Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector
Abstract
1. Introduction
2. Physicochemical Properties of Hydrogen
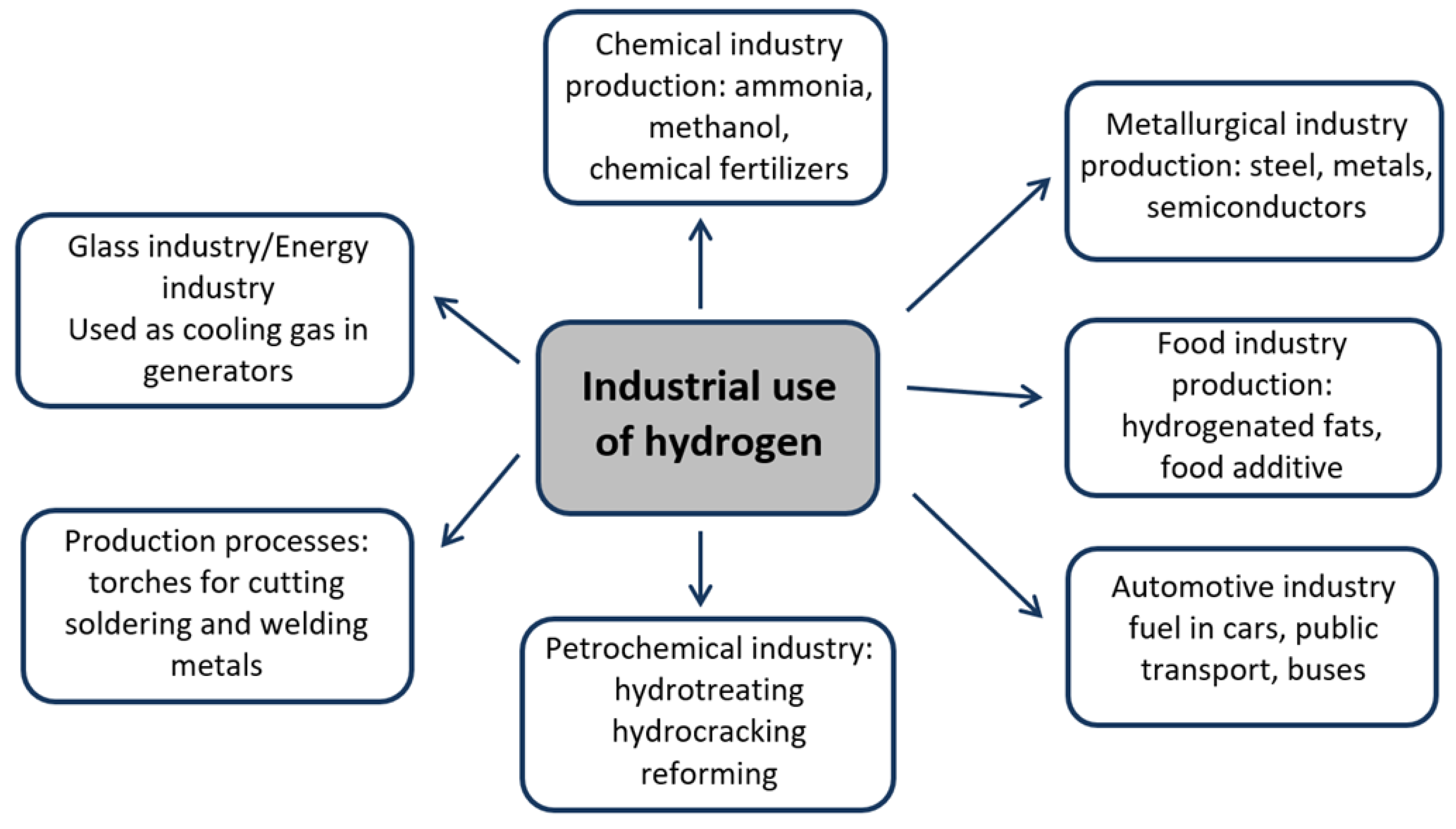
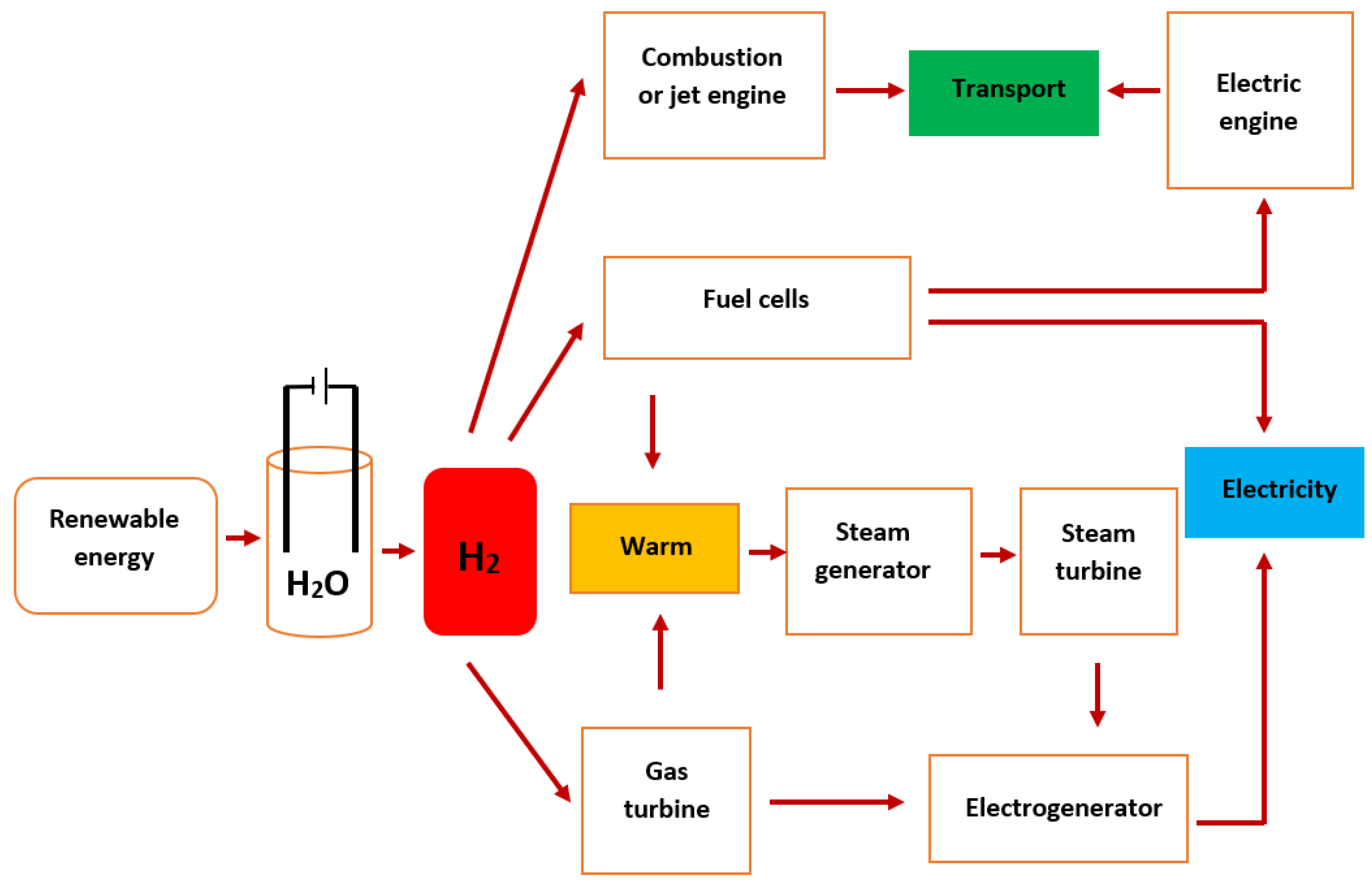
