Microstructural Evaluation of Dental Implant Success Using Micro-CT: A Comprehensive Review
Abstract
1. Introduction
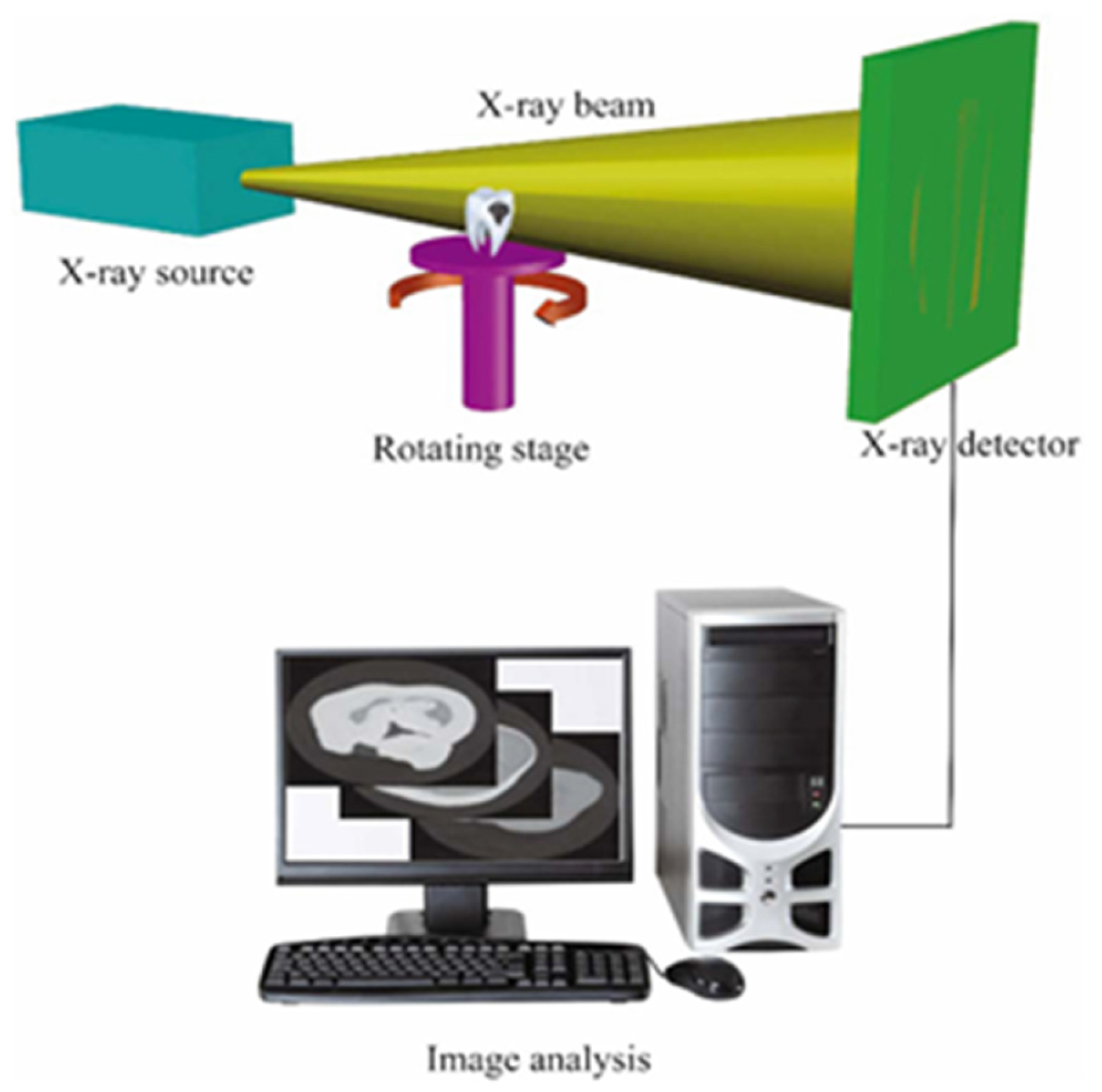
2. Principles and Applications of Micro-CT in Dental Implant Evaluation
- Sub-micron resolution;
- Non-destructive analysis;
- Three-dimensional visualization;
- Accurate quantification;
- Bone–implant interface analysis;
- Integration with advanced analyses, such as finite element analysis (FEA).
