Abstract
The antidiabetic drugs metformin, glipizide and gliclazide have been used for many years to control blood glucose levels. In recent years, they have gained importance in non-diabetic pharmacological purposes including cancer and hearing loss treatment. Co-administration of these therapeutics represents a challenge to some clinicians seeking an efficient, sensitive and rapid analytical method to use in the pharmacokinetic studies and the therapeutic monitoring of these agents. This research outlines the development and validation of a new precise, robust, sensitive, selective and rapid ion-pairing reversed-phase HPLC method for the simultaneous determination of a ternary mixture of metformin, glipizide and gliclazide in the same isocratic chromatographic run within 5 min. The limits of detection were 59.22 ng/mL for metformin, 169.48 ng/mL for glipizide and 151.29 ng/mL for gliclazide. The method was applied in quantifying metformin uptake by the auditory cell line HEI-OC1, to gain an insight into the kinetics of this biguanide in the organ of Corti. Metformin exhibited a concentration-dependent uptake by HEI-OC1 cells up to 5 mM, after which, saturation of the uptake was noticed. When HEI-OC1 cells were subjected to diabetes-simulated conditions, metformin was able to mitigate the hyperglycaemic stress and revealed a protective role in this cell line.
1. Introduction
Metformin (a biguanide) and sulfonylureas (such as glipizide and gliclazide) were clinically proven as efficient therapeutics for diabetes decades ago []. The literature reveals that these agents have a good safety profile and could be repurposed for various pharmacological applications beyond glycaemic control [,,]. Glipizide was recently reported to ameliorate some neurodegenerative disorders []. Suppression of tumour growth and inhibition of angiogenesis were also reported with glipizide [], which has been investigated in breast cancer [], lung cancer [] and prostate cancer []. Gliclazide was reported to have a unique antioxidant profile [,], with some studies highlighting its vascular benefits [,] and neuroprotective effect in peripheral neuropathy [] and diabetic atherosclerosis []. Recent studies revealed the potential therapeutic role of gliclazide in lung adenocarcinoma [] and colorectal cancer []. Metformin, on the other hand, is a pleotropic agent that plays a multifaceted beneficial role in aging, cancer, neuroprotection, inflammation and polycystic ovary syndrome, and ameliorating the oxidative stress [].
Co-administration of biguanides and sulfonylureas in diabetes management is frequently reported. The new pharmacological potentials of these therapeutics necessitate the availability of a quick, reliable, sensitive and easily accessible analytical tool for the analysis or the therapeutic monitoring of these agents. Several HPLC methods were reported for the analysis of these analytes, either individually [,,] or with other therapeutics [,,,,,]. Some of these reported methods are complicated [,], or are time consuming [,], or require special columns [] or exhibit unsatisfactory detection sensitivity for the analyte(s) of interest []. Unlike gas chromatography and mass spectroscopy, reversed-phase HPLC methods are still the preferred tool for quality control and clinical laboratories for routine analysis and therapeutic drug monitoring of various pharmacological agents, as they do not require special instrumentation, and the analysis could be achieved in a reasonable time [,]. The most common HPLC approaches in speeding up the analysis run are either increasing the flow rate of the mobile phase or shortening the stationary phase length (chromatographic columns) []. In fact, these approaches could partially enhance the throughput of individual analytes without improving the analytical run time for samples of mixed agents [].
Conventional reversed-phase HPLC methods on C8 and C18 columns can be satisfactorily used in the analysis of semi-polar and ionic apolar agents such as sulfonylureas or even non-ionic substrates [,]. However, retaining metformin on reversed-phase chromatographic columns is quite challenging due to its high polarity and small molecular size (minimal interaction/partitioning with the non-polar free silanol groups of the stationary phase) [,]. The reversed-phase ion-pairing HPLC technique is an alternative approach that has versatile applications in the separation of neutral analytes and inorganic, organic or zwitterionic substrates, and was historically known as soap chromatography and often called ion-interaction chromatography or ion-pair adsorption [,,]. The technique primarily relies on the adsorption of hydrophobic surfactant ions known as ion-pairing solute (added to the mobile phase) to dynamically modify the surface of the stationary phase so that they can interact/couple with analyte ions of opposite charge to improve their retention on C18 columns [,,]. on the other hand, the choice of the organic modifier (methanol or acetonitrile) in the mobile phase helps to predict and customise the elution pattern of analytes of same charge by improving their solubility as well as the wettability of the stationary phase [,,]. Some studies reported the retention and analysis of metformin using this technique [,,]; however, delayed metformin retention time and the limited sensitivity of some methods are considered among the method’s limitations [,,].
One objective of this study was to achieve a cost-effective, rapid and sensitive HPLC method for the analysis of the ternary mixture of metformin, glipizide and gliclazide on the same chromatographic run and validate this method as per the International Conference of Harmonisation (ICH) []. The other objective was to investigate the uptake of metformin by an auditory cell line HEI-OC1 using this method and eventually to study the effect of metformin in these cells when exposed to simulated diabetes conditions. Previous in vitro studies on the auditory cell line HEI-OC1 have revealed the protective role of metformin against the ototoxicity caused by aminoglycoside [,,,] and platinum-based anti-cancer therapeutics [,]. These protective effects of metformin could be attributed to the antioxidant effect of this biguanide []; however, the exact mechanism of metformin’s action and its pharmacokinetics in the organ of Corti are still unknown. Of importance, some histopathological reports and recent molecular and biochemical studies shed light on the deteriorating impact of diabetes on auditory function [,]. Neuropathy and microangiopathy were reported as major etiologies of hearing malfunctioning in diabetes [,]. However, the literature showed a dearth in knowledge about the vasculoprotective and neuroprotective effects of metformin in the diabetic inner ear in vitro and in vivo. To the best of our knowledge, this paper is the first to quantify metformin uptake by the auditory cell line HEI-OC1 in vitro and is the first to investigate the effect of this interesting biguanide in an in vitro diabetic inner ear model. This could potentially assist in designing a reliable and well-characterised in vitro diabetic model using the HEI-OC1 cell line to help investigate auditory malfunctioning caused by diabetes and give an insight into the role of metformin in auditory organ protection, the molecular mechanisms of metformin and its pharmacokinetics in the inner ear niche.
2. Materials, Chemicals, Methods and Cell Line
Glipizide > 98%, gliclazide > 98%, metformin hydrochloride 97% and HPLC-grade acetonitrile were purchased form Thermo Fisher Scientific (Melbourne, Australia). Analytical reagent-grade potassium dihydrogen orthophosphate (KH2PO4), DMEM and sodium dodecyl sulfate (SDS) were procured from Sigma-Aldrich (St. Louis, MO, USA). Minidiab® (glipizide 5 mg tablets, Pfizer pharmaceuticals, Sydney, Australia), APX-gliclazide® (gliclazide 80 mg tablets, Arrotex pharmaceuticals, Cremorne, Australia) and Diaformin® (metformin 500 mg tablets, Viatris pharmaceuticals, Carole Park, Australia) were obtained from a local pharmacy. Sodium hydroxide (NaOH, analytical grade) was obtained from Ajax Fine Chemicals Pty Ltd. (Melbourne, Australia). Deionised water (from a Milli-Q ultra-pure water system (Millipore, Australia) was used in the mobile phase preparation and sterilised for the cell culture experiments. HEI-OC1 cell lines were obtained from ATCC (the American Tissue Culture Collection, Gaithersburg, MD, USA). A CellTitre-Blue kit was obtained from (Promega, NSW, Australia).