3. Hydrogen Production Methods
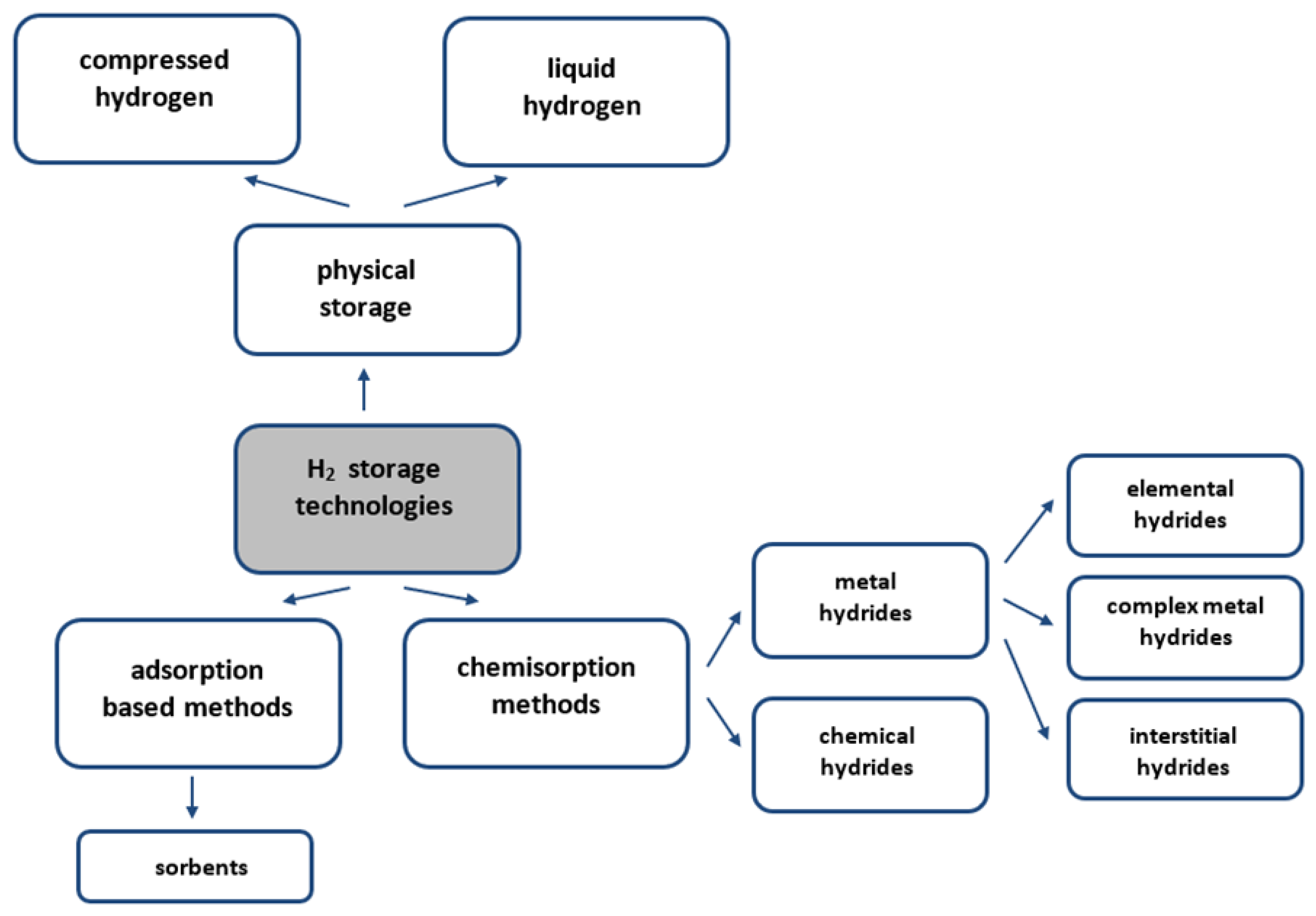
4. Hydrogen Storage
- −
- Physical methods that involve storing compressed or liquefied molecular hydrogen;
- −
- Methods on the basis of the adsorption processes of molecular hydrogen on materials having a well-developed surface using weak intermolecular van der Waals interactions;
- −
- Chemisorption methods, which involve the chemical binding (absorption) of atomic hydrogen.
