Preferred Chemical Agent for Electrochemical Modification of Physical and Mechanical Parameters of Mudstone
Abstract
1. Introduction
2. Research Program and Methodology
2.1. Theoretical Basis for Electrochemical Modification of Mudstones
2.2. Mechanism of Action of Electrochemically Modified Mudstone
2.2.1. Electro-Osmotic Drainage Consolidation
2.2.2. Chemical Reaction Cementation
2.3. Preferred Solution for Electrochemical Modification of Mudstone Agents
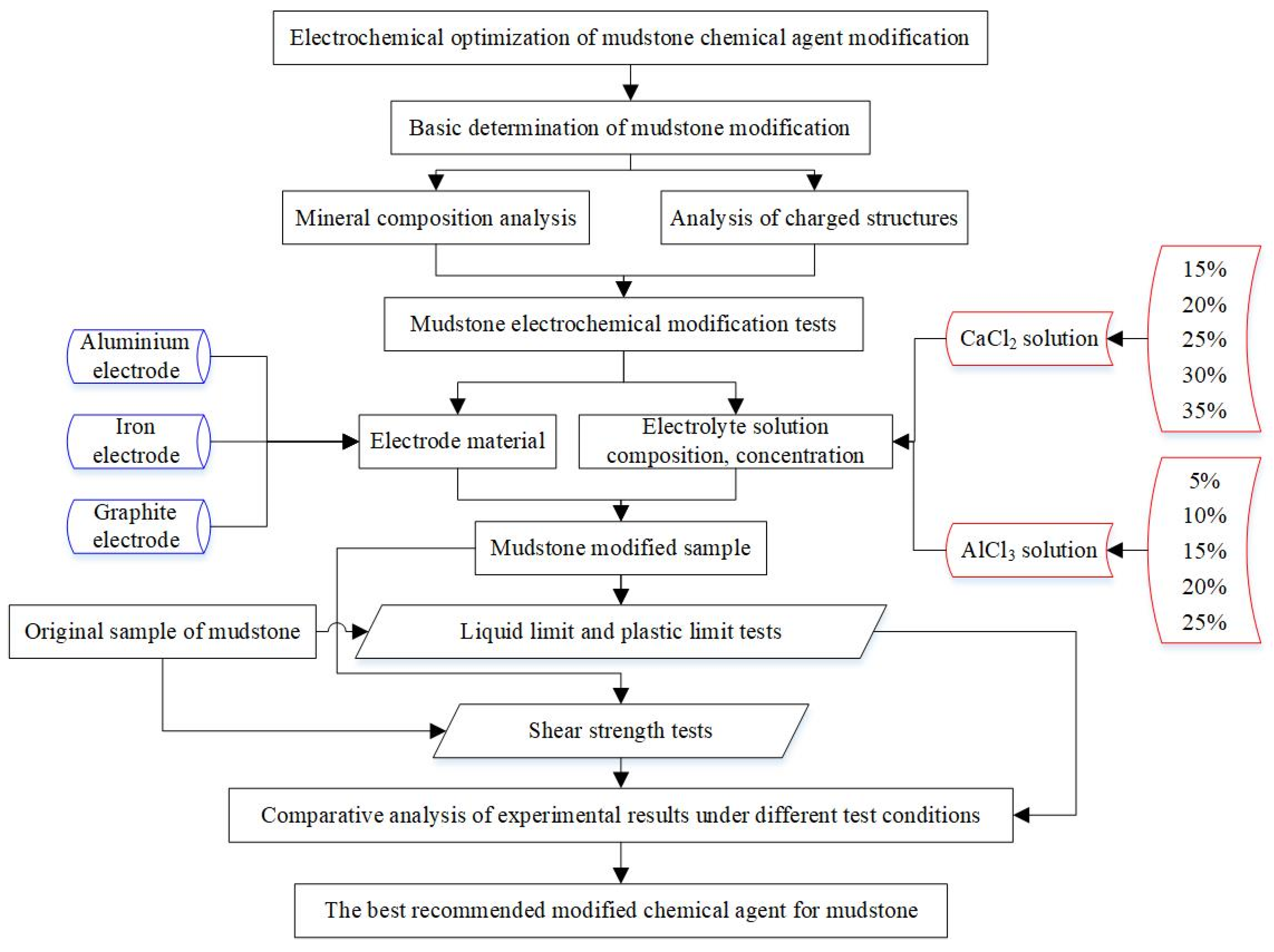
3. Mudstone Electrochemical Modification Tests Program
3.1. Mudstone Samples Characteristics
3.2. Test Condition Setting
3.3. Test Setup and Procedure
3.4. Determination of the Electrolyte Solution Amount
- (1)
- Begin with a given initial volume of electrolytes for testing. When the current stabilizes at zero and no longer changes, remove the sample, weigh it to determine the decrease in mass, and then conduct a direct shear test on the sample.
- (2)
- Replenish the electrolyte solution with the same mass as the initial mass reduction in the specimen. Inject the electrolyte into the specimen that has completed the initial modification 4–5 times, allowing it to stand so that the electrolyte is uniformly distributed. Conduct the electrochemical modification again until the current stabilizes at zero. To determine the mass reduction value, remove the secondary modified specimen and weigh it. Maintain the same vertical direction as the initial test specimen and conduct a direct shear test on the secondary specimen.
- (3)
- Repeat the above process until the modification experiment no longer changes the shear strength of the mudstone. The total amount of electrolyte consumed in this repeated process is the volume required for a mudstone sample to achieve complete modification.
