; Wilcock, G.; Newton, P.; Butler, S. Visual mismatch negativity highlights abnormal pre-attentive visual processing in mild cognitive impairment and Alzheimer’s disease. Neuropsychologia 2008, 46, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.; Yücel, M.; Yap, M.B.; Allen, N.B. Sex differences in the neural correlates of emotion: Evidence from neuroimaging. Biol. Psychol. 2011, 87, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Chen, L.; Sun, H.; Qiao, Z.; Qiu, X.; Zhang, C.; Wang, L.; Zhu, X.; He, J.; et al. Gender differences in pre-attentive change detection for visual but not auditory stimuli. Clin. Neurophysiol. 2016, 127, 431–441. [Google Scholar] [CrossRef]
- Näätänen, R. Somatosensory mismatch negativity: A new clinical tool for developmental neurological research? Dev. Med. Child Neurol. 2009, 51, 930–931. [Google Scholar] [CrossRef]
- Shen, G.; Weiss, S.M.; Meltzoff, A.N.; Marshall, P.J. The somatosensory mismatch negativity as a window into body representations in infancy. Int. J. Psychophysiol. 2018, 134, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, N.; Yabe, H.; Sutoh, T.; Hiruma, T.; Kaneko, S. Somatosensory automatic responses to deviant stimuli. Brain Res. Cogn. Brain Res. 1998, 7, 165–171. [Google Scholar] [CrossRef]
- Tamura, Y.; Hoshiyama, M.; Inui, K.; Nakata, H.; Wasaka, T.; Ojima, S.; Inoue, K.; Kakigi, R. Cognitive processes in two-point discrimination: An ERP study. Clin. Neurophysiol. 2004, 115, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Korpilahti, P.; Martynova, O.; Lang, A.H. Mismatch Negativity and Late Discriminative Negativity in Investigating Speech Perception and Learning in Children and Infants. Audiol. Neurotol. 2001, 6, 2–11. Available online: https://karger.com/aud/article-pdf/6/1/2/2249646/000046804.pdf (accessed on 26 October 2023). [CrossRef] [PubMed]
- Petermann, M.; Kummer, P.; Burger, M.; Lohscheller, J.; Eysholdt, U.; Döllinger, M. Statistical detection and analysis of mismatch negativity derived by a multi-deviant design from normal hearing children. Hear. Res. 2009, 247, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Hardiman, M.J.; Barry, J.G. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev. Sci. 2011, 14, 402–416. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1467-7687.2010.00990.x (accessed on 27 October 2023). [CrossRef] [PubMed]
- Čeponienė, R.; Lepistö, T.; Soininen, M.; Aronen, E.; Alku, P.; Näätänen, R. Event-related potentials associated with sound discrimination versus novelty detection in children. Psychophysiology 2004, 41, 130–141. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1469-8986.2003.00138.x (accessed on 26 October 2023). [CrossRef]
- Schulte-Körne, G.; Deimel, W.; Bartling, J.; Remschmidt, H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN). Int. J. Psychophysiol. 2001, 40, 77–87. [Google Scholar] [CrossRef]
- Kropotov, J.D. Chapter 18—Schizophrenia; Academic Press: San Diego, CA, USA, 2009; pp. 420–431. [Google Scholar] [CrossRef]
- Rüsseler, J.; Münte, T. Kognitive Potenziale (ereigniskorrelierte Potenziale, EKP). Evozierte Potenziale, Neurovegetative Diagnostik, Okulographie; Buchner, H., Noth, J., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2005; Chapter 7; pp. 80–94. [Google Scholar] [CrossRef]
- Kurz, M.J.; Heinrichs-Graham, E.; Arpin, D.J.; Becker, K.M.; Wilson, T.W. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J. Neurophysiol. 2014, 111, 573–579. [Google Scholar] [CrossRef]
- Hoon, A.H., Jr.; Stashinko, E.E.; Nagae, L.M.; Lin, D.D.M.; Keller, J.; Bastian, A.; Campbell, M.L.; Levey, E.; Mori, S.; Johnston, M.V. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev. Med. Child Neurol. 2009, 51, 697–704. [Google Scholar] [CrossRef]