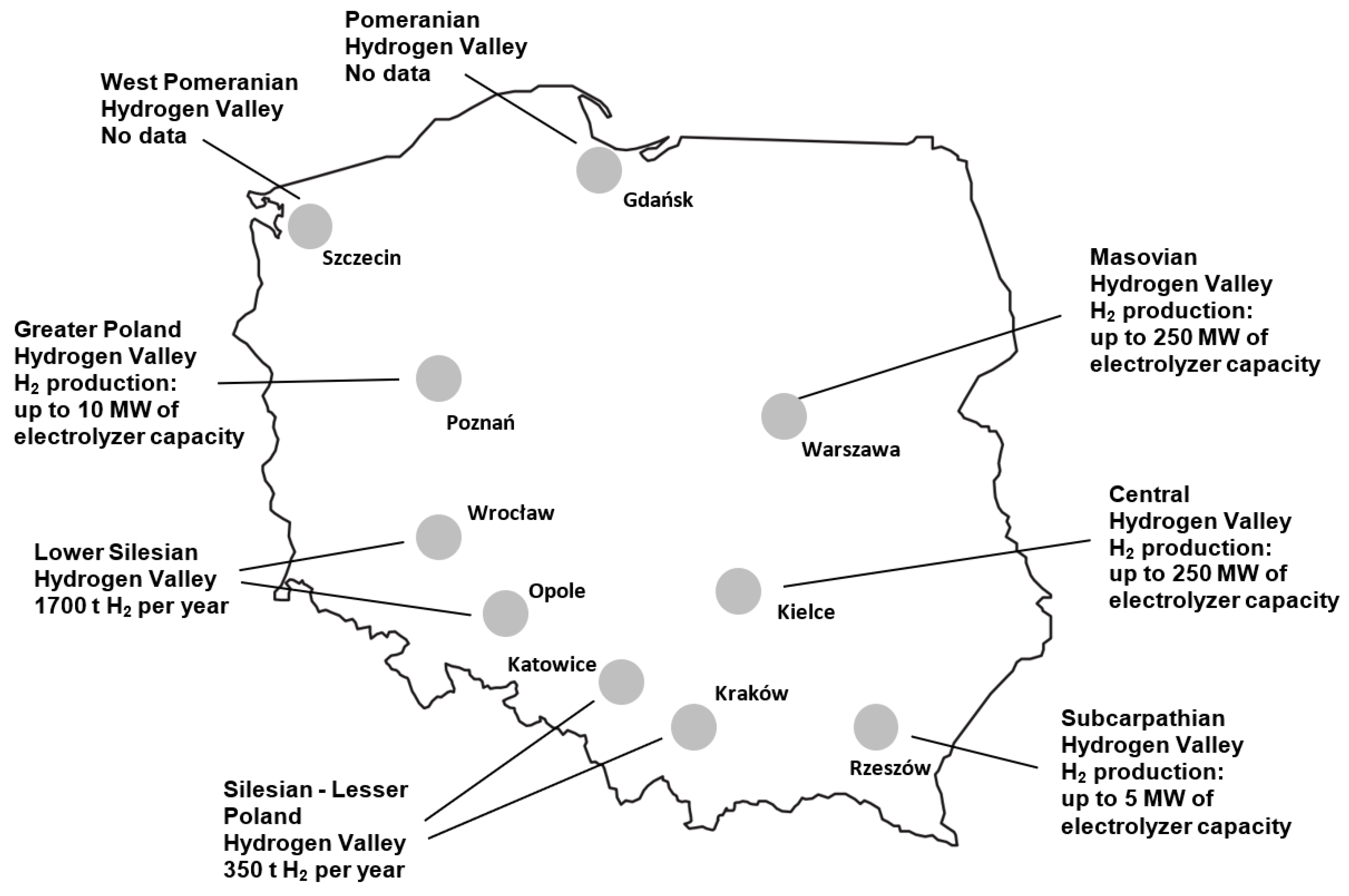
5. Polish Hydrogen Valleys
6. The Future of Hydrogen in Poland
7. Polish Prototype Installations for Production and Storage of Hydrogen
8. Conclusions and Prospects
- −
- The technologies and materials used in green hydrogen energy are more environmentally friendly but require large investment outlays, supplies of critical raw materials for electrolysers and fuel cells, and the consumption of large amounts of water.