2.1. Instrumentation and Mobile Phase Used
The chromatographic conditions for the optimised run and HPLC instrumentation is summarised in Table 1.

Table 1.
Optimised chromatographic conditions.
2.2. Preparation of Stock Solutions of Individual Analytes
In a 100 mL volumetric flask, an accurate weight of 50 mg of metformin was dissolved in 50 mL of mobile phase mixture and sonicated for 10 min, and the final volume was made up to 100 mL using the mobile phase mixture to obtain metformin stock solution, (MT flask). Glipizide stock solution (GP flask) and gliclazide stock solution (GC flask) were prepared the same way, by dissolving 50 mg of glipizide and 50 mg of gliclazide, respectively, in their own flasks.
2.3. Preparation of Working Solutions of Individual Analytes and the Ternary Mixture
An aliquot of 1 mL from MT flask was transferred into a 20 mL flask, and the final volume was made up to 20 mL with the mobile phase mixture to create Metformin 25 µg/mL. Similarly, glipizide 25 µg/mL and gliclazide 25 µg/mL were prepared by transferring an aliquot of 1 mL from GP flask and 1 mL from GC flask, respectively, into two different 20 mL flasks and the final volume was made up to 20 mL with the mobile phase mixture. The ternary mixture flask was made by transferring 1 mL of each analyte (1 mL from MT flask + 1 mL from GP flask + 1 mL from GC flask) into a 20 mL flask, and the final volume was made up to 20 mL with the mobile phase mixture.
2.4. Method Development and Validation
2.4.1. Method Development, Wavelength Selection and Optimisation
The physicochemical properties of metformin (basic, water soluble) and the sulfonylureas (acidic, water insoluble) constitute a major challenge in the chromatographic separation of these analytes in the same chromatographic run. The polar hydrophilic nature of metformin (pKa = 2.8, 11.5) [] constituted another level of challenge to retain such small molecules on conventional C18 columns. The separation of both glipizide and gliclazide in the same chromatographic run seems to be problematic, too, which could be attributed to the similar dissociation constants they possess (pKa = 5.8 for gliclazide, pKa = 5.9 for glipizide) []. A substantial goal of this paper is to achieve a short-time HPLC separation of metformin, glipizide and gliclazide in the same chromatographic run. The decision was made to try the ion-pairing chromatography on a conventional reversed-phase C18 column to achieve this goal due to reported versatility of this technique in the chromatographic separation. The selection of the detection wavelength (λ = 233 nm) was based on a satisfactory response obtained with respect to the previous literature of reported λmax for the three therapeutics. λmax for metformin was reported to be within 233 nm–237 nm [,]. Gliclazide was reported to have λmax at 227 nm–228 nm [,], while 273 nm was reported to be the λmax for glipizide []. Table 2 summarises some of previously reported chromatographic methods for the analysis of metformin, glipizide and gliclazide.

Table 2.
Some reported chromatographic methods used in the analysis of metformin (Mt), glipizide (Gp) and gliclazide (Gc).
2.4.2. Analytical Method Validation
The optimised chromatographic method for the simultaneous analysis of metformin, glipizide and gliclazide was validated in compliance with the International Conference of Harmonisation (ICH) guidelines Q2 (R1) [] with respect to accuracy, specificity, repeatability, limit of detection, precision, linearity, robustness, system suitability and limit of quantification.
2.4.3. Chromatographic System Suitability
Six replicates of the ternary mixture (metformin 25 µg/mL, glipizide 25 µg/mL and gliclazide 25 µg/mL) were injected to evaluate the repeatability of the peak retention time on the chromatographic run, the peak tailing, number of the theoretical plates and the resolution between drug peaks. Data were recorded as mean (n = 6, ±SD), and a % RSD of <2 was set as the acceptance limit for validated results.
2.4.4. Intra-Day Precision (Repeatability) and Intermediate Precision (Inter-Day Precision)
On the same day, and under same operating chromatographic conditions, six independent ternary mixture samples (containing 25 µg/mL of each analyte) were injected to investigate the method and chromatographic system precision (intra-day). The results of six independent ternary mixtures analysed over 3 consecutive days were compared to investigate inter-day precision of the analytical method and the chromatographic system employed. Data were recorded as mean (n = 6, ±SD), and a % RSD of <2 was set as the acceptance limit for validated results.
2.4.5. Analytical Method Linearity and Calibration Curve
The linearity of the analytical method in analysing metformin, glipizide and gliclazide was determined through analysing each analyte separately over two different concentration ranges, an upper limit (1–100 µg/mL) and a lower limit (0.5–10 µg/mL). Triplicate determinations of each concentration were plotted on a calibration curve, and the linearity of the analytical method was assessed from the linear regression equation and the correlation coefficient R2 produced in these two concentration ranges.
2.4.6. Detection and Quantification Limits of the Analytical Method
The slope the calibration curve (S) and the standard deviation of the intercept (Q) obtained from the linear regression equation were used to determine the sensitivity of the analytical procedure for detection (LOD, the limit of detection) and quantification (LOQ, the limit of quantification) of the three analytes using Equations (1) and (2).
2.4.7. Method Robustness
Slight alterations to the chromatographic conditions (buffer pH, mobile phase ratio and flow rate) were made to ensure method robustness through evaluation of the peak tailing, number of theoretical plates and resolution between the three analytes. Data were recorded as mean (n = 3, ±SD) and a % RSD of <2 was set as the acceptance limit for validated results.
2.4.8. Method Selectivity, Specificity, Accuracy and Recovery from Market Tablets
The selectivity, specificity and ability of the analytical method to quantify the individual analytes in marketed products were evaluated using the addition method []. Briefly, 10 tablets of each formulation were separately crushed, and a known amount of the pure therapeutic was added to the powder of the relevant crushed tablets containing the same therapeutic (metformin was added to crushed Diaformin® 500 mg tablets, glipizide was added to crushed Minidiab® 5 mg tablets and gliclazide was added to crushed APX-Gliclazide® 80 mg tablets). The accuracy of the analytical method to quantify and recover every therapeutic agent among tablet excipients was assessed at 3 concentration levels—1 µg/mL, 2 µg/mL and 5 µg/mL—of every analyte separately. The % RSD was recorded for the triplicate determinations (n = 3, ±SD) of theses concentration levels.
2.4.9. Quantification of Metformin Uptake by the House Ear Institute-Organ of Corti HEI-OC1 Cell Line
Using T75 flasks and under permissive conditions (10% CO2 and 33 °C, Heracell VIOS 160i CO2 incubator, ThermoScientific, Langenselbold, Germany), cells were cultured in high glucose (Dulbecco’s Modified Eagle’s Medium, DMEM, Sigma Aldrich) supplemented with 1% penicillin/streptomycin solution and 10% foetal bovine serum. The medium was changed every two days until the cells reached 75% confluency. Cells were trypsinised, collected and seeded in a 96-well plate at 1000 cell/well density for 24 h to attach. Cells were then treated for 24 h with metformin at different concentrations (1 mM, 2.5 mM, 5 mM and 10 mM) in quadruplets for every concentration. DMEM containing residual metformin from the 96-well plate was collected and transferred into HPLC vials; 1 mL of mobile phase mixture was added, vortexed for 1 min and freeze-dried for 24 h using (ScanVac Coolsafe 55-4 pro freeze dryer, Lynge, Denmark). The dried content of the HPLC vials was reconstituted in 1 mL of the mobile phase mixture with vigorous shaking, vortexed for 5 min and filtered through a syringe filter before HPLC quantification. For reliability and reproducibility, the cell seeding and metformin treatment procedures were conducted on two separate experimental days, and metformin uptake by HEI-OC1 was calculated through Equations (3) and (4). Data are presented as mean (n = 4, ±SD) for every metformin concentration over the two experimental days.