4. Results
4.1. Comparison of Mudstone Liquid/Plastic Limit Modification
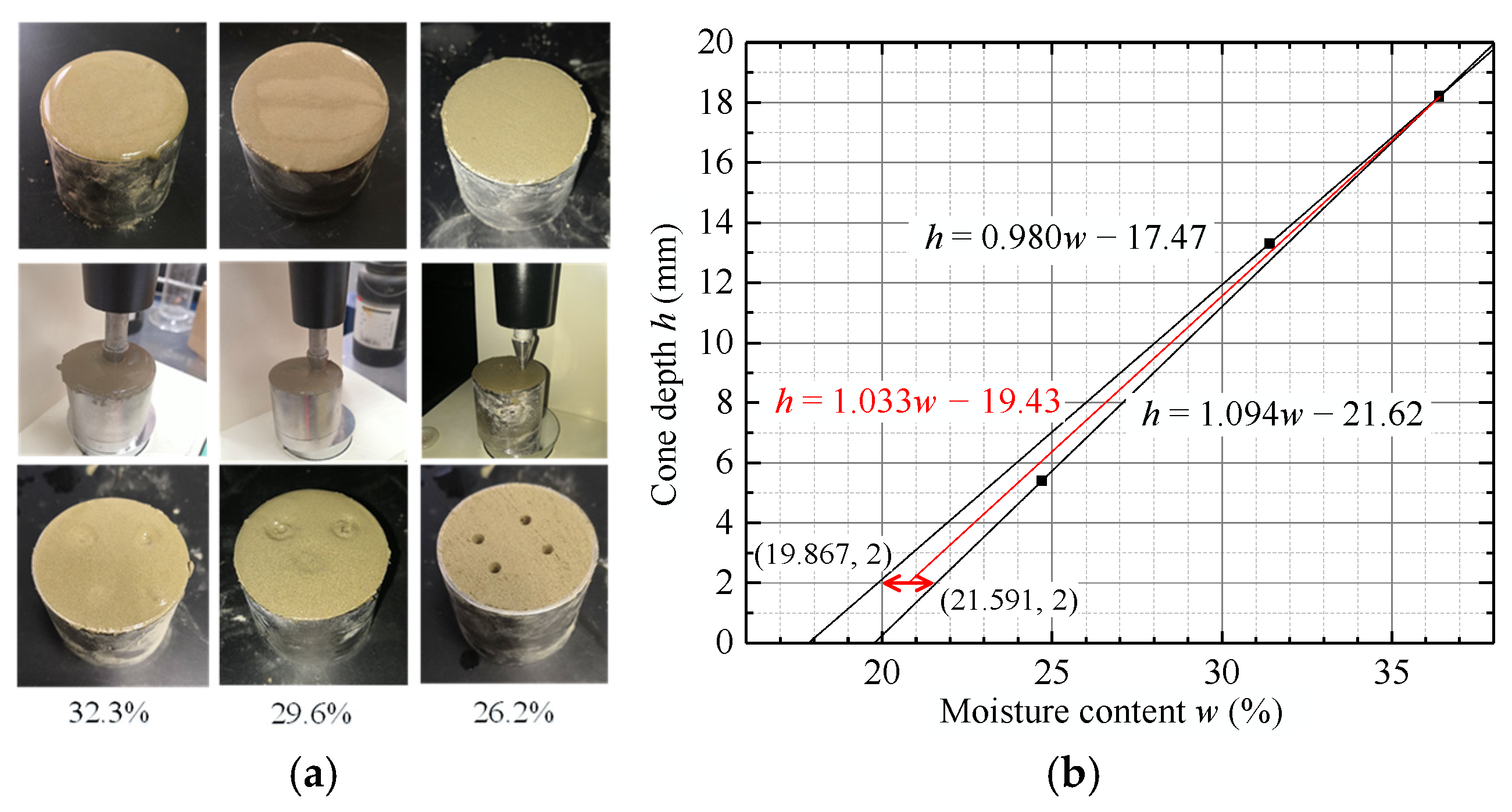
4.1.1. Determination of the In Situ Mudstone Liquid/Plastic Limit
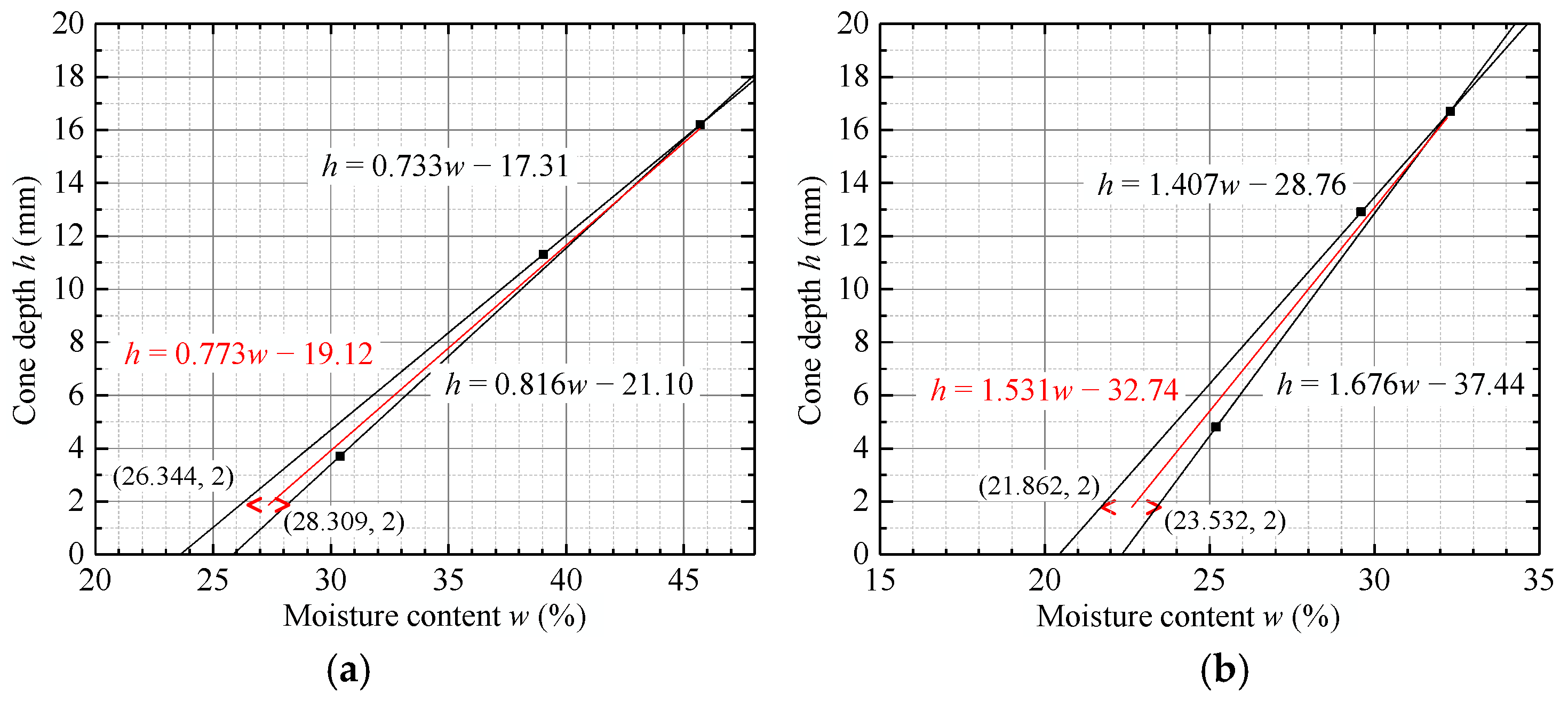
4.1.2. Changes in the Liquid/Plastic Limit of the Modified Mudstone