- −
- The steam reforming of methane will be a bridge method of hydrogen production in the pursuit of zero emissions. With the drastically increasing costs of CO2 emissions, the use of CCS/CCUS technology will become economically profitable and will be an essential activity to carry out the energy transformation in Poland.
- −
- Gasification as a technology for the thermal processing of biogenic waste and one of the closed loop technologies has great potential for hydrogen production and can play an important role in achieving the sustainable development goals in Poland. This technology is currently at the level of research and development projects and implementations.
- −
- The Polish hydrogen economy is in the initial stage of development. Currently, the main barriers to the development of hydrogen energy in Poland are the lack of appropriate legal regulations enabling and supporting the development of an ecological hydrogen industry, high investment and operating costs of a hydrogen infrastructure, and the limited availability of electrolysers.
- −
- The condition for the development of the hydrogen economy in Poland is the simultaneous development of new capacities of renewable energy sources, primarily offshore and onshore wind farms. Nuclear power plants will provide significant support for the development of hydrogen energy.
- −
- In the long-term perspective of the development of the hydrogen market in Poland, the construction of a hydrogen terminal (similar to the LNG terminal), as well as the expansion of hydrogen pipelines, will be necessary to handle export/import trade.
- −
- Although there is currently no underground hydrogen storage facility in Poland, its construction should not pose any problems, taking into account the experience in the construction and operation of underground natural gas storage facilities.
- −
- Creating hydrogen valleys in Poland will support the use of research and development potential and should contribute to significant economic growth and the creation of new jobs.
- −
- Taking into account the growing demand for energy storage, it can be concluded that there is still a need to look for new technological solutions that are more economically beneficial and environmentally friendly.
- −
- To accelerate the development of hydrogen storage systems, it is recommended to use machine learning techniques, which will reduce the time and costs associated with conducting experiments on a large number of potential materials.
- −
- Hydrogen storage is recommended for use in the transport sector, seasonal energy storage, the flexibility of network operations, and for the integration of the electricity grid with the gas network.
- −
- The use of waste biomass or seawater for hydrogen production will certainly bring significant social and environmental benefits.
- −
- The development of hydrogen energy requires the drafting of international standards and regulations that are currently not available, as well as cooperation integrating the scientific community, industries, and the government.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0301 (accessed on 6 February 2024).
- Available online: https://www.gov.pl/attachment/06213bb3-64d3-4ca8-afbe-2e50dadfa2dc (accessed on 6 February 2024).
- Available online: https://www.gov.pl/attachment/1b590d54-fa1e-49fe-9096-b2d0c6a4fe59 (accessed on 24 October 2024).
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.A.J. Hydrogen Energy—Challenges and Prospects; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Lewandowski, W.M. Proecological Renewable Energy Sources; WNT: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Surygała, J. Hydrogen as a Fuel; WNT: Warszawa, Poland, 2008. (In Polish) [Google Scholar]
- Available online: https://www.idealhy.eu (accessed on 6 February 2024).
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liang, Y.; Li, J. CFD analysis of leakage and diffusion characteristics in the buried hydrogen-blended natural gas pipeline. Int. J. Hydrogen Energy 2024, 60, 354–368. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production (accessed on 30 October 2024).