3. Critical Bone Microstructure Parameters for Implant Success
4. Evaluation of Bone Around Implants Using Micro-CT
5. Correlation of Bone Microstructure with Implant Stability
6. Micro-CT Application in Bone Quality Assessment
7. Micro-CT in Biomaterial Evaluation and Bone Augmentation
8. Challenges and Limitations of Using Micro-CT in Dental Implant Evaluation
9. Recent Innovations and Future Prospects
10. Clinical Implications and Recommendations
11. Discussion
12. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of Titanium, Titanium Alloy and Zirconia Dental Implants: Current Knowledge and Open Questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Haj Husain, A.; De Cicco, O.; Stadlinger, B.; Bosshard, F.A.; Schmidt, V.; Özcan, M.; Valdec, S. A Survey on Attitude, Awareness, and Knowledge of Patients Regarding the Use of Dental Implants at a Swiss University Clinic. Dent. J. 2023, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S. Dental Implants: The Last 100 Years. J. Oral Maxillofac. Surg. 2018, 76, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Poli, P.P.; Giboli, L.; Beretta, M.; Maiorana, C.; Pellegrini, M. Clinical Factors on Dental Implant Fractures: A Systematic Review. Dent. J. 2024, 12, 200. [Google Scholar] [CrossRef]
- Howe, M.S.; Keys, W.; Richards, D. Long-Term (10-Year) Dental Implant Survival: A Systematic Review and Sensitivity Meta-Analysis. Journal of Dentistry. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Hare, A.; Bird, S.; Wright, S.; Ucer, C.; Khan, R.S. Current Undergraduate Dental Implantology Teaching in UK. Dent. J. 2022, 10, 127. [Google Scholar] [CrossRef]
- Da Silva Brum, I.; Elias, C.N.; Lopes, J.C.A.; Frigo, L.; dos Santos, P.G.P.; de Carvalho, J.J. Clinical Analysis of the Influence of Surface Roughness in the Primary Stability and Osseointegration of Dental Implants: Study in Humans. Coatings 2024, 14, 951. [Google Scholar] [CrossRef]
- Abu Alfaraj, T.; Al-Madani, S.; Alqahtani, N.S.; Almohammadi, A.A.; Alqahtani, A.M.; AlQabbani, H.S.; Bajunaid, M.K.; Alharthy, B.A.; Aljalfan, N. Optimizing Osseointegration in Dental Implantology: A Cross-Disciplinary Review of Current and Emerging Strategies. Cureus 2023, 15, e47943. [Google Scholar] [CrossRef]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical Evaluations of the Long-Term Stability of Dental Implant Using Finite Element Modeling Method: A Systematic Review. J. Adv. Prosthodont. 2022, 14, 182–202. [Google Scholar] [CrossRef]
- Zanetti, E.M.; Pascoletti, G.; Calì, M.; Bignardi, C.; Franceschini, G. Clinical Assessment of Dental Implant Stability during Follow-up: What Is Actually Measured, and Perspectives. Biosensors 2018, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Dura Haddad, C.; Andreatti, L.; Zelezetsky, I.; Porrelli, D.; Turco, G.; Bevilacqua, L.; Maglione, M. Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro. Bioengineering 2024, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Kittur, N.; Oak, R.; Dekate, D.; Jadhav, S.; Dhatrak, P. Dental Implant Stability and Its Measurements to Improve Osseointegration at the Bone-Implant Interface: A Review. Mater. Today Proc. 2020, 43, 1064–1070. [Google Scholar] [CrossRef]
- Mangal, K.; Dhamande, M.M.; Sathe, S.; Godbole, S.; Patel, R.M. An Overview of the Implant Therapy: The Esthetic Approach. Int. J. Curr. Res. Rev. 2021, 13, 106–112. [Google Scholar] [CrossRef]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone Beam Computed Tomography in Implant Dentistry: Recommendations for Clinical Use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef]
- Salian, S.S.; Subhadarsanee, C.P.; Patil, R.T.; Dhadse, P.V. Radiographic Evaluation in Implant Patients: A Review. Cureus 2024, 16, e54783. [Google Scholar] [CrossRef]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone Quality Evaluation at Dental Implant Site Using Multislice CT, Micro-CT, and Cone Beam CT. Clin. Oral Implant. Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef]
- Báskay, J.; Pénzes, D.; Kontsek, E.; Pesti, A.; Kiss, A.; Guimarães Carvalho, B.K.; Szócska, M.; Szabó, B.T.; Dobó-Nagy, C.; Csete, D.; et al. Are Artificial Intelligence-Assisted Three-Dimensional Histological Reconstructions Reliable for the Assessment of Trabecular Microarchitecture? J. Clin. Med. 2024, 13, 1106. [Google Scholar] [CrossRef]
- Campioni, I.; Pecci, R.; Bedini, R. Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview. Appl. Sci. 2020, 10, 4328. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.D.; et al. Micro-ct for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. [Google Scholar] [CrossRef]