4.2. Modification Analysis of the Mudstone Shear Strength
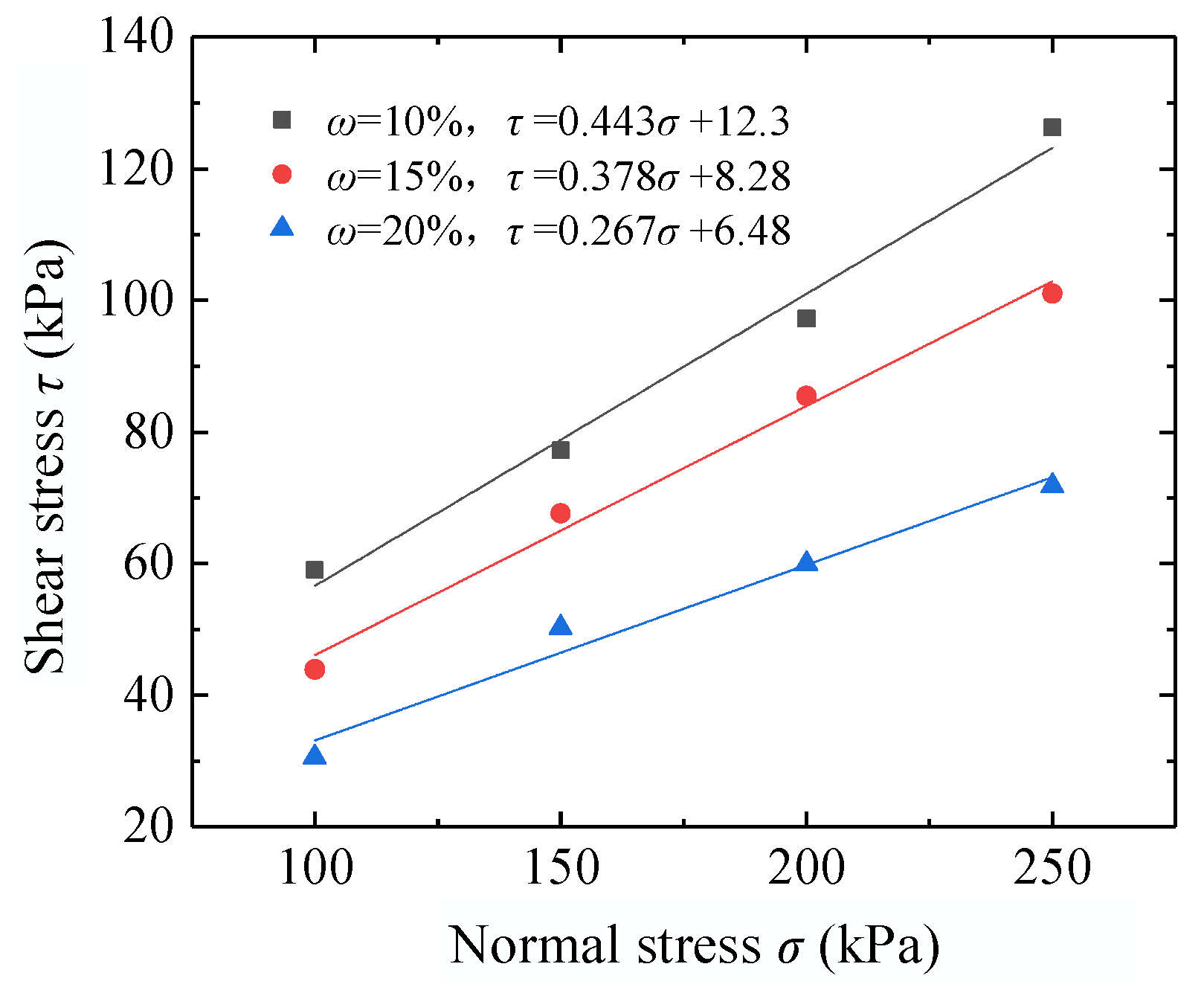
4.2.1. Determination of In Situ Mudstone Shear Strength
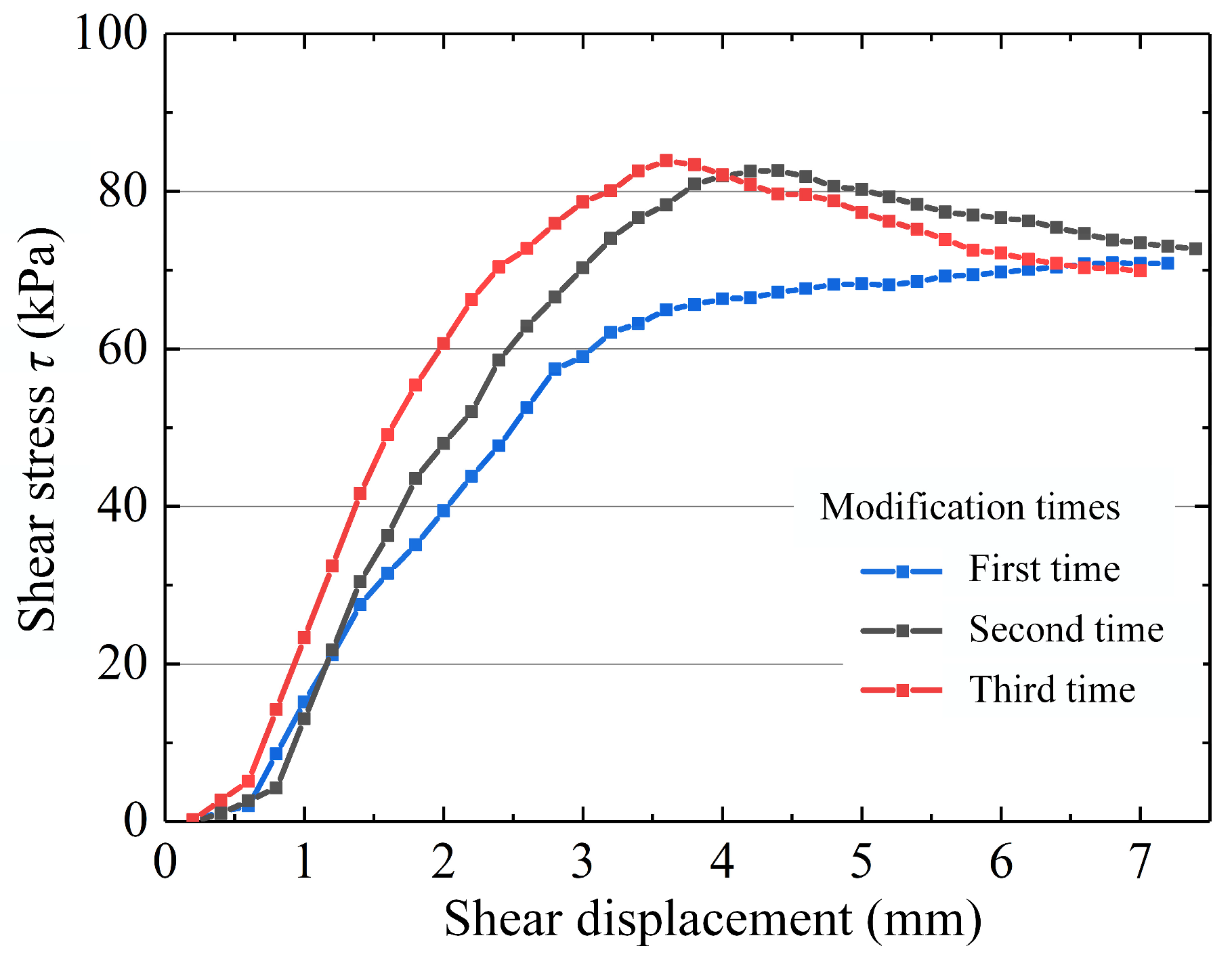
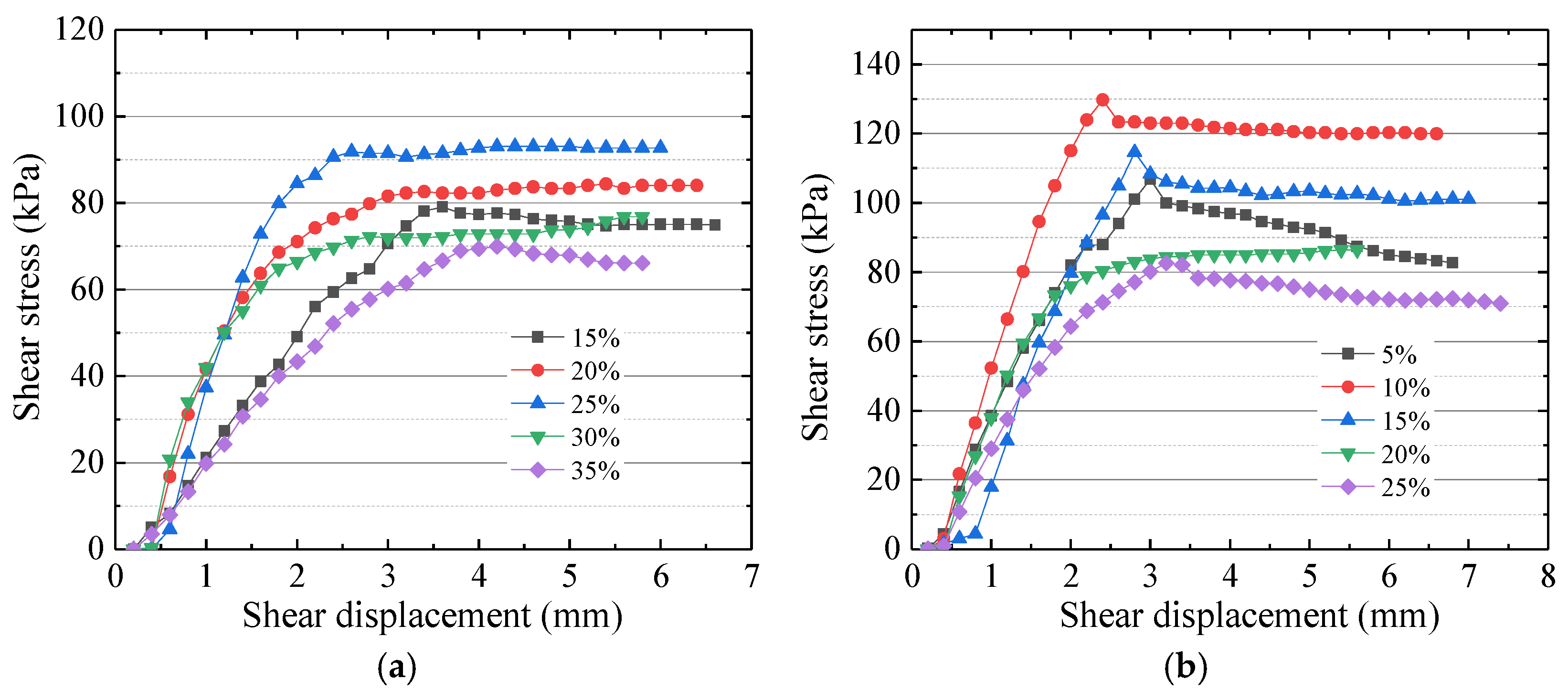
4.2.2. Variation in the Shear Strength of Modified Mudstone
5. Discussion
5.1. Electrolyte Solution Optimization