- Miao, H.; Lu, L.; Huang, Z. Flammability limits of hydrogen-enriched natural gas. Int. J. Hydrogen Energy 2011, 36, 6937–6947. [Google Scholar] [CrossRef]
- Kordylewski, W. Combustion and Fuel; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2005. (In Polish) [Google Scholar]
- Folentarska, A.; Ciesielski, W.; Pavlyuk, V. Modern materials for storage of hydrogen as fuels of the future. Chem. Enviroment Biotechnol. 2016, 19, 125–130. [Google Scholar] [CrossRef]
- Nanda, S.; Vo, D.-V.N.; Nguyen, P. New Dimensions in Production and Utilization of Hydrogen; Elsevier: Cambridge, UK, 2020. [Google Scholar]
- Meija, J. Atomic weights of the elements. Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Young, K.H.; Nei, J. The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- El Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen production technologies overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–452. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Milewski, J.; Kupecki, J.; Szczesniak, A.; Uzunow, N. Hydrogen production in solid oxide electrolyzers coupled with nuclear reactors. Int. J. Hydrogen Energy 2021, 46, 35765–35776. [Google Scholar] [CrossRef]
- Available online: https://ptx-hub.org/water-electrolysis-explained (accessed on 6 February 2024).
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Hydrogen and Fuel Cell Technologies Office Accomplishments and Progress. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-and-fuel-cell-technologies-office-accomplishments-and-progress (accessed on 30 October 2024).
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Li, Y.-C.; Liu, J.; He, J.; Wang, L.-Y.; Le, J.-D. Recent developments in high-performance Nafion membranes for hydrogen fuel cells applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Andersson, J.; Gronkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Tietze, V.; Luhr, S.; Stolten, D. Bulk Storage Vessels for Compressed and Liquid Hydrogen. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 659–690. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Zhang, Z.; Li, L.; Jiang, D.; Fan, J.; Chen, J.; Zhang, X.; Wan, J.; Li, Z. Optimization of operating pressure of hydrogen storage salt cavern in bedded salt rock with multi-interlayers. Int. J. Hydrogen Energy 2024, 58, 974–986. [Google Scholar] [CrossRef]
- Mehr, A.S.; Phillips, A.D.; Brandon, M.P.; Pryce, M.T.; Carton, J.G. Recent challenges and development of technical and technoeconomic aspects for hydrogen storage, insights at different scales; A state of art review. Int. J. Hydrogen Energy 2024, 70, 786–815. [Google Scholar] [CrossRef]
- Wolf, E. Large-Scale Hydrogen Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 129–142. [Google Scholar]
- Moseley, P.T.; Garche, J.; Adelmann, P. Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 129–142. [Google Scholar]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/hpwgw_airprod_remp.pdf (accessed on 11 April 2024).
- Available online: https://www.cder.dz/A2H2/Medias/Download/Proc%20PDF/posters/%5bGIV%5d%20Liquid%20&%20gaseous%20storage,%20delidevy,%20safety,%20RCS/222.pdf (accessed on 30 October 2024).
- Leighty, W.; Holloway, J.; Merer, R.; Somerday, B.; San Marchi, C.; Keith, G.; White, D. Compressorless Hydrogen Transmission Pipelines Deliver Largescale Stranded Renewable Energy at Competitive Cost. In Proceedings of the 16th World Hydrogen Energy Conference, Lyon, France, 13–16 June 2006; Available online: http://www.leightyfoundation.org/wp-content/uploads/whec16-lyon/WHEC16-Ref022.pdf (accessed on 30 October 2024).