where Mu is Metformin uptake (µg/mL)
- Mi is the Initial metformin added to treat HEI-OC1 cells (µg/mL)
- Md is the metformin detected in DMEM dried vials (µg/mL)
2.4.10. Cell Viability Assay
HEI-OC1 cells were cultured under the conditions outlined in Section 2.4.9. Cells were trypsinised, collected and seeded in a 96-well plate at 1000 cell/well density for 24 h to attach prior to subsequent treatment. HEI-OC1 cells were exposed to 25 mM glucose (HG) to mimic diabetic conditions [,,], to metformin only at concentrations 2.5 mM, 5 mM and 10 mM (M2.5, M5 and M10, respectively) and to combination treatments (HG + M2.5), (HG + M5) and (HG + M10). Control cells were only exposed to fresh culture medium without any other treatments (CTRL). A viability assay of HEI-OC1 cells was determined by the CellTitre-Blue kit (Promega, NSW, Australia) at 560–590 nm wavelength [] using a PerkinElmer microplate reader (PerkinElmer, Waltham, MA, USA). The viability of HEI-OC1 cells was determined as mean % (n = 4, ±SD) of treated cells with respect to CTRL as per Equation (5).
Viability assay of treated HEI-OC1 cells was conducted after 2 days, 3 days, 4 days and 5 days from treatment. And the whole experiment was repeated one more time for reproducibility.
3. Results and Discussion
3.1. Method Development and Optimisation
Many trials were made on different C18 columns, such as (Econosil silica (250 × 4.6 mm, 10 µm, Econosil C18 (250 × 4.6 mm, 10 µm) and Phenomenex LUNA C18 (250 × 4.6 mm, 10 µm); the chromatographic separation of the three analytes in the same chromatographic run could not be achieved. However, sharp peaks in short time were obtained on Alltech Apollo C18 (150 mm × 4.6 mm, 5µm). The use of acetonitrile in the presented ion-pair HPLC method gave better chromatographic and analytical outcomes over methanol. Initial attempts at pH (7–7.5) close to the pKa of the phosphate (to have a good buffering capacity) did not give satisfactory chromatographic separation of the ternary mixture, although optimisation for individual analytes seemed to be achievable.
In their optimisation of an ion-pairing method for the separation of metformin (pKa = 2.8, 11.5) and rosiglitazone (pKa = 6.1, 6.8) [] on a similar column, Kolte et al. reported that good resolution between metformin and rosiglitazone peaks was obtained by decreasing the pH from 7.5 to 7.1 []. Thus, it was decided, in this study, to decrease the pH to 5.5, where metformin HCl is reported to be fully ionised in its cationic form [], which enables the ionised biguanide to couple/ion pair and efficiently retained with the anionic SDS moieties adsorbed on the silica of the C18 column. Also, at pH = 5.5, which is very close to the pKa of gliclazide and glipizide, 5.8 and 5.9, respectively, around 50% of the sulfonylureas are ionised and can be retained satisfactorily with ion-pairing chromatography on the same column. We found that the concentration of SDS of 0.03% is optimal for ion-pairing metformin in a very short time without affecting the retention times of glipizide or gliclazide. Also, this low concentration of SDS did not cause the precipitation (snow-like effect) reported by Troja et al. (resulting from a side interaction of SDS with KH2PO4 buffer) []. Below 0.03%, we could not retain metformin. Also, concentrations above 0.03% resulted in delayed metformin retention and sulfonylureas peaks brought forwards (overlapped), and had a bad resolution with metformin peak; similar findings were reported by Kolte et al. and others [,,,].
In this paper, a very short HPLC run for the individual analytes was shown in Figure 1, and a chromatogram of the ternary mixture was presented in Figure 2, showing sharp and symmetric peaks with good separation between the three therapeutics analysed, with the optimised chromatographic conditions outlined in Table 1 and eluted within 5 min.
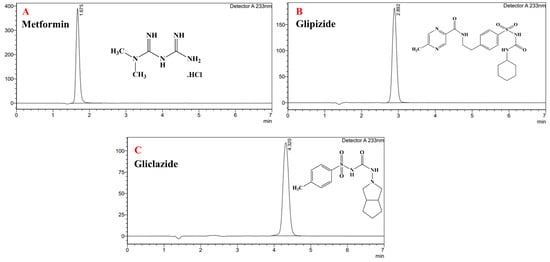
Figure 1.
Individual peaks for metformin 25 µg/mL (A), glipizide 25 µg/mL (B) and gliclazide 25 µg/mL (C).
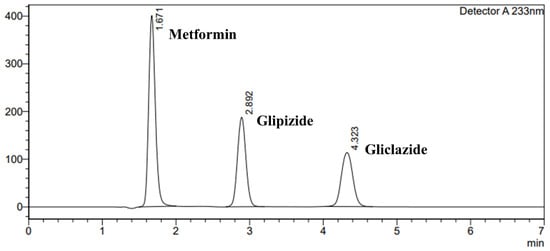
Figure 2.
Chromatogram of ternary mixture of metformin 25 µg/mL:glipizide 25 µg/mL:gliclazide 25 µg/mL.
3.2. Method Validation
3.2.1. Chromatographic System Suitability
Metformin, glipizide and gliclazide were repeatedly retained at 1.675 min ± 0.002, 2.895 min ± 0.004 and 4.329 min ± 0.010, respectively. The metformin peak exhibited a tailing factor of 1.200 ± 0.002, the glipizide peak had a tailing factor of 1.055 ± 0.002, while that for gliclazide was 1.058 ± 0.004, indicating sharp and symmetric drug peaks. The three analyte peaks were well separated, where the resolution between the metformin and glipizide peaks was 6.153 ± 0.020, and the resolution between the glipizide and gliclazide peaks was 5.658 ± 0.006. In all reported results, % RSD never exceeded 0.006%, indicating the suitability of the chromatographic conditions and the HPLC system for analysis of metformin, glipizide and gliclazide. Results are recorded in Table 3 and presented in Figure 2.

Table 3.
System suitability results for the analysis of the ternary mixture of metformin (Mt), glipizide (Gp) and gliclazide (Gc).
3.2.2. Intra-Day Precision (Repeatability) and Intermediate Precision (Inter-Day Precision)
Repeatable and precise peak areas of the three analytes were obtained, where intra-day peak areas for metformin, glipizide and gliclazide were 2,524,883 ± 2430.12, 1,497,714.17 ± 3171.46 and 1,225,869.50 ± 2266.39, respectively.
Inter-day peak areas of the three analytes also showed high precision and repeatability of the analytical method where % RSD never exceeded 0.006% over 3 consecutive days in accordance with ICH [] (% RSD < 2). Intra and inter-day precision are presented in Table 4.

Table 4.
Repeatability results and inter-day precision results of the produced peak areas of independent replicates of metformin (Mt), glipizide (GP) and gliclazide (Gc) over 3 consecutive days.
3.2.3. Analytical Method Linearity and Calibration Curve
The developed method showed good linearity response for all tested analytes over the tested concentration ranges verified by the correlation coefficients (R2 close to 1) for all of the constructed calibration curves.
Over the upper limit (1–100 µg/mL), the linear regression equations were (y = 97,631x + 10,949, R2 = 1) for metformin, (y = 57,676x + 14,145, R2 = 0.9997) for glipizide and (y = 48,226x − 15,022, R2 = 0.9999) for gliclazide.