- Kawata, N.; Teplov, A.; Ntiamoah, P.; Shia, J.; Hameed, M.; Yagi, Y. Micro-Computed Tomography: A Novel Diagnostic Technique for the Evaluation of Gastrointestinal Specimens. Endosc. Int. Open 2021, 09, E1886–E1889. [Google Scholar] [CrossRef] [PubMed]
- Erpaçal, B.; Adıgüzel, Ö.; Cangül, S. The Use of Micro-Computed Tomography in Dental Applications. Int. Dent. Res. 2019, 9, 78–91. [Google Scholar] [CrossRef]
- Rahman, F.U.A.; Azhari, A.; Epsilawati, L.; Firman, R.N.; Pramanik, F. Micro-Computed Tomography: Teknologi Pencitraan Mikroskopis Berbasis Computed Tomography Dan Pengunaannya Dalam Analisis Kualitas Tulang. J. Radiol. Dentomaksilofasial Indones. (JRDI) 2020, 4, 111. [Google Scholar] [CrossRef]
- Bohner, L.; Tortamano, P.; Gremse, F.; Chilvarquer, I.; Kleinheinz, J.; Hanisch, M. Assessment of Trabecular Bone During Dental Implant Planning Using Cone-Beam Computed Tomography with High-Resolution Parameters. Open Dent. J. 2021, 15, 57–63. [Google Scholar] [CrossRef]
- Orhan, K.; Büyüksungur, A. Fundamentals of Micro-CT Imaging. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–33. [Google Scholar] [CrossRef]
- Nolte, P.; Dullin, C.; Svetlove, A.; Brettmacher, M.; Rußmann, C.; Schilling, A.F.; Alves, F.; Stock, B. Current Approaches for Image Fusion of Histological Data with Computed Tomography and Magnetic Resonance Imaging. Radiol. Res. Pract. 2022, 2022, 6765895. [Google Scholar] [CrossRef]
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, Á.; Vajtai, R. Microcomputed Tomography–Based Characterization of Advanced Materials: A Review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- Ghavami-Lahiji, M.; Davalloo, R.T.; Tajziehchi, G.; Shams, P. Micro-Computed Tomography in Preventive and Restorative Dental Research: A Review. Imaging Sci. Dent. 2021, 51, 341–350. [Google Scholar] [CrossRef]
- Gregor, T.; Kochov, P.; Eberlov, L.; Nedorost, L.; Proseck, E.; Lika, V.; Mrka, H.; Kachlk, D.; Pirner, I.; Zimmermann, P.; et al. Correlating Micro-CT Imaging with Quantitative Histology. In Injury and Skeletal Biomechanics; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Roque-Torres, G.D. Application of Micro-CT in Soft Tissue Specimen Imaging. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 139–170. [Google Scholar] [CrossRef]
- Elkhoury, J.E.; Shankar, R.; Ramakrishnan, T.S. Resolution and Limitations of X-Ray Micro-CT with Applications to Sandstones and Limestones. Transp. Porous Media 2019, 129, 413–425. [Google Scholar] [CrossRef]
- Hunter, L.; Dewanckele, J. Evolution of Micro-CT: Moving from 3D to 4D. Microsc. Today 2021, 29, 28–34. [Google Scholar] [CrossRef]
- Ijiri, T.; Todo, H.; Hirabayashi, A.; Kohiyama, K.; Dobashi, Y. Digitization of Natural Objects with Micro CT and Photographs. PLoS ONE 2018, 13, e0195852. [Google Scholar] [CrossRef]
- Akhter, M.P.; Recker, R.R. High Resolution Imaging in Bone Tissue Research-Review. Bone 2021, 143, 115620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, D.; Zhou, R.; Zhang, Y. 3D X-Ray Micro-Computed Tomography Imaging for the Microarchitecture Evaluation of Porous Metallic Implants and Scaffolds. Micron 2021, 142, 102994. [Google Scholar] [CrossRef] [PubMed]
- Kerberger, R.; Brunello, G.; Drescher, D.; van Rietbergen, B.; Becker, K. Micro finite element analysis of continuously loaded mini-implant—A micro-CT study in the rat tail model. Bone 2023, 177, 116912. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiao, W.; Liu, X.; Bian, D.; Shen, D.; Zheng, Y.; Wu, J.; Kwan, K.Y.H.; Wong, T.M.; Cheung, K.M.C.; et al. Biomimicking bone–implant interface facilitates the bioadaption of a new degradable magnesium alloy to the bone tissue microenvironment. Adv. Sci. 2021, 8, 2102035. [Google Scholar] [CrossRef]
- de Freitas, R.B.; Aredes, G.D.A.; Cicareli, A.J.; Idalgo, F.A.; Kassis, E.N. Dental implant and aesthetics: A systematic review. MedNEXT J. Med. Health Sci. 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Poilliot, A.; Gay-Dujak, M.H.P.; Müller-Gerbl, M. The quantification of 3D-trabecular architecture of the fourth cervical vertebra using CT osteoabsorptiometry and micro-CT. J. Orthop. Surg. Res. 2023, 18, 297. [Google Scholar] [CrossRef]
- El-Gizawy, A.S.; Ma, X.; Pfeiffer, F.; Schiffbauer, J.D.; Selly, T. Characterization of microarchitectures, stiffness and strength of human trabecular bone using micro-Computed Tomography (micro-CT) scans. BioMed 2023, 3, 89–100. [Google Scholar] [CrossRef]