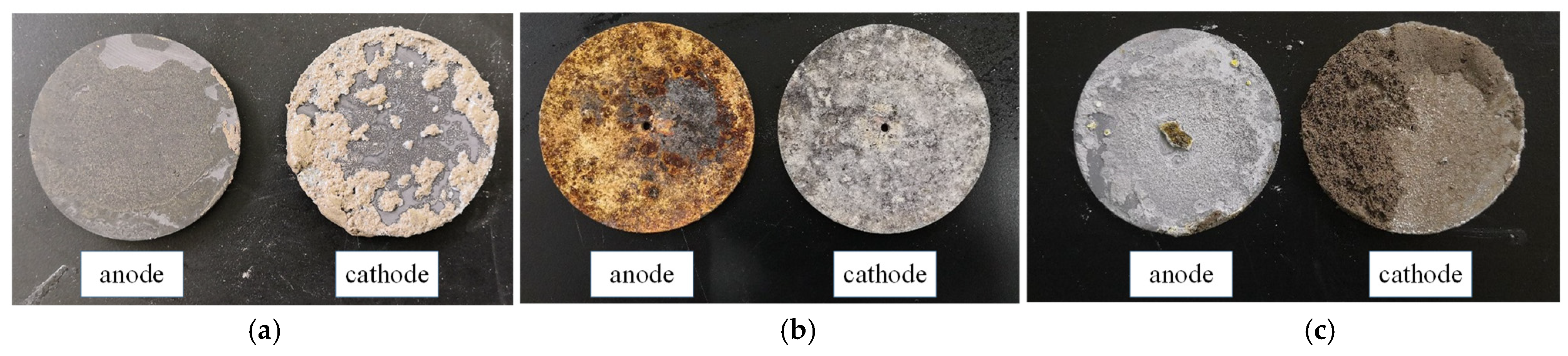
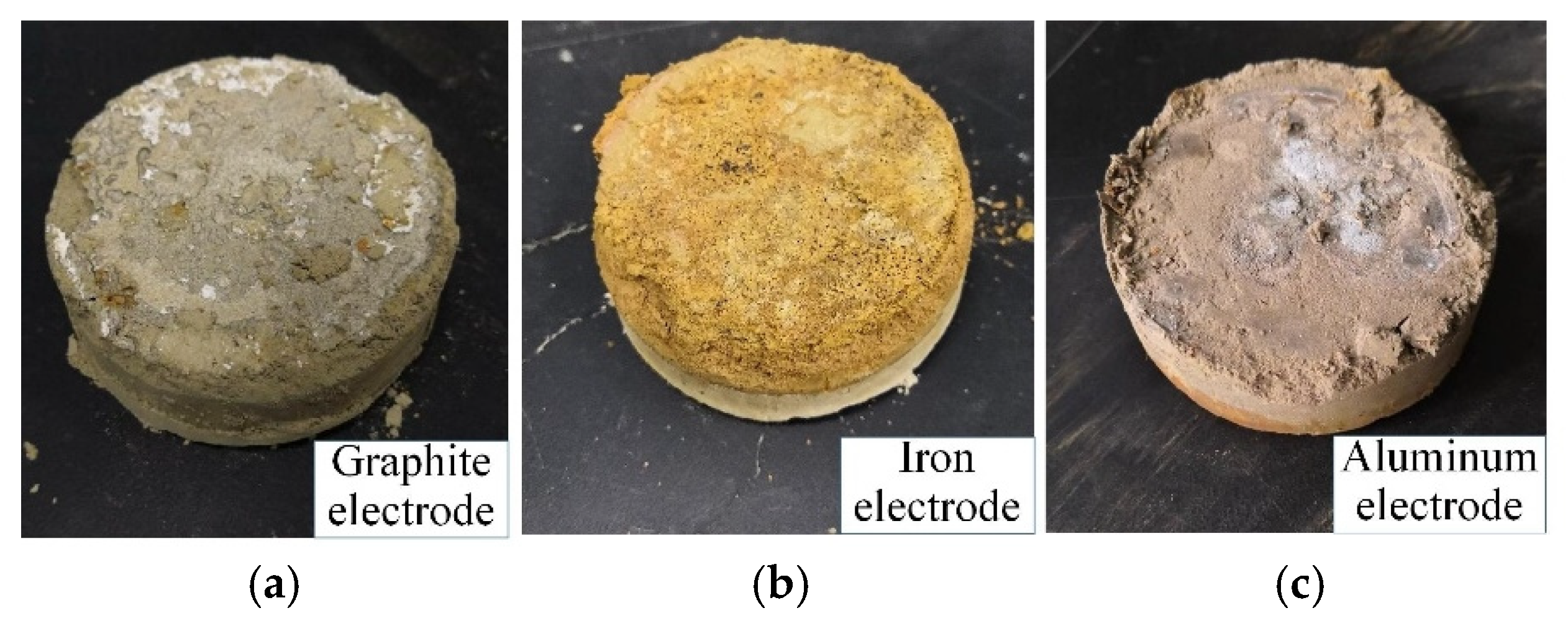
5.2. Electrode Material Optimization
6. Conclusions
- (1)
- From the standpoint of theoretical analysis, the mechanism of the modification effect of electrochemical technology on mudstone is explained, focusing primarily on electro-osmotic drainage consolidation and electrochemical reaction cementation. After testing and comparing the liquid and plastic limits of mudstone samples before and after modification, it was found that the liquid limit of the modified mudstone samples decreased by 7.874%, while the plastic limit increased by 9.499%.