The linear regression equations over the lower limit (0.5–10 µg/mL) were (y = 94,142x + 4364.1, R2 = 0.9998) for metformin, (y = 61,174x + 2038.8, R2 = 0.9996) for glipizide and y = 47,137x − 12,977, R2 = 0.9965) for gliclazide. Figure 3A,B exhibit the calibration curves constructed for the three analytes over the upper and lower concentration ranges, respectively.
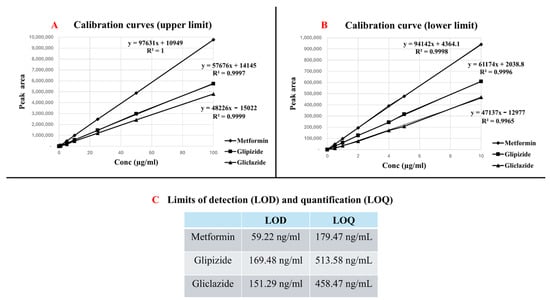
Figure 3.
HPLC method linearity and sensitivity. (A) represents calibration curves (upper limit), (B) represents calibration curves (lower limit), and (C) represents limit of detection (LOD) and limit of quantification (LOQ) for the three analytes.
3.2.4. Detection and Quantification Limits of the Analytical Method
The standard deviation of the intercept and the slope of the calibration obtained from the linear regression equation revealed good sensitivity of the analytical method, where the estimated limits of detection (LODs) were 59.22 ng/mL, 169.48 ng/mL and 151.29 ng/mL for metformin, glipizide and gliclazide, respectively, while the limits of quantification (LOQs) were 179.47 ng/mL for metformin, 513.58 ng/mL for glipizide and 458.47 ng/mL for gliclazide. Figure 3C represents the LODs and LOQs for the three analytes.
3.2.5. Method Robustness
The changes introduced to the chromatographic conditions (flow rate, pH and mobile phase ratio) did not reveal any significant changes to peak retention times, sharpness of the peaks, peak symmetry (tailing factor is still <2) or the resolution between the analyte peaks of the ternary mixture, indicating robustness of the HPLC method in analysis and separation of metformin, glipizide and gliclazide. The % RSD in all cases never exceeded 0.009% (acceptance limit % RSD < 2). The results of the robustness studies are presented in Table 5.

Table 5.
Robustness results showing the chromatographic parameters of metformin (Mt), glipizide (GP) and gliclazide (Gc) following the changes made to the pH of the mobile phase, changes made to the ratio of the mobile phase components (A and B) and the flow rate of the chromatographic run.
3.2.6. Method Selectivity, Specificity, Accuracy/Trueness and Recovery from Market Tablets
The proposed analytical method showed good selectivity and specificity to detect and quantify metformin, glipizide and gliclazide among the unknown excipients used in the marketed tablets of these agents. The method also showed good accuracy/trueness in quantifying and recovering these analytes from crushed tablet excipients. Method trueness/recovery was verified by the close experimental data detected for these agents to the claimed theoretical values at three different concentration levels (1 µg/mL, 2 µg/mL and 5 µg/mL) determined in triplicates. Across these concentration levels, metformin recovery % ranged between 99.01% ± 1.01 and 100.01% ± 0.82, glipizide had a recovery % that ranged between 99.61% ± 0.52 and 100.31% ± 0.96 while gliclazide was recovered at values ranged between 97.62% ± 2.04 and 98.52% ± 0.92. Recovery % results are presented in Figure 4.
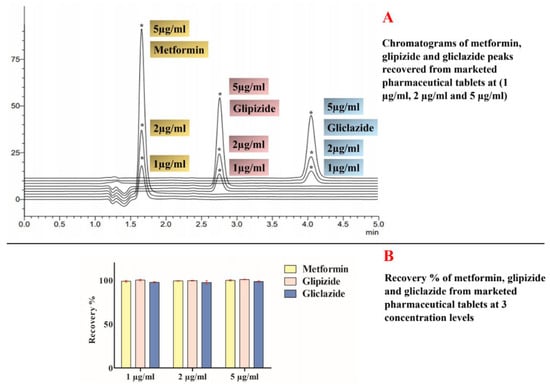
Figure 4.
Recovery results for metformin, glipizide and gliclazide at three different concentrations (1 µg/mL, 2 µg/mL and 5 µg/mL). (A) represents chromatograms of recovered analytes from marketed pharmaceutical products and (B) represents recovery % of these analytes from marketed tablets at three different concentration levels (1 µg/mL, 2 µg/mL and 5 µg/mL). (*) in this image stands for the peaks of metformin, glipizide and gliclazide recovered from their marketed pharmaceutical products at different concentration levels and appearing at the same retention time for every analyte.
3.2.7. Quantification of Metformin Uptake by HEI-OC1
Good viability of HEI-OC1 to different metformin concentrations was noticed with respect to control non-treated cells (Figure 5). Two-way ANOVA results revealed that metformin concentrations are significant variables in the experimental uptake results, while the experimental day outcomes are not. Bonferroni post hoc tests also revealed that increasing metformin concentration resulted in significant uptake of HEI-OC1 cells of the supplemented biguanide up to 5 mM concentration, after which, 10 mM metformin did not improve the cellular uptake by HEI-OC1 cells (Figure 5A,B). Exposure of HEI-OC1 cells to 1 mM metformin resulted in 19.34 µg/mL ± 2.23 uptake, which was increased to 51.67 µg/mL ± 7.96 upon exposure to 2.5 mM metformin (Figure 5A). When HEI-OC1 cells were exposed to 5 mM metformin, the cellular uptake of the biguanide significantly increased to 373.32 µg/mL ± 24.03, after which, the cellular uptake of HEI-OC1 to metformin slightly increased to 377.82 µg/mL ± 5.06 upon exposure to 10 mM concentration (Figure 5A).
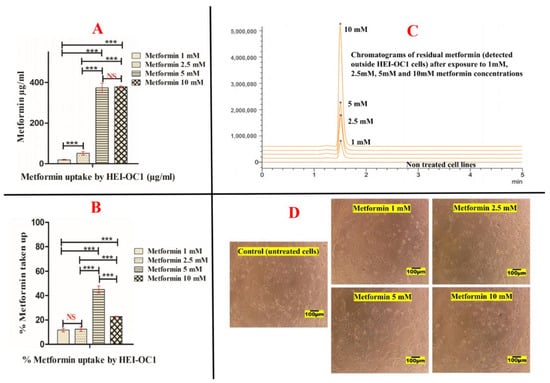
Figure 5.
Uptake of metformin by the auditory cell line (HEI-OC1). (A) represents metformin cellular uptake in µg/mL, (B) represents metformin cellular uptake in %, (C) represents chromatograms of residual metformin detected outside the auditory cells and (D) represents images of untreated (HEI-OC1) and cells exposed to different concentrations of metformin (1 mM, 2.5 mM, 5 mM and 10 mM). (***) and (NS) in subfigures (A,B) represent the level of statistical significance of the results. (***) means highly significant when p-value < 0.001. (NS) means statistically non significant. (*) in subfigure (C) represent the peaks produced by metformin detected outside HEI-OC1 cells after exposure to different concentrations at the same retention time.