- Kivell, T.L. A Review of Trabecular Bone Functional Adaptation: What Have We Learned from Trabecular Analyses in Extant Hominoids and What Can We Apply to Fossils? J. Anat. 2016, 228, 569–594. [Google Scholar] [CrossRef]
- Tian, T.; Liu, H.; Zhang, H.; Han, Q.; Chen, J. Correlation between bone volume fraction in posterior implant area and initial implant stability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 396–401. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S.; Atanasov, D. Association between Bone Density Values, Primary Stability and Histomorphometric Analysis of Dental Implant Osteotomy Sites on the Upper Jaw. Folia Medica 2020, 62, 563–571. [Google Scholar] [CrossRef]
- Bruno, V.; Berti, C.; Barausse, C.; Badino, M.; Gasparro, R.; Ippolito, D.R.; Felice, P. Clinical Relevance of Bone Density Values from CT Related to Dental Implant Stability: A Retrospective Study. BioMed Res. Int. 2018, 2018, 6758245. [Google Scholar] [CrossRef] [PubMed]
- Sabeva, E.; Peev, S.; Miteva, M.; Georgieva, M. Bone characteristics and implant stability. Scr. Sci. Med. Dent. 2017, 3, 18–22. [Google Scholar] [CrossRef]
- Giner, M.; Miranda, C.; Vázquez-Gámez, M.A.; Altea-Manzano, P.; Miranda, M.J.; Casado-Díaz, A.; Pérez-Cano, R.; Montoya-García, M.J. Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus. Diagnostics 2021, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Boutroy, S.; Chapurlat, R. Prediction of Fractures in Men Using Bone Microarchitectural Parameters Assessed by High-Resolution Peripheral Quantitative Computed Tomography—The Prospective STRAMBO Study. J. Bone Miner. Res. 2018, 33, 1470–1479. [Google Scholar] [CrossRef]
- Tabassum, A.; Chainchel Singh, M.K.; Ibrahim, N.; Ramanarayanan, S.; Mohd Yusof, M.Y.P. Quantifications of Mandibular Trabecular Bone Microstructure Using Cone Beam Computed Tomography for Age Estimation: A Preliminary Study. Biology 2022, 11, 1521. [Google Scholar] [CrossRef]
- Tsegai, Z.J.; Skinner, M.M.; Pahr, D.H.; Hublin, J.-J.; Kivell, T.L. Ontogeny and Variability of Trabecular Bone in the Chimpanzee Humerus, Femur and Tibia. Am. J. Phys. Anthropol. 2018, 167, 713–736. [Google Scholar] [CrossRef]
- Doershuk, L.J.; Saers, J.P.P.; Shaw, C.N.; Jashashvili, T.; Carlson, K.J.; Stock, J.T.; Ryan, T.M. Complex Variation of Trabecular Bone Structure in the Proximal Humerus and Femur of Five Modern Human Populations. Am. J. Phys. Anthropol. 2018, 168, 104–118. [Google Scholar] [CrossRef]
- Parkinson, I.H.; Fazzalari, N.L. Characterisation of Trabecular Bone Structure. In Skeletal Aging and Osteoporosis; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; Volume 5, pp. 31–51. [Google Scholar] [CrossRef]
- Cooper, D.M.L.; Kawalilak, C.E.; Harrison, K.; Johnston, B.D.; Johnston, J.D. Cortical Bone Porosity: What Is It, Why Is It Important, and How Can We Detect It? Current Osteoporosis Reports. Curr. Osteoporos. Rep. 2016, 14, 187–198. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone Mechanical Properties and Changes with Osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Yun, J.H. Three-Dimensional Microstructure of Human Alveolar Trabecular Bone: A Micro-Computed Tomography Study. J. Periodontal Implant. Sci. 2017, 47, 20–29. [Google Scholar] [CrossRef]
- Putri, A.; Pramanik, F.; Azhari, A. The Suitability of trabecular patterns in the assessment of dental implant osseointegration process through 2D digital and 3D CBCT radiographs. Eur. J. Dent. 2024, 18, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Z.; Su, Y.; Liu, Q.; Ge, Y.; Shan, Z. Osseointegration of a novel dental implant in canine. Sci. Rep. 2021, 11, 4317. [Google Scholar] [CrossRef] [PubMed]
- Bregoli, C.; Biffi, C.A.; Tuissi, A.; Buccino, F. Effect of trabecular architectures on the mechanical response in osteoporotic and healthy human bone. Med. Biol. Eng. Comput. 2024, 62, 3263–3281. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.; Synek, A.; Pahr, D.H. Comparison of different microCT-based morphology assessment tools using human trabecular bone. Bone Rep. 2020, 12, 100261. [Google Scholar] [CrossRef]
- Klintström, E.; Klintström, B.; Spångeus, A.; Sandborg, M.; Woisetschläger, M. Trabecular bone microstructure analysis on data from a novel twin robotic X-ray device. Acta Radiol. 2023, 64, 1566–1572. [Google Scholar] [CrossRef]