- (2)
- Using a method with controlled variables, the laws of the electrolyte solution and electrode materials on the shear strength of modified mudstone were investigated. It was found that the type of ions added to the electrolyte solution affected the cementation strength of the soil. The concentration of ions in the electrolyte solution affected the electro-osmotic efficiency and the amount of residual ions in the soil after electrolysis. The best solution to match the mudstone samples in the test was an AlCl3 solution with a mass fraction of 10%.
- (3)
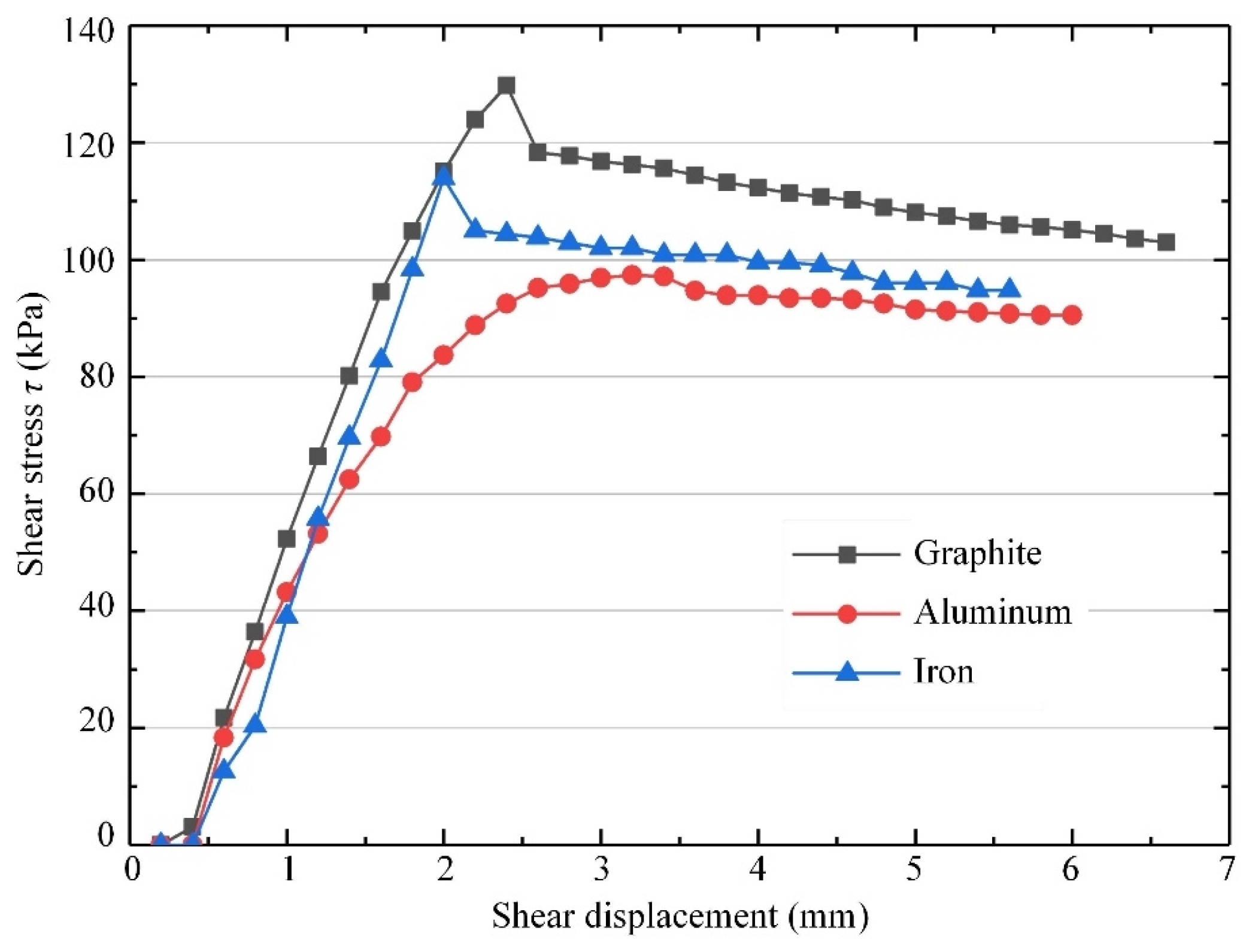
- Three common electrode materials were chosen for electrochemical modification tests: graphite, iron, and aluminum. The electrolytically oxidizable metal electrodes (iron and aluminum) produced passivation, resulting in higher water content in the modified mudstone specimens compared to the graphite electrode group. The internal friction angle of the graphite electrode-modified mudstone specimen was 26.7° and the cohesion was 34.4 kPa, which was nearly three times that of the original mudstone specimen with 15% water content. For real-world applications involving electrochemical changes to mudstones in the study area, it is suggested to use electrodes made of non-metallic materials and 10% AlCl3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.H.; Chen, P.Y.; Wu, J.H.; Chen, H.L.; Yang, Y.E. Method of mitigating the surface erosion of a high-gradient mudstone slope in southwest Taiwan. Bull. Eng. Geol. Environ. 2013, 72, 533–545. [Google Scholar] [CrossRef]
- Higuchi, K.; Chigira, M.; Lee, D.; Wu, J. Pore-water chemistry and its influence on rock mechanical properties and hydrogeophysical processes in a mudstone slope in the southwestern Taiwan badlands. Catena 2020, 190, 104533. [Google Scholar] [CrossRef]
- Han, L.; Shu, J.S.; Hanif, N.R.; Xi, W.J.; Li, X.; Jing, H.W.; Ma, L. Influence law of multipoint vibration load on slope stability in Xiaolongtan open pit mine in Yunnan, China. J. Cent. South Univ. 2015, 22, 4819–4827. [Google Scholar] [CrossRef]
- Lu, J.K.; Xu, T.; Tang, X.H.; Heap, M.J.; Xu, J.J.; Yang, T.H.; Zhao, X. Nanoindentation-based characterization of micromechanical properties of greenish mudstone from deep Fushun West open-pit mine (Fushun city, China). Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 59. [Google Scholar] [CrossRef]
- Wang, Y.K.; Han, M.S.; Li, B.; Wan, Y.K. Stability evaluation of earth-rock dam reinforcement with new permeable polymer based on reliability method. Constr. Build. Mater. 2022, 320, 126294. [Google Scholar] [CrossRef]
- Liu, F.Y.; Chen, W.Y.; Yang, Z.Q.; Deng, W.X.; Li, H.; Yang, T.H. Landslide Characteristics and Stability Control of Bedding Rock Slope: A Case Study in the Sijiaying Open-Pit Mine. Min. Metall. Explor. 2024. [Google Scholar] [CrossRef]
- Yu, M.Y.; Liu, B.G.; Liu, K.Y.; Sun, J.L.; Deng, T.B.; Wang, Q. Creep behavior of carbonaceous mudstone under triaxial hydraulic coupling condition and constitutive modelling. Int. J. Rock Mech. Min. Sci. 2023, 164, 105357. [Google Scholar] [CrossRef]
- Alikhani, A.; Taheri Moghadder, M.; Mohammadi, H. Investigation of Bishop’s and Janbu’s Models Capabilities on Slope Stability Problems with Special Consideration to Open-Pit Mining Operations. J. Min. Environ. 2020, 11, 161–170. [Google Scholar]
- Yang, G.; Chen, Y.; Liu, X.; Yang, R.; Zhang, Y.; Zhang, J. Stability analysis of a slope containing water-sensitive mudstone considering different rainfall conditions at an open-pit mine. Int. J. Coal Sci. Technol. 2023, 10, 64. [Google Scholar] [CrossRef]
- Alzo’Ubi, A.K.; Alneasan, M.; Ibrahim, F. The Mechanical Behavior of Mudstone Under Coupling Effect of Temperature and Confining Pressure: A Comprehensive Investigation. Rock Mech. Rock Eng. 2024, 57, 2325–2338. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Ma, J.; Yuan, S.; Zhang, L.; Chen, F. Multiscale investigation on the shear behaviour of lime-basalt fibre modified red mudstone as fill material for high-speed railway. Constr. Build. Mater. 2024, 456, 138961. [Google Scholar] [CrossRef]