These results were also presented in Figure 5B, which shows that exposure of HEI-OC1 to 1 mM resulted in 11.68% ± 1.34 uptake of metformin with respect to original metformin supplemented. The uptake was slightly increased to 12.48% ± 1.93 with exposure to 2.5 mM metformin. Cellular uptake of metformin by HEI-OC1 was significantly increased to 45.08% ± 2.90 when metformin concentration increased to 5 mM. On the contrary, when HEI-OC1 cells were exposed to 10 mM metformin, their cellular uptake was only 22.81% ± 0.31 with respect to the initial metformin concentration added (Figure 5B). Although 10 mM metformin did not harmfully affect the cell viability of HEI-OC1 cells, metformin cellular uptake was not significantly improved. These data suggest saturation of the cellular uptake of HEI-OC1 to the biguanide above 5 mM concentration.
The otoprotective effect of metformin was previously investigated on HEI-OC1 at several concentrations (1 mM) [], (2 mM) [] and (5 mM) []. In this paper, we tried 10 mM metformin concentration to investigate the cellular uptake of metformin by HEI-OC1 cell lines in addition to the previously reported concentrations. Cellular metformin uptake was reported to be mediated through different types of cellular transporters such as the organic cation transporters (OCTs), carnitine/organic cation transporter (OCTN1), thiamine transporter (THTR-2), serotonin reuptake transporter (SERT), plasma membrane monoamine transporter (PMAT) and multidrug and toxin extrusion transporters (MATEs) []. Kimura et al. investigated the transport/uptake of radiolabelled metformin on HEK-293 cell lines (a transformed type of embryonic human kidney cell line) []. They reported concentration-dependent uptake of the biguanide mediated through OCT2 that is saturated by increasing metformin concentration []. To a lesser extent, OCT1-mediated uptake of metformin was also reported in HEK-293 cell lines transfected with both OCT1 and OCT2 [].
In a similar investigation on the Chinese hamster ovary cell lines (CHO-K1) transfected with rat OCT1, Wang et al. reported a saturable and time-dependent uptake of metformin []. Their in vivo results also revealed that metformin uptake was 30 times higher in wild-type mice compared to the OCT1 knocked-down group []. OCT1 was also involved in the lactic acidosis associated with metformin []. Altered metformin pharmacokinetics were also reported by ablation of both OCT1 and OCT2 in another study [].
Of significance, OCT2 was reported to be expressed in the cochlear organ of Corti [,]. The ototoxicity and nephrotoxicity associated with cisplatin chemotherapy is reported to be mediated through OCT2 [,]. Ciarimboli et al. highlighted the significance of OCT2 as a therapeutic target for cisplatin toxicity through competitive cation trafficking []. Quantitative PCR results also revealed the expression of OCT1 in HEI-OC1 cells and localisation of OCT1 as well as OCT2 in mouse cochlea []. In their investigation of the metformin otoprotective effect against cisplatin toxicity on HEI-OC1 cells, Chang et al. suggested the possibility of competitive uptake between the hydrophilic cations (metformin and cisplatin) for mediating transporter(s) []. To date, the exact uptake mechanism of metformin by HEI-OC1 has not been reported, and the exact transporters/carriers involved in this uptake by HEI-OC1 cells are not fully understood. Our results showed a concentration-dependent and saturable pattern of metformin uptake by HEI-OC1 cells (Figure 5), and further investigations are needed to reveal whether metformin uptake by HEI-OC1 cells is mediated by OCT1, OCT2 or both or different transporter(s), and what factors could affect this uptake in healthy and diseased conditions. The validated HPLC method outlined herein could be a useful tool in the quantification process of metformin uptake in HEI-OC1 cells or other cell lines and can be trialled for the quantification of the uptake of glipizide and gliclazide in similar cell culture experiments.
3.2.8. Cell Viability Results
Initial investigation of exposing HEI-OC1 cells for 24 h to diabetic conditions (25 mM glucose, HG; 2.5 mM metformin, M2.5; 5 mM metformin, M5; and 10 mM metformin, M10) as well as the combined treatments (HG + M2.5, HG + M5 and HG + M10) did not reveal any changes to the cell viability detected by CellTitre-Blue assay. The decision was made to expose these auditory cell lines to all treatment conditions over a few days (longer duration) to allow the cells to metabolise the high glucose added and to investigate whether metformin could mitigate the stressing conditions created by the simulated diabetic state. This constitutes a more clinically relevant setting of hyperglycaemia. In general, treatment type (drug dose) results were considered highly significant (p < 0.0001). Metformin was not cytotoxic up to the concentration 10 mM to HEI-OC1 after 4 days of exposure (Figure 6).
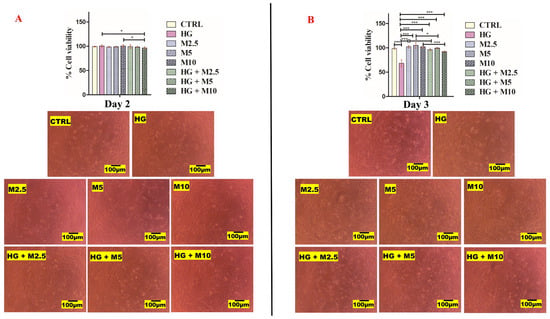
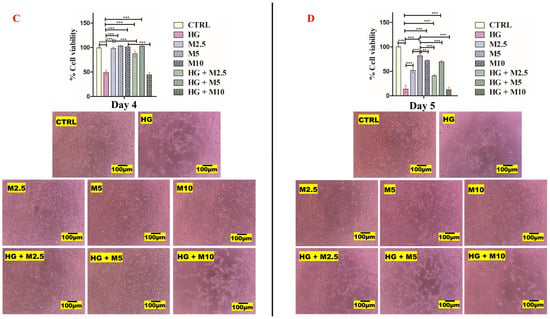
Figure 6.
(A,B). HEI-OC1 cell viability results. (A) After 2 days and (B) after 3 days of treatment with: no treatment (CTRL), high glucose, 25 mM (HG), metformin 2.5 mM (M2.5), metformin 5 mM (M5), metformin 10 mM (M10) and combination treatments (HG + M2.5), (HG + M5) and (HG + M10). (C,D). HEI-OC1 cell viability results. (C) After 4 days and (D) after 5 days of treatment with: no treatment (CTRL), high glucose, 25 mM (HG), metformin 2.5 mM (M2.5), metformin 5 mM (M5), metformin 10 mM (M10) and combination treatments (HG + M2.5), (HG + M5) and (HG + M10). (*), (**) and (***) represent level of statistical significance of results. (*) when p-value < 0.05, statistical results are significant. (**) when p-value < 0.01, statistical results are very significant and more significant than (*). (***) when p-value < 0.001, statistical results are highly significant and more significant than (*) and (**).
After 2 days of HEI-OC1 exposure to all treatments, all cells showed more than 96.5% (±2.52) viability. Cells exposed to 25 mM glucose (HG treated cells) showed slightly higher viability 99.75% (±1.71). All other treated cells showed cell viability ranging between 96.5% (±2.52) and 100.5% (±3). Neither the experimental day results nor the treatment type after 2 days of treatment were considered significant. The Bonferroni post hoc test revealed that only (HG versus HG + M10) and (M10 versus HG + M10) results were statistically significant, where the p value was <0.05 (Figure 6A).
Day 3 results post treatment revealed that cells exposed to 25 mM glucose (HG treated cells) had the lowest viability of all treatments: 68% (±7.26). Cells exposed to metformin 5 mM (M5) showed the highest cell viability of all treatments: 103% (±3.50). The cell viability of M2.5 and M10 were 99% (±2.58) and 102% (±2.83), respectively. Metformin seemed to mitigate the stressing effect of HG added. We noticed that, when metformin was co-added into HG treated cells, viability was improved to 95.5% (±1.92) in HG + M2.5, to 98% (±1.63) in HG + M5 and to 90.5% (±1.0) in HG + M10 (Figure 6B).