- Ariyachaipanich, A.; Kaya, E.; Statum, S.; Biswas, R.; Tran, B.; Bae, W.C.; Chung, C.B. MR imaging pattern of tibial subchondral bone structure: Considerations of meniscal coverage and integrity. Skelet. Radiol. 2020, 49, 2019–2027. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, J.; Zhang, P.; Chen, X.; Jiang, H. Trabeculae microstructure parameters serve as effective predictors for marginal bone loss of dental implant in the mandible. Sci. Rep. 2020, 10, 18437. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, U.G.; Yeo, I.S.L. Comparison of three-dimensional digital analyses and two-dimensional histomorphometric analyses of the bone-implant interface. PLoS ONE 2022, 17, e0276269. [Google Scholar] [CrossRef]
- Lyu, H.Z.; Lee, J.H. Correlation between two-dimensional micro-CT and histomorphometry for assessment of the implant osseointegration in rabbit tibia model. Biomater. Res. 2021, 25, 11. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, J.I.; Chae, J.S.; Yeo, I.S.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone–implant contact ratios. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.G.V.; Santos, F.T.; Allegrini, J.S. The importance of prosthetic planning for implant-supported dentures in esthetic zones—A case report. Int. J. Surg. Case Rep. 2019, 54, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bhawnani, D.; Bhasinn, A.; Mantri, S.; Gupta, P. Prosthetic consideration in implant prostheses treatment planning: A review. South Asian Res. J. Oral Dent. Sci. 2021, 3, 99–103. [Google Scholar] [CrossRef]
- Su, Y.H.; Peng, B.Y.; Wang, P.D.; Feng, S.W. Evaluation of the implant stability and the marginal bone level changes during the first three months of dental implant healing process: A prospective clinical study. J. Mech. Behav. Biomed. Mater. 2020, 110, 103899. [Google Scholar] [CrossRef]
- Wang, S.H.; Fuh, L.J.; Chen, M.Y.C.; Tsai, M.T.; Huang, H.L.; Peng, S.L.; Hsu, J.-T. Preoperative assessment of bone density for dental implantation: A comparative study of three different ROI methods. Head Face Med. 2024, 20, 33. [Google Scholar] [CrossRef]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Catena, A.; Pérez-Sayáns, M.; Fernández-Barbero, J.E.; O’Valle, F.; Padial-Molina, M. Early Marginal Bone Loss around Dental Implants to Define Success in Implant Dentistry: A Retrospective Study. Clin. Implant. Dent. Relat. Res. 2022, 24, 630–642. [Google Scholar] [CrossRef]
- Cruz, R.S.; Lemos, C.A.A.; de Luna Gomes, J.M.; Fernandes e Oliveira, H.F.; Pellizzer, E.P.; Verri, F.R. Clinical Comparison between Crestal and Subcrestal Dental Implants: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2022, 127, 408–417. [Google Scholar] [CrossRef]
- Stacchi, C.; Lamazza, L.; Rapani, A.; Troiano, G.; Messina, M.; Antonelli, A.; Giudice, A.; Lombardi, T. Marginal Bone Changes around Platform-Switched Conical Connection Implants Placed 1 or 2 Mm Subcrestally: A Multicenter Crossover Randomized Controlled Trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 398–408. [Google Scholar] [CrossRef]
- Fernández-Figares-Conde, I.; Castellanos-Cosano, L.; Fernandez-Ruiz, J.A.; Soriano-Santamaria, I.; Hueto-Madrid, J.A.; Gómez-Lagunas, J.; Romano-Laureato, R.; Torres-Lagares, D. Multicentre Prospective Study Analysing Relevant Factors Related to Marginal Bone Loss: A Two-Year Evolution. Dent. J. 2023, 11, 185. [Google Scholar] [CrossRef]
- Wang, S.H.; Hsu, J.T.; Fuh, L.J.; Peng, S.L.; Huang, H.L.; Tsai, M.T. New classification for bone type at dental implant sites: A dental computed tomography study. BMC Oral Health 2023, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.S.; Rabelo, G.D.; Spin-Neto, R.; Dechichi, P.; Borges, J.S.; Soares, P.B.F. Use of micro-computed tomography for bone evaluation in dentistry. Braz. Dent. J. 2018, 29, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Assari, A.; Al Bukairi, M.; Al Saif, R. Micro-Computed Tomography applications in dentistry. Open J. Stomatol. 2024, 14, 32–41. [Google Scholar] [CrossRef]
- Lee, Y.K.; Wadhwa, P.; Cai, H.; Jung, S.U.; Zhao, B.C.; Rim, J.S.; Kim, D.-H.; Jang, H.-S.; Lee, E.-S. Micro-CT and histomorphometric study of bone regeneration effect with autogenous tooth biomaterial enriched with platelet-rich fibrin in an animal model. Scanning 2021, 2021, 6656791. [Google Scholar] [CrossRef] [PubMed]
- Bedini, R.; Pecci, R.; Meleo, D.; Campioni, I. Bone substitutes scaffold in human bone: Comparative evaluation by 3D micro-CT technique. Appl. Sci. 2020, 10, 3451. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT—A digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef]