- Matusz, P.J.; Key, A.P.; Gogliotti, S.; Pearson, J.; Auld, M.L.; Murray, M.M.; Maitre, N.L. Somatosensory Plasticity in Pediatric Cerebral Palsy following Constraint-Induced Movement Therapy. Neural Plast. 2018, 2018, 1891978. [Google Scholar] [CrossRef]
- Restuccia, D.; Della Marca, G.; Valeriani, M.; Leggio, M.G.; Molinari, M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain 2007, 130 Pt 1, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, E.; Pirilä, S.; Kaartinen, J.; Meere, J. Stimulus evaluation, event preparation, and motor action planning in young patients with mild spastic cerebral palsy: An event-related brain potential study. J. Child Neurol. 2012, 27, 465–470. [Google Scholar] [CrossRef]
- Lackner, C.; Gorter, J.; Segalowitz, S. Cognitive Event-Related Potentials in Young Adults With Cerebral Palsy: A Proof-of-Concept Study. Clin. EEG Neurosci. 2024, 55, 64–75. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, G.; Dunne, S.; Farres, E.; Marco-Pallares, J.; Ray, C.; Mendoza, E.; Silva, R.; Grau, C. A dry electrophysiology electrode using CNT arrays. Sens. Actuators A Phys. 2006, 132, 34–41. [Google Scholar] [CrossRef]
- Garrido, M.; Kilner, J.; Stephan, K.; Friston, K. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef]
- Butler, J.; Molholm, S.; Fiebelkorn, I.; Mercier, M.; Schwartz, T.; Foxe, J. Common or redundant neural circuits for duration processing across audition and touch. J. Neurosci. 2011, 31, 3400–3406. [Google Scholar] [CrossRef]
- Giard, M.; Perrin, F.; Pernier, J.; Bouchet, P. Brain generators implicated in the processing of auditory stimulus deviance: A topographic event-related potential study. Psychophysiology 1990, 27, 627–640. [Google Scholar] [CrossRef]
- Akatsuka, K.; Wasaka, T.; Nakata, H.; Kida, T.; Kakigi, R. The effect of stimulus probability on the somatosensory mismatch field. Exp. Brain Res. 2007, 181, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Jung, T.; Bell, A.; Gharemani, D.; Sejnowski, T. Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. USA 1997, 94, 10979–10984. [Google Scholar] [CrossRef] [PubMed]
- Xide, Y.; Tao, L.; Dingguo, G. The Mismatch Negativity: An Indicator of Perception of Regularities in Music. Behav. Neurol. 2015, 2015, 469508. [Google Scholar] [CrossRef]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef] [PubMed]

| Participant | Gender | Age | GMFCS | MACS | MACS | Diagnosis |
|---|---|---|---|---|---|---|
| Year | L | R | ||||
| P01 | Female | 23 | I | 4 | 1 | Unilateral CP |
| P02 | Male | 45 | III | 1 | 1 | Bilateral CP |
| P03 | Male | 39 | III | 3 | 3 | Bilateral CP |
| P04 | Male | 36 | IV | 4 | 4 | Bilateral CP |
| P05 | Female | 53 | IV | 2 | 2 | Ataxic CP |
| P06 | Female | 47 | II | 2 | 4 | Unilateral spastic CP |
| P07 | Male | 25 | III | 2 | 2 | Bilateral spastic CP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Ehrlich, S.K.; Lampe, R. Somatosensory Mismatch Response in Patients with Cerebral Palsy. Appl. Sci. 2024, 14, 1030. https://doi.org/10.3390/app14031030
Roy S, Ehrlich SK, Lampe R. Somatosensory Mismatch Response in Patients with Cerebral Palsy. Applied Sciences. 2024; 14(3):1030. https://doi.org/10.3390/app14031030
Chicago/Turabian StyleRoy, Susmita, Stefan K. Ehrlich, and Renée Lampe. 2024. "Somatosensory Mismatch Response in Patients with Cerebral Palsy" Applied Sciences 14, no. 3: 1030. https://doi.org/10.3390/app14031030
APA StyleRoy, S., Ehrlich, S. K., & Lampe, R. (2024). Somatosensory Mismatch Response in Patients with Cerebral Palsy. Applied Sciences, 14(3), 1030. https://doi.org/10.3390/app14031030