- Feng, X.; Huang, P.; Carvelli, V.; Lin, G.; Zhu, C.; He, F. Can laser irradiation improve the strength of weak rock mass? J. Rock Mech. Geotech. Eng. 2024, in press. [Google Scholar] [CrossRef]
- Cameselle, C.; Reddy, K.R. Development and enhancement of electro-osmotic flow for the removal of contaminants from soils. Electrochim. Acta 2012, 86, 10–22. [Google Scholar] [CrossRef]
- Jones, C.J.F.P.; Lamont-Black, J.; Glendinning, S.; White, C.; Alder, D. The environmental sustainability of electrokinetic geosynthetic strengthened slopes. Proc. Inst. Civ. Eng. Eng. Sustain. 2014, 167, 95–107. [Google Scholar] [CrossRef]
- Lamont-Black, J.; Jones, C.; Alder, D. Electrokinetic strengthening of slopes—Case history. Geotext. Geomembr. 2016, 44, 319–331. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Y.L.; Xu, C.J.; Gong, X.N.; Hu, P.C. Electro-osmotic strengthening of silts based on selected electrode materials. Soils Found. 2015, 55, 1171–1180. [Google Scholar] [CrossRef]
- Chai, Z.Y.; Sun, Y.H.; Zhang, Y.T.; Yang, D. Influence of electrode material on argillaceous rock electrochemical modified effects. J. China Coal Soc. 2017, 42, 68–75. [Google Scholar]
- Ou, C.; Chien, S.; Chang, H. Soil improvement using electroosmosis with the injection of chemical solutions: Field tests. Can. Geotech. J. 2009, 46, 727–733. [Google Scholar] [CrossRef]
- Chapelain, J.; Faure, S.; Beneventi, D. Clay Flotation: Effect of TTAB Cationic Surfactant on Foaming and Stability of Illite Clay Microaggregates Foams. Ind. Eng. Chem. Res. 2016, 55, 2191–2201. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Chai, S.; Han, X.; Zhang, Q.; Bao, X.; Guo, Y.; Zhang, X.; Zhou, X.; Guo, S.; et al. Review—Clay Mineral Materials for Electrochemical Capacitance Application. J. Electrochem. Soc. 2021, 168, 70558. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N.; Sheahan, T.C.; Wu, X. Coupling of electrochemical and mechanical processes in soils under DC fields. Mech. Mater. 2004, 36, 453–465. [Google Scholar] [CrossRef]
- Peng, F.; Sun, D.; Chen, B.; Gao, Y. Effective approach to predict soil-water retention curve of bentonites considering adsorption and capillarity. J. Hydrol. 2024, 641, 131799. [Google Scholar] [CrossRef]
- Shang, J.Q.; Tang, Q.H.; Xu, Y.Q. Consolidation of marine clay using electrical vertical drains. Geomech. Eng. 2009, 1, 275–289. [Google Scholar] [CrossRef]
- Chai, Z.Y.; Kang, T.H.; Chen, W.Y. Effects of organic silicone additive material on physical and mechanical properties of mudstone. Geomech. Eng. 2014, 6, 139–151. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, Y.; Chen, M.; Li, W. Experimental analysis of the effect of mineral composition and water content of clay soil on electroosmotic efficiency. Bull. Eng. Geol. Environ. 2021, 80, 705–715. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Kang, G.; Kang, T. Mechanism and Effect of Electrochemical Modification on Physicochemical Soft Rock. Adv. Mater. Res. 2011, 415–417, 2275–2280. [Google Scholar] [CrossRef]
- Wang, D.; Kang, T.; Han, W.; Liu, Z. Electrochemical modification of tensile strength and pore structure in mudstone. Int. J. Rock Mech. Min. Sci. 2011, 48, 687–692. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.F.; Fan, J.; Zhou, C.Y. Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions. J. Mar. Sci. Eng. 2019, 7, 235. [Google Scholar] [CrossRef]
- Tenodi, K.Z.; Tenodi, S.; Nikic, J.; Mohora, E.; Agbaba, J.; Roncevic, S. Optimizing arsenic removal from groundwater using continuous flow electrocoagulation with iron and aluminum electrodes: An experimental and modeling approach. J. Water Process Eng. 2024, 66, 1–50. [Google Scholar] [CrossRef]
- Chen, S.B.; Sun, C.Y.; Zhang, H.A.; Yu, H.; Wang, W.T. Electrochemical Deposition of Bismuth on Graphite Felt Electrodes: Influence on Negative Half-Cell Reactions in Vanadium Redox Flow Batteries. Appl. Sci. 2024, 14, 3316. [Google Scholar] [CrossRef]