- Zou, W.; Li, X.; Li, N.; Guo, T.; Cai, Y.; Yang, X.; Liang, J.; Sun, Y.; Fan, Y. A comparative study of autogenous, allograft and artificial bone substitutes on bone regeneration and immunotoxicity in rat femur defect model. Regen. Biomater. 2021, 8, rbaa040. [Google Scholar] [CrossRef]
- Kivovics, M.; Szabó, B.T.; Németh, O.; Iványi, D.; Trimmel, B.; Szmirnova, I.; Orhan, K.; Mijiritsky, E.; Szabó, G.; Dobó-Nagy, C. Comparison between micro-Computed Tomography and Cone-Beam Computed Tomography in the assessment of bone quality and a long-term volumetric study of the augmented sinus grafted with an albumin impregnated allograft. J. Clin. Med. 2020, 9, 303. [Google Scholar] [CrossRef]
- Beitlitum, I.; Rayyan, F.; Pokhojaev, A.; Tal, H.; Sarig, R. A novel micro-CT analysis for evaluating the regenerative potential of bone augmentation xenografts in rabbit calvarias. Sci. Rep. 2024, 14, 4321. [Google Scholar] [CrossRef]
- Orhan, K.; de Faria, V.K.; Gaêta-Araujo, H. Artifacts in Micro-CT. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 35–48. [Google Scholar] [CrossRef]
- Kowalski, J.; Puszkarz, A.K.; Radwanski, M.; Sokolowski, J.; Cichomski, M.; Bourgi, R.; Hardan, L.; Sauro, S.; Lukomska-Szymanska, M. Micro-CT evaluation of microgaps at implant–abutment connection. Materials 2023, 16, 4491. [Google Scholar] [CrossRef]
- Soares, A.P.; Blunck, U.; Bitter, K.; Paris, S.; Rack, A.; Zaslansky, P. Hard X-ray phase-contrast-enhanced micro-CT for quantifying interfaces within brittle dense root-filling-restored human teeth. J. Synchrotron Radiat. 2020, 27, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Hristov, K.; Gigova, R.; Gateva, N.; Angelova, L. Micro-computed tomography (micro-CT) evaluation of root canal morphology in immature maxillary third molars. J. Clin. Pediatr. Dent. 2024, 48, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, S.; Fan, Y.; Wang, G.; Li, J.; Liu, C.; Li, Z.; Sun, J. Large-factor Micro-CT super-resolution of bone microstructure. Front. Phys. 2022, 10, 997582. [Google Scholar] [CrossRef]
- Prasaanth, S.A.; Reddy, T.V.K.; Mitthra, S.; Venkatesh, K.V. Applications of micro-Computed Tomography in dentistry. Int. J. Pharm. Res. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Micro-CT of Rodents: State-of-the-Art and Future Perspectives. Phys. Medica 2014, 30, 619–634. [Google Scholar] [CrossRef]
- Muller, F.M.; Maebe, J.; Vanhove, C.; Vandenberghe, S. Dose reduction and image enhancement in micro-CT using deep learning. Med. Phys. 2023, 50, 5643–5656. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Advances in Micro-CT Imaging of Small Animals. Phys. Medica 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Maewi, H.; Al-Mahalawy, H. Micro-computed tomographic evaluation of osseointegration of trabecular dental implants in a rabbit model. Egypt Dent. J. 2018, 64, 3125–3134. [Google Scholar]
- Vilardell, A.M.; Cinca, N.; Barriuso, E.; Frigola, J.; Dosta, S.; Cano, I.G.; Guilemany, J.M. X-ray microtomographic characterization of highly rough titanium cold gas sprayed coatings for identification of effective surfaces for osseointegration. Microscopy 2019, 68, 413–416. [Google Scholar] [CrossRef]
- Dudak, J.; Karch, J.; Holcova, K.; Zemlicka, J. X-ray imaging with sub-micron resolution using large-area photon counting detectors Timepix. J. Instrum. 2017, 12, C12024. [Google Scholar] [CrossRef]
- Yakovlev, M.A.; Vanselow, D.J.; Ngu, M.S.; Zaino, C.R.; Katz, S.R.; Ding, Y.; Parkinson, D.; Wang, S.Y.; Ang, K.C.; La Riviere, P.J.; et al. A wide-field micro-Computed Tomography detector: Micron resolution at half-centimeter scale. J. Synchrotron Radiat. 2022, 29, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Algabri, R.S.; Elawady, D.; Ibrahim, W.I. Advancements in artificial intelligence algorithms for dental implant identification: A systematic review with meta-analysis. J. Prosthet. Dent. 2023, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moufti, M.A.; Trabulsi, N.; Ghousheh, M.; Fattal, T.; Ashira, A.; Danishvar, S. Developing an artificial intelligence solution to autosegment the edentulous mandibular bone for implant planning. Eur. J. Dent. 2023, 17, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Santos, N.; Jacobs, R.; Picoli, F.F.; Lahoud, P.; Niclaes, L.; Groppo, F.C. Automated segmentation of the mandibular canal and its anterior loop by deep learning. Sci. Rep. 2023, 13, 10819. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, B.A.; Galluci, G.O.; Att, W.; Krishnamurthy, V.R. Artificial Intelligence Applications in Implant Dentistry: A Systematic Review. J. Prosthet. Dent. 2023, 129, 293–300. [Google Scholar] [CrossRef]