Likewise, day 4 results post treatment revealed that cells exposed to 25 mM glucose (HG-treated cells) appeared more stressed and had a viability of 48.75% (±4.79). However, all metformin-treated cells looked healthy and attached to each other. Cells exposed to metformin 5 mM (M5) showed the highest cell viability of all treatments: 101.75% (±1.26). The cell viability of M2.5 and M10 were 97.5% (±1.91) and 99% (±2.0), respectively. Metformin at the concentrations 2.5 mM and 5 mM seemed to mitigate the deteriorating effect of HG in HEI-OC1 cells. However, this ameliorating effect of metformin was not noticed at 10 mM concentration, and the cells looked as stressed as HG-only-treated cells. When metformin was co-added into HG treated cells, viability was improved to 87.25% (±6.08) in HG + M2.5, and to 102% (±1.41) in HG + M5. However, cell viability dropped to 44.5% (±4.44) in HG + M10 (Figure 6C).
After 5 days of exposure of HEI-OC1 cells to all treatments, only cells exposed to metformin 5 mM (M5) looked viable (more attached and confluent) than M2.5- and M10-treated cells, where cell viability of M5-treated cells was 77.5% (±4.12). The cell viability of M2.5 and M10 were 48.5% (±3.42) and 67.5% (±3.42), respectively. Cells exposed to 25 mM glucose (HG-treated cells) looked very stressed and had a cell viability of only 13.5 % (±5.45). Metformin seemed to partially mitigate the harmful effect of HG at the concentration of 5 mM, where the cell viability of (HG + M5)-treated cells was 67.5% (±3.42). This beneficial effect of metformin was not noticed in other concentrations, where the cell viability of (HG + M2.5) and (HG + M10) did not exceed 35.5% (±2.52) and 10% (±4.32), respectively (Figure 6D).
Activating AMPK and inhibiting complex I of the mitochondria by metformin was shown to play a crucial role in protecting the auditory function from insults to the organ of Corti []. Improved mitochondrial function, mitochondrial biogenesis and proliferation are evidenced effects of metformin [,]. The antioxidant characteristics of metformin and its role in controlling the oxidative surge (uncontrolled production of free radicals) are also well reported [,]. Prevention of AGE accumulation and suppression of the AGE/RAGE pathway are also reported effects with metformin therapy [,]. Inhibitory action to caspase 3 activation and poly-ADP-ribose polymerase levels was reported as a protective effect of metformin from the toxicity caused by cisplatin to HEI-OC1 cell lines [], while activation of the autophagy process by AMPK/FOXO3a was reported as another protective pathway of metformin against cisplatin toxicity by others []. Jung et al. reported that the inhibited expression of caspase 3 and the cleavage of poly-ADP ribose polymerase as a protective pathway of metformin from gentamicin toxicity to HEI-OC1 cell lines []. Our results reveal the novel protective effect of metformin to HEI-OC1 cell lines from hyperglycaemic conditions, and further studies are needed to elucidate the exact biochemical interventions that metformin exerts in HEI-OC1 cell lines under simulated diabetes conditions and the exact pathway(s) involved in this protective effect.
4. Statistical Analysis
Microsoft Excel (Microsoft Office, version 2402) and GraphPad Prism software (GraphPad Inc., La Jolla, CA, USA, version 5) were used for the statistical analysis and graphical presentation of the statistical analysis of mean ± SD triplicate data for chromatographic results and mean ± SD quadruplet data for cell line work using raw means/totals and two-way ANOVA with Bonferroni post-test, considering a p value < 0.05 to be statistically significant.
5. Conclusions and Future Directions
A precise, accurate, selective and cost-effective ion-pair HPLC tool was developed and validated for the simultaneous analysis of a ternary mixture of metformin, glipizide and gliclazide. The method can be applied for the detection of these analytes either individually or in combinations (in bulk or in pharmaceutical formulations). The process was also able to quantify the metformin uptake by the auditory cell line HEI-OC1 that showed a concentration-dependent and saturable pattern. This analytical procedure can be trialled and customised for similar experiments in other cell lines, and even in vivo, for the therapeutic monitoring of these drugs. Metformin does not seem to be cytotoxic to HEI-OC1 cells at concentrations of 2.5 mM, 5 mM and 10 mM for up to 4 days of treatment. Metformin seemed to exert an otoprotective effect against high glucose-induced stress and maintained the cell viability of HEI-OC1 cell lines at 2.5 mM and 5 mM concentrations after 4 days of exposure, after which, that effect declined noticeably. Our results warrant further studies to elaborate the exact mechanism(s) of this interesting biguanide in the protection of the inner ear environment against diabetes, and necessitate the evolution of novel delivery systems that target metformin directly to the inner ear niche to maximise its therapeutic effects and overcome the pharmacokinetic limitations associated with its oral administration.
Author Contributions
Conceptualization, A.G.; Methodology, A.G.; Software, A.G.; Validation, A.G.; Formal analysis, A.G.; Investigation, A.G.; Resources, H.A.-S. and C.R.D.; Data curation, A.G.; Writing—original draft, A.G.; Writing—review & editing, H.A.-S. and C.R.D.; Project administration, C.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The Authors thank Federico Kalinec (University of California, USA) and Young Joon Seo (University of Wonju, South Korea) for their assistance in acquiring the HEI-OC1 cell line. AG was supported by an Australian government RTP scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quianzon, C.C.; Cheikh, I.E. History of current non-insulin medications for diabetes mellitus. J. Community Hosp. Intern. Med. Perspect 2012, 2, 19081. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kim, J.-R.; Choi, H.C. Gliclazide, a KATP channel blocker, inhibits vascular smooth muscle cell proliferation through the CaMKKβ–AMPK pathway. Vascul. Pharmacol. 2018, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Li, B.; Yang, Y.; Yang, Y.; Li, J.; Zhou, Q.; Wen, Y.; Zeng, C.; Zheng, L.; Zhang, Q. Glipizide suppresses prostate cancer progression in the TRAMP model by inhibiting angiogenesis. Sci. Rep. 2016, 6, 27819. [Google Scholar] [CrossRef] [PubMed]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Role of metformin in various pathologies: State-of-the-art microcapsules for improving its pharmacokinetics. Ther. Deliv. 2020, 11, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Sarkar, S. Glipizide ameliorates human poly (Q) mediated neurotoxicity by upregulating insulin signalling in Drosophila disease models. Biochem. Biophys. Res. Commun. 2023, 645, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhou, Q.; Li, B.; Yang, Y.; Cao, L.; Ye, Y.; Li, J.; Ding, Y.; Wang, H.; Wang, J. Glipizide, an antidiabetic drug, suppresses tumor growth and metastasis by inhibiting angiogenesis. Oncotarget 2014, 5, 9966–9979. [Google Scholar] [CrossRef]