- Chai, Z.Y.; Sun, Y.H.; Bai, J.B. Experimental study on pore and strength change of mudstone from the coal measure strata under electro-chemical. J. China Coal Soc. 2018, 43, 667–674. [Google Scholar]
- Yoshida, N.; Hosokawa, K. Compression and shear behavior of mudstone aggregates. J. Geotech. Geoenviron. Eng. 2004, 130, 519–525. [Google Scholar] [CrossRef]
- Shang, J.Q.; Mohamedelhassan, E.; Ismail, M. Electrochemical cementation of offshore calcareous soil. Can. Geotech. J. 2004, 41, 877–893. [Google Scholar] [CrossRef]
- Ozkan, S.; Gale, R.J.; Seals, R.K. Electrokinetic stabilization of kaolinite by injection of Al and PO43− ions. Proc. Inst. Civ. Eng. Ground Improv. 1999, 3, 135–144. [Google Scholar] [CrossRef]
- Lo, K.Y.; Ho, K.S.; Inculet, I.I. Field test of electroosmotic strengthening of soft sensitive clay. Can. Geotech. J. 1991, 28, 74–83. [Google Scholar] [CrossRef]
- Chien, S.C.; Ou, C.Y.; Wang, Y.H. Soil improvement using electroosmosis with the injection of chemical solutions: Laboratory tests. J. Chin. Inst. Eng. 2011, 34, 863–875. [Google Scholar] [CrossRef]
- Chien, S.; Teng, F.; Ou, C. Soil improvement of electroosmosis with the chemical treatment using the suitable operation process. Acta Geotech. 2015, 10, 813–820. [Google Scholar] [CrossRef]
- Bergado, D.T.; Sasanakul, I.; Horpibulsuk, S. Electro-Osmotic Consolidation of Soft Bangkok Clay Using Copper and Carbon Electrodes with PVD. Geotech. Test. J. 2003, 26, 277–288. [Google Scholar] [CrossRef]
- Tonnizam, E.; Othman, M.; Adnan, S.; Gofar, N.; Saad, R. The Effectiveness of Electrodes Types on Electro-Osmosis of Malaysian Soil. Electron. J. Geotech. Eng. 2011, 16, 887–898. [Google Scholar]



| Electrode | Electrolyte | Mass Fraction of CaCl2 Solution | Mass Fraction of AlCl3 Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | AlCl3 | |||||||||||
| Iron | Test 1 | Test 2 | - | - | - | - | - | - | - | - | - | - |
| Aluminum | Test 3 | Test 4 | - | - | - | - | - | - | - | - | - | - |
| Graphite | Test 5 | Test 6 | 15% | 20% | 25% | 30% | 35% | 5% | 10% | 15% | 20% | 25% |
| Water Content | 15% | 20% | 25% | |
|---|---|---|---|---|
| Shear strength parameters | φ (°) | 23.9 | 20.7 | 14.9 |
| C (kPa) | 12.3 | 8.28 | 6.48 | |
| Specimens | Electrolytic Conditions I | Electrolytic Conditions II |
|---|---|---|
| Specimen 1 | Samples with 25% water content, electrolysis time 3.5 h | - |
| Specimen 2 | Samples with 25% water content, electrolysis time 3.5 h | 25 mL of solution in 3 drops, electrolysis over 5.5 h |
| Specimen 3 | Samples with 25% water content, electrolysis time 3.5 h | 50 mL of solution in 6 drops, electrolysis over 7.5 h |
| Electrode | Sample Mass/g | Sample Mass After Drying/g | Moisture Content/% |
|---|---|---|---|
| Graphite | 87.535 | 79.433 | 10.2 |
| Iron | 85.427 | 73.809 | 15.7 |
| Aluminum | 91.168 | 76.855 | 18.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Han, L.; Tovele, G.S.V.; Kong, J.; Yang, H. Preferred Chemical Agent for Electrochemical Modification of Physical and Mechanical Parameters of Mudstone. Appl. Sci. 2024, 14, 11789. https://doi.org/10.3390/app142411789
Chen X, Han L, Tovele GSV, Kong J, Yang H. Preferred Chemical Agent for Electrochemical Modification of Physical and Mechanical Parameters of Mudstone. Applied Sciences. 2024; 14(24):11789. https://doi.org/10.3390/app142411789
Chicago/Turabian StyleChen, Xiangchen, Liu Han, Gerson S. V. Tovele, Jiangrong Kong, and Han Yang. 2024. "Preferred Chemical Agent for Electrochemical Modification of Physical and Mechanical Parameters of Mudstone" Applied Sciences 14, no. 24: 11789. https://doi.org/10.3390/app142411789
APA StyleChen, X., Han, L., Tovele, G. S. V., Kong, J., & Yang, H. (2024). Preferred Chemical Agent for Electrochemical Modification of Physical and Mechanical Parameters of Mudstone. Applied Sciences, 14(24), 11789. https://doi.org/10.3390/app142411789