- Senthil, R.S.R.; Kumar, K.H.S.; Sekhar, A.; Nadakkavukaran, D.; Feroz, S.M.A.; Gangadharappa, P. Evaluating the Role of AI in Predicting the Success of Dental Implants Based on Preoperative CBCT Images: A Randomized Controlled Trial. J. Pharm. Bioallied Sci. 2024, 16, S889–S891. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Kunam, A.; Rashme, R.; Sudarsanam, P.P.; Gupta, A.; Kiran Kumar, H.S. AI Assisted Treatment Planning for Dental Implant Placement: Clinical vs AI Generated Plans. J. Pharm. Bioallied Sci. 2024, 16, S942–S944. [Google Scholar] [CrossRef]
- Chavez, M.B.; Chu, E.Y.; Kram, V.; de Castro, L.F.; Somerman, M.J.; Foster, B.L. Guidelines for micro–Computed Tomography analysis of rodent dentoalveolar tissues. JBMR Plus 2021, 5, e10474. [Google Scholar] [CrossRef]
- Chackartchi, T.; Romanos, G.E.; Parkanyi, L.; Schwarz, F.; Sculean, A. Reducing errors in guided implant surgery to optimize treatment outcomes. Periodontology 2000 2022, 88, 64–72. [Google Scholar] [CrossRef]
- Schmidt, A.; Billig, J.W.; Schlenz, M.A.; Wöstmann, B. A new 3D-method to assess the inter implant dimensions in patients—A pilot study. J. Clin. Exp. Dent. 2020, 12, 187–192. [Google Scholar] [CrossRef]
- Huang, S.; Wei, H.; Li, D. Additive manufacturing technologies in the oral implan clinic: A review of current applications and progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, C.; Serrano-Sosa, M.; Hu, J.; Zhu, J.; Tang, G.; Huang, C.; Huang, M. Bone microarchitectural parameters can detect oxytocin induced changes prior to bone density on mitigating bone deterioration in rabbit osteoporosis model using micro-CT. BMC Musculoskelet. Disord. 2019, 20, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rytky, S.J.O.; Tiulpin, A.; Finnilä, M.A.J.; Karhula, S.S.; Sipola, A.; Kurttila, V.; Valkealahti, M.; Lehenkari, P.; Joukainen, A.; Kröger, H.; et al. Clinical Super-Resolution Computed Tomography of Bone Microstructure: Application in Musculoskeletal and Dental Imaging. Ann. Biomed. Eng. 2024, 52, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.D.; Egito, M.; Castro, C.; Groisman, S.; Basílio, M.; da Penha, N.L. About the elemental analysis of dental implants. Radiat. Phys. Chem. 2019, 154, 53–57. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Gadyukov, A.; Chaushu, G.; Chaushu, L. Micro-CT Analysis of Microgap at a Novel Two-Piece Dental Implant Comprising a Replaceable Sleeve In Vitro. Int. J. Oral Maxillofac. Implants 2021, 36, 451–459. [Google Scholar] [CrossRef]
- Cobos, S.F.; Norley, C.J.; Pollmann, S.I.; Holdsworth, D.W. Cost-effective micro-CT system for non-destructive testing of titanium 3D printed medical components. PLoS ONE 2022, 17, e0275732. [Google Scholar] [CrossRef]
- Li, M.; Fang, Z.; Cong, W.; Niu, C.; Wu, W.; Uher, J.; Bennett, J.; Rubinstein, J.T.; Wang, G. Clinical Micro-CT Empowered by Interior Tomography, Robotic Scanning, and Deep Learning. IEEE Access 2020, 8, 229018–229032. [Google Scholar] [CrossRef]
- Fidan, S. The use of Micro-CT in Materials Science and Aerospace Engineering. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 267–276. [Google Scholar] [CrossRef]
- Nanthakumar, R.; Sivakumaran, N. Role of Biomedical Engineering for Diagnose and Treatment. Int. J. Adv. Sci. Res. Eng. 2018, 4, 94–112. [Google Scholar] [CrossRef]
- Vallathan, G.; Rajamani, V.; Harinee, M.P. Enhanced Medical Data Security and Perceptual Quality for Healthcare services. In Proceedings of the 2020 International Conference on System, Computation, Automation and Networking, ICSCAN 2020, Pondicherry, India, 3–4 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Krishna, A.; Tanveer, A.; Bhagirath, P.; Gannepalli, A. Role of artificial intelligence in diagnostic oral pathology—A modern approach. J. Oral Maxillofac. Pathol. 2020, 24, 152–156. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Xiao, L. Artificial Intelligence in Digital Pathology Image Analysis; Frontiers Media SA: Lausanne, Switzerland, 2023; pp. 5–187. [Google Scholar] [CrossRef]
- Martín-Noguero, T.; Paulano-Godino, F.; López-Ortega, R.; Górriz, J.M.; Riascos, R.F.; Luna, A. Artificial intelligence in radiology: Relevance of collaborative work between radiologists and engineers for building a multidisciplinary team. Clin. Radiol. 2021, 76, 317–324. [Google Scholar] [CrossRef]