- Mao, G.; Zheng, S.; Li, J.; Liu, X.; Zhou, Q.; Cao, J.; Zhang, Q.; Zheng, L.; Wang, L.; Qi, C. Glipizide combined with ANP suppresses breast cancer growth and metastasis by inhibiting angiogenesis through VEGF/VEGFR2 signaling. Anticancer Agents Med. Chem. 2022, 22, 1735–1741. [Google Scholar] [CrossRef]
- Nazim, U.M.; Moon, J.-H.; Lee, Y.-J.; Seol, J.-W.; Kim, Y.J.; Park, S.-Y. Glipizide sensitizes lung cancer cells to TRAIL-induced apoptosis via Akt/mTOR/autophagy pathways. Oncotarget 2017, 8, 100021–100033. [Google Scholar] [CrossRef]
- Alp, H.; Varol, S.; Celik, M.M.; Altas, M.; Evliyaoglu, O.; Tokgoz, O.; Tanrıverdi, M.H.; Uzar, E. Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. J. Diabetes Res. 2012, 2012, 230342. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Shi, L.-L.; Wu, Y.-J.; Xu, W.-H.; Wang, L.; Ren, M.-S. Protective effect of gliclazide on diabetic peripheral neuropathy through Drp-1 mediated-oxidative stress and apoptosis. Neurosci. Lett. 2012, 523, 45–49. [Google Scholar] [CrossRef]
- Jennings, P.E. Vascular benefits of gliclazide beyond glycemic control. Metabolism 2000, 49, 17–20. [Google Scholar] [CrossRef]
- Mamputu, J.-C. Gliclazide decreases vascular smooth muscle cell dysfunction induced by cell-mediated oxidized low-density lipoprotein. Metab. Clin. Exp. 2001, 50, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hou, K.; Wang, Y.; Chen, Y.; Zheng, X.; Qi, J.; Yang, B.; Tang, S.; Han, X.; Shi, D. Identification of prognostic signature and gliclazide as candidate drugs in lung adenocarcinoma. Front. Oncol. 2021, 11, 665276. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Zhang, D.; Wang, H.; Cui, X.; Zhang, C.; Xin, Y. Gliclazide Reduces Colitis-Associated Colorectal Cancer Formation by Deceasing Colonic Inflammation and Regulating AMPK-NF-κB Signaling Pathway. Dig. Dis. Sci. 2024, 69, 453–462. [Google Scholar] [CrossRef]
- Umapathi, P.; Ayyappan, J.; Quine, S.D. Quantitative determination of metformin hydrochloride in tablet formulation containing croscarmellose sodium as disintegrant by HPLC and UV spectrophotometry. Trop. J. Pharm. Res. 2012, 11, 107–116. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A. Performance liquid chromatographic analysis of glipizide: Application to in vitro and in vivo studies. J. Chromatogr. Sci. 2003, 41, 295–300. [Google Scholar] [CrossRef]
- Foroutan, S.; Zarghi, A.; Shafaati, A.; Khoddam, A. Application of monolithic column in quantification of gliclazide in human plasma by liquid chromatography. J. Pharm. Biomed. Anal. 2006, 42, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. J. Food Drug Anal. 2019, 27, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Advanced and multifaceted stability profiling of the first-line antidiabetic drugs metformin, gliclazide and glipizide under various controlled stress conditions. Saudi Pharm. J. 2020, 28, 362–368. [Google Scholar] [CrossRef]
- Lakshmi, K.; Rajesh, T. Development and validation of RP-HPLC method for simultaneous determination of glipizide, rosiglitazone, pioglitazone, glibenclamide and glimepiride in pharmaceutical dosage forms and human plasma. J. Iran. Chem. Soc. 2011, 8, 31–37. [Google Scholar] [CrossRef]
- Sultana, N.; Naveed, S.; Arayne, M. Development and validation of a simple and efficient RPLC method for analysis of captopril, metformin, pioglitazone and glibenclamide in API, formulations and human serum. Pharm. Anal. Acta. 2013, 4, 2. [Google Scholar] [CrossRef]
- Sultana, N.; Saeed Arayne, M.; Ali, S.N.; Zuberi, M.H. Simultaneous determination of glipizide and glimepride by Rp-Hplc in dosage formulations and in human serum. Med. Chem. Res. 2012, 21, 2443–2448. [Google Scholar] [CrossRef]
- Cruz-Angeles, J.; Martínez, L.M.; Videa, M.; Rodríguez-Rodríguez, J.; Martínez-Jiménez, C. Development and Validation of a Rapid Analytical Method for the Simultaneous Quantification of Metabolic Syndrome Drugs by HPLC-DAD Chromatography. Sci. Pharm. 2021, 89, 8. [Google Scholar] [CrossRef]
- Elkady, E.F.; El-Zaher, A.A.; Elwy, H.H.; Saleh, M.A. Validated liquid chromatographic method for simultaneous determination of metformin, pioglitazone, sitagliptin, repaglinide, glibenclamide and gliclazide-application for counterfeit drug analysis. J. Anal. Bioanal. Tech. 2015, S13, 1–8. [Google Scholar] [CrossRef]
- El-Wasseef, D.R. Simultaneous determination of metformin, nateglinide and gliclazide in pharmaceutical preparations using micellar liquid chromatography. Int. J. Biomed. Sci. 2012, 8, 144–151. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614865/ (accessed on 15 September 2024). [CrossRef]
- AbuRuz, S.; Millership, J.; McElnay, J. The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma. J. Chromatogr. B. 2005, 817, 277–286. [Google Scholar] [CrossRef]
- Kolte, B.; Raut, B.; Deo, A.; Bagool, M.; Shinde, D. Simultaneous determination of metformin in its multicomponent dosage forms with glipizide and gliclazide using micellar liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 1117–1133. [Google Scholar] [CrossRef]
- Sebaiy, M.M.; El-Adl, S.M.; Baraka, M.M.; Hassan, A.A. Rapid RP-HPLC method for simultaneous estimation of some antidiabetics; Metformin, Gliclazide and Glimepiride in Tablets. Egypt. J. Chem. 2019, 62, 429–440. [Google Scholar] [CrossRef]
- Troja, E.; Deda, L.; Boçari, G. Ion-pair HPLC method for the quantification of metformin in human urine. J. Appl. Bioanal. 2016, 2, 16–24. [Google Scholar] [CrossRef]
- AbuRuz, S.; Millership, J.; McElnay, J. Determination of metformin in plasma using a new ion pair solid phase extraction technique and ion pair liquid chromatography. J. Chromatogr. B. 2003, 798, 203–209. [Google Scholar] [CrossRef]
- Bandarkar, F.; Khattab, I. Simultaneous estimation of glibenclamide, gliclazide, and metformin hydrochloride from bulk and commercial products using a validated ultra fast liquid chromatography technique. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1814–1830. [Google Scholar] [CrossRef]
- Pani, N.R.; Acharya, S.; Patra, S. Development and validation of RP-HPLC method for quantification of glipizide in biological macromolecules. Int. J. Biol. Macromol. 2014, 65, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Porwal, P.K.; Talele, G.S. Development of validated HPLC-UV method for simultaneous determination of metformin, amlodipine, glibenclamide and atorvastatin in human plasma and application to protein binding studies. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 129–139. [Google Scholar] [CrossRef][Green Version]
- Cecchi, T. Ion pairing chromatography. Crit. Rev. Anal. Chem. 2008, 38, 161–213. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, J. Chromatography: Liquid|ion pair liquid chromatography. Encycl. Sep. Sci. 2000. Available online: https://www.sciencedirect.com/topics/chemistry/ion-pair-chromatography (accessed on 15 September 2024).