- Pascadopoli, M.; Zampetti, P.; Nardi, M.G.; Pellegrini, M.; Scribante, A. Smartphone Applications in Dentistry: A Scoping Review. Dent. J. 2023, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.; Ruiz, A.J.; Pogue, B.W. Smartphone-Based Imaging Systems for Medical Applications: A Critical Review. J. Biomed. Opt. 2021, 26, 040902. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.N.; Kunze, K.N.; Haeberle, H.S.; Karnuta, J.M.; Luu, B.C.; Nwachukwu, B.U.; Williams, R.J. Clinical and Research Medical Applications of Artificial Intelligence. Arthroscopy 2021, 37, 1694–1697. [Google Scholar] [CrossRef] [PubMed]

| µCT | VV | PR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||||
| Inferior | Superior | Inferior | Superior | Inferior | Superior | |||||
| BV.TV | ||||||||||
| G1 | 53.17 ± 12.5 * | 46.13 | 60.21 | 45.37 ± 10.8 * | 38.91 | 51.84 | 37.86 ± 9.1 * | 32.45 | 43.27 | |
| G2 | 33.51 ± 3.78 * | 26.47 | 40.54 | 32.57 ± 5.20 * | 26.10 | 39.03 | 36.55 ± 4.2 * | 31.14 | 41.96 | |
| BS.BV | ||||||||||
| G1 | 52.98 ± 12.4 * | 43.19 | 62.78 | 20.05 ± 3.77 * | 16.32 | 23.78 | 34.65 ± 5.7 * | 30.02 | 39.28 | |
| G2 | 72.03 ± 11.6 * | 62.87 | 81.19 | 27.25 ± 5.15 * | 23.76 | 30.74 | 27.44 ± 5.5 * | 23.11 | 31.77 | |
| Tb. Th | ||||||||||
| G1 | 0.06 ± 0.01 * | 0.05 | 0.07 | 0.24 ± 0.03 * | 0.22 | 0.26 | 0.15 ± 0.01 * | 0.13 | 0.17 | |
| G2 | 0.05 ± 0.01 * | 0.04 | 0.06 | 0.19 ± 0.01 * | 0.17 | 0.21 | 0.18 ± 0.02 * | 0.17 | 0.20 | |
| Tb. Sp | ||||||||||
| G1 | 0.08 ± 0.02 * | 0.06 | 0.10 | 0.27 ± 0.08 * | 0.21 | 0.33 | 0.19 ± 0.06 * | 0.14 | 0.24 | |
| G2 | 0.12 ± 0.02 * | 0.11 | 0.14 | 0.29 ± 0.06 * | 0.24 | 0.35 | 0.28 ± 0.07 * | 0.23 | 0.33 | |
| Patient | Clinical (x, y, z) | AI-Generated (x, y, z) | Deviation (x, y, z) |
|---|---|---|---|
| 1 | (12, 4, −3) | (11, 5, −2) | (1, 1, 1) |
| 2 | (9, 6, −2) | (10, 5, −3) | (1, 1, 1) |
| 3 | (11, 3, −4) | (11, 4, −4) | (0, 1, 0) |
| 20 | (10, 5, −3) | (10, 5, −3) | (0, 0, 0) |
| Mean | (10.5, 4.5, −3) | (10.5, 4.5, −3) | (0.5, 0.5, 0.5) |
| Std dev | (0.8, 0.6, 0.8) | (0.7, 0.6, 0.7) | (0.3, 0.2, 0.3) |
| Patient | Clinical Angulation | AI-Generated Angulation | Deviation |
|---|---|---|---|
| 1 | 25 | 26 | 1 |
| 2 | 30 | 29 | 1 |
| 3 | 20 | 20 | 0 |
| 20 | 28 | 28 | 0 |
| Mean | 27.5 | 27.4 | 0.1 |
| Std dev | 3.2 | 2.9 | 0.2 |
| Patient | Clinical Depth | AI-Generated Depth | Deviation |
|---|---|---|---|
| 1 | 12 | 11 | 1 |
| 2 | 14 | 13 | 1 |
| 3 | 10 | 10 | 0 |
| 20 | 11 | 11 | 0 |
| Mean | 12.2 | 12.1 | 0.1 |
| Std dev | 1.3 | 1.2 | 0.1 |
| Imaging Technique | Resolution | Three-Dimensional Capabilities | Usability | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Micro-CT | 1–10 µm | Yes | Research and laboratory application | High resolution, detailed microstructure analysis, non-destructive | Time consuming, limited sample size, expensive |
| CBCT | 0.1–0.3 mm | Yes | Clinical application (dentist, etc.) | Lower radiation dose than conventional CT, cost effective, quick scan time | Limited field of view, still involves ionizing radiation, affected by metal artifacts |
| MRI | 0.1–1 mm | Yes | Clinical application (soft tissue, etc.) | Excellent soft tissue contrast, no ionizing radiation, detailed soft tissue imaging | Affected by metal artifacts, expensive, longer scan times |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setiawan, K.; Primarti, R.S.; Sitam, S.; Suridwan, W.; Usri, K.; Latief, F.D.E. Microstructural Evaluation of Dental Implant Success Using Micro-CT: A Comprehensive Review. Appl. Sci. 2024, 14, 11016. https://doi.org/10.3390/app142311016
Setiawan K, Primarti RS, Sitam S, Suridwan W, Usri K, Latief FDE. Microstructural Evaluation of Dental Implant Success Using Micro-CT: A Comprehensive Review. Applied Sciences. 2024; 14(23):11016. https://doi.org/10.3390/app142311016
Chicago/Turabian StyleSetiawan, Krisnadi, Risti Saptarini Primarti, Suhardjo Sitam, Wawan Suridwan, Kosterman Usri, and Fourier Dzar Eljabbar Latief. 2024. "Microstructural Evaluation of Dental Implant Success Using Micro-CT: A Comprehensive Review" Applied Sciences 14, no. 23: 11016. https://doi.org/10.3390/app142311016
APA StyleSetiawan, K., Primarti, R. S., Sitam, S., Suridwan, W., Usri, K., & Latief, F. D. E. (2024). Microstructural Evaluation of Dental Implant Success Using Micro-CT: A Comprehensive Review. Applied Sciences, 14(23), 11016. https://doi.org/10.3390/app142311016