- Ståhlberg, J. Retention models for ions in chromatography. J. Chromatogr. A. 1999, 855, 3–55. [Google Scholar] [CrossRef]
- Troja, E.; Deda, L.; Boçari, G. Ion-pair HPLC method for the quantification of metformin in human plasma and its application to a pharmacokinetic study. Br. J. Pharm. Res. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Vasudevan, M.; Ravi, J.; Ravisankar, S.; Suresh, B. Ion-pair liquid chromatography technique for the estimation of metformin in its multicomponent dosage forms. J. Pharm. Biomed. Anal. 2001, 25, 77–84. [Google Scholar] [CrossRef]
- Rao, B.U.; Nikalje, A.P. Determination of gliclazide in a tablet dosage form in the presence of metformin hydrochloride by ion pair reversed phase liquid chromatographic technique. Afr. J. Pharm. Pharmacol. 2011, 5, 1331–1337. [Google Scholar] [CrossRef]
- Brocks, D.; Gabr, R.Q.; Padwal, R.S. Determination of metformin in human plasma and urine by high-performance liquid chromatography using small sample volume and conventional octadecyl silane column. J. Pharm. Pharm. Sci. 2010, 13, 486–494. [Google Scholar] [CrossRef]
- El-Gindy, A.; Nassar, M.W.; El-Abasawy, N.M.; Attia, K.A.-S.; Al-Shabrawi, M. Optimization and validation of an RP-HPLC method for direct determination of metformin hydrochloride in human urine and in a dosage form. J. AOAC. Int. 2010, 93, 1821–1828. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- Jung, H.H.; Chang, J.; Yang, J.Y.; Choi, J.; Im, G.J.; Chae, S.W. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear. Res. 2011, 282, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kendall, A.; Schacht, J. Metformin protects against gentamicin-induced hair cell death in vitro but not ototoxicity in vivo. Neurosci. Lett. 2014, 583, 65–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glutz, A.; Leitmeyer, K.; Setz, C.; Brand, Y.; Bodmer, D. Metformin protects auditory hair cells from gentamicin-induced toxicity in vitro. Audiol. Neurotol. 2015, 20, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, S.H.; Chang, J.W.; Song, J.-J.; Jung, H.H.; Im, G.J. Protective effect of metformin on gentamicin-induced vestibulotoxicity in rat primary cell culture. Clin. Exp. Otorhinolaryngol. 2014, 7, 286–294. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, T.; Zhan, T.; Cheng, G.; Zhang, W.; Jia, H.; Yang, H. Metformin alleviates cisplatin-induced ototoxicity by autophagy induction possibly via the AMPK/FOXO3a pathway. J. Neurophysiol. 2021, 125, 1202–1212. [Google Scholar] [CrossRef]
- Chang, J.; Jung, H.H.; Yang, J.Y.; Lee, S.; Choi, J.; Im, G.J.; Chae, S.W. Protective effect of metformin against cisplatin-induced ototoxicity in an auditory cell line. J. Assoc. Res. Otolaryngol. 2014, 15, 149–158. [Google Scholar] [CrossRef][Green Version]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Diabetes-induced cellular changes in the inner ear. Diabet. Epidemiol. Manag. 2023, 13, 100183. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Biochemical changes to the inner ear contributing to diabetes-induced hearing loss: Possible pharmacological targets for therapy. J. Pharm. Pharmacol. 2024, 76, 295–306. [Google Scholar] [CrossRef]
- Schönherr, D.; Wollatz, U.; Haznar-Garbacz, D.; Hanke, U.; Box, K.; Taylor, R.; Ruiz, R.; Beato, S.; Becker, D.; Weitschies, W. Characterisation of selected active agents regarding pKa values, solubility concentrations and pH profiles by SiriusT3. Eur. J. Pharm. Biopharm. 2015, 92, 155–170. [Google Scholar] [CrossRef]
- Tahara, K.; Yonemoto, A.; Yoshiyama, Y.; Nakamura, T.; Aizawa, M.; Fujita, Y.; Nishikawa, T. Determination of antihyperglycemic biguanides in serum and urine using an ion-pair solid-phase extraction technique followed by HPLC-UV on a pentafluorophenylpropyl column and on an octadecyl column. Biomed. Chromatogr. 2006, 20, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Harisudhan, T.; Choudhury, H.; Mullangi, R.; Srinivas, N.R. Simultaneous estimation of six anti-diabetic drugs—Glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: Development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomed. Chromatogr. 2006, 20, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Kolte, B.; Raut, B.; Deo, A.; Bagool, M.; Shinde, D. Simultaneous determination of metformin in combination with rosiglitazone by reversed-phase liquid chromatography. J. Chromatogr. Sci. 2004, 42, 70–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xing, Y.; Ji, Q.; Li, X.; Ming, J.; Zhang, N.; Zha, D.; Lin, Y. Asiaticoside protects cochlear hair cells from high glucose-induced oxidative stress via suppressing AGEs/RAGE/NF-κB pathway. Biomed. Pharmacother. 2017, 86, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Gu, J.; He, T. Apelin-13 reduces high glucose-induced mitochondrial dysfunction in cochlear hair cells by inhibiting endoplasmic reticulum stress. Exp. Ther. Med. 2024, 27, 226. [Google Scholar] [CrossRef]
- Xing, Y.; Ming, J.; Liu, T.; Zhang, N.; Zha, D.; Lin, Y. Decreased expression of TRPV4 channels in HEI-OC1 cells induced by high glucose is associated with hearing impairment. Yonsei Med. J. 2018, 59, 1131–1137. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. PEDF attenuates insulin-dependent molecular pathways of glucose homeostasis in skeletal myocytes. Mol. Cell Endocrinol. 2016, 422, 115–124. [Google Scholar] [CrossRef]
- Rao, C.V. Biguanides. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 452–455. [Google Scholar]
- Mujica-Mota, M.A.; Salehi, P.; Devic, S.; Daniel, S.J. Safety and otoprotection of metformin in radiation-induced sensorineural hearing loss in the guinea pig. Otolaryngol. Head Neck Surg. 2014, 150, 859–865. [Google Scholar] [CrossRef]
- Kimura, N.; Okuda, M.; Inui, K.-i. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm. Res. 2005, 22, 255–259. [Google Scholar] [CrossRef]
- Kimura, N.; Masuda, S.; Tanihara, Y.; Ueo, H.; Okuda, M.; Katsura, T.; Inui, K.-i. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab. Pharmacokinet. 2005, 20, 379–386. [Google Scholar] [CrossRef]
- Wang, D.-S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Kusuhara, H.; Kato, Y.; Jonker, J.W.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol. Pharmacol. 2003, 63, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.W.; Bedwell, D.W.; Zamek-Gliszczynski, M.J. Ablation of both organic cation transporter (OCT) 1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab. Dispos. 2012, 40, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; am Zehnhoff-Dinnesen, A.; Schinkel, A.H. Organic cation transporter 2 mediates cisplatin-induced oto-and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- Kros, C.J.; Steyger, P.S. Aminoglycoside-and cisplatin-induced ototoxicity: Mechanisms and otoprotective strategies. Cold Spring Harb. Perspect. Med. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- More, S.S.; Akil, O.; Ianculescu, A.G.; Geier, E.G.; Lustig, L.R.; Giacomini, K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010, 30, 9500–9509. [Google Scholar] [CrossRef]
- Föller, M.; Jaumann, M.; Dettling, J.; Saxena, A.; Pakladok, T.; Munoz, C.; Ruth, P.; Sopjani, M.; Seebohm, G.; Rüttiger, L. AMP-activated protein kinase in BK-channel regulation and protection against hearing loss following acoustic overstimulation. FASEB J. 2012, 26, 4243–4253. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Dubinin, M.V. Diabetes mellitus, mitochondrial dysfunction and Ca2+-dependent permeability transition pore. Int. J. Mol. Sci. 2020, 21, 6559. [Google Scholar] [CrossRef]
- Choi, S.W.; Ho, C.K. Antioxidant properties of drugs used in Type 2 diabetes management: Could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018, 23, 1–24. [Google Scholar] [CrossRef]
- Bulterijs, S. Metformin as a geroprotector. Rejuvenation Res. 2011, 14, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).