- Fekete, J.R.; Sowards, J.W.; Amaro, R.L. Economic impact of applying high strength steels in hydrogen gas pipelines. Int. J. Hydrogen Energy 2015, 40, 10547–10558. [Google Scholar] [CrossRef]
- Klell, M. Storage of hydrogen in the pure form. In Handbook of Hydrogen Storage: New Materials for Future Energy Storage; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Yin, L.; Yang, H.; Ju, Y. Review on the key technologies and future development of insulation structure for liquid hydrogen storage tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1315. [Google Scholar] [CrossRef]
- Kuendig, A.; Loehlein, K.; Kramer, G.J.; Huijsmans, J. Large scale hydrogen liquefaction in combination with LNG re-gasification. In Proceedings of the 16th World Hydrogen Energy Conference, Lyon, France, 13–16 June 2006; pp. 3326–3333. [Google Scholar]
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrogen Energy 2017, 42, 13329–13338. [Google Scholar] [CrossRef]
- Xia, Y.D.; Yang, Z.X.; Zhu, Y.Q. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; MacA Gray, E.; Webb, C.J. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- Mehdi, S.; Alizadeh, S.; Parhizi, Z.; Alibak, A.H.; Vaferi, B.; Hosseini, S. Predicting the hydrogen uptake ability of a wide range of zeolites utilizing supervised machine learning methods. Int. J. Hydrogen Energy 2022, 47, 21782–21793. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Balahmar, N.; Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Hurst, K.E.; Parilla, P.A.; Gennett, T.; Brown, C.M.; Zacharia, R.; Tylianakis, E.; Klontzas, E.; Froudakis, G.E.; et al. Outlook and challenges for hydrogen storage in nanoporous materials. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–21. [Google Scholar] [CrossRef]
- Rathi, B.; Agarwal, S.; Shrivastava, K.; Kumar, M.; Jain, A. Recent advances in designing metal oxide-based catalysts to enhance the sorption kinetics of magnesium hydride. Int. J. Hydrogen Energy 2024, 53, 131–162. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum hydride for solid-state hydrogen storage: Structure, synthesis, thermodynamics, kinetics, and regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.W.; Aguey-Zinsou, K.F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloys Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Fabian, T.; Petrie, M.; Crouch-Baker, S.; Fong, H. Low-Cost Alpha Alane for Hydrogen Storage; Ardica Technologies: San Francisco, CA, USA, 2017. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 11 April 2024).
- Giza, K.; Pospiech, B.; Gęga, J. Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges. Energies 2023, 16, 5777. [Google Scholar] [CrossRef]
- Harries, D.N.; Paskevicius, M.; Sheppard, D.A.; Price, T.E.; Buckley, C.E. Concentrating solar thermal heat storage using metal hydrides. Proc. IEEE 2012, 100, 539–549. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Davids, M.W.; Klochko, Y.V.; Parsons, A.; Swanepoel, D.; Ehlers, R.; Louw, G.; van der Westhuizen, B.; Smith, F.; et al. Metal hydride hydrogen storage and supply systems for electric forklift with low-temperature proton exchange membrane fuel cell power module. Int. J. Hydrogen Energy 2016, 41, 13831–13842. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and potential as hydrogen storage medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Han, Y.; Chen, X.; Sheng, P.; Wang, S.; Huang, X.; Wang, X.; Lu, C.; Luo, H.; et al. Development of a gaseous and solid-state hybrid system for stationary hydrogen energy storage. Green Energy Environ. 2021, 6, 528–537. [Google Scholar] [CrossRef]
- Tarhan, C.; Cil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Gerard, F.; van Nuffel, L.; Smith, T.; Yearwood, J.; Cerny, O.; Michalski, J.; Altmann, M. Opportunities for Hydrogen Energy Technologies Considering the National Energy & Climate Plans Final Report. 2020. Available online: https://www.lei.lt/wp-content/uploads/2020/09/Final-Report-Hydrogen-in-NECPs-28-8-2020-ID-9474232.pdf (accessed on 30 October 2024).
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review. Membranes 2021, 11, 127. [Google Scholar] [CrossRef]
- Roszak, W. Wodór w grupie ZE PAK i PAK PCE. Energetyka Ciepl. Zawodowa 2022, 6, 64–70. [Google Scholar]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef]
- Available online: https://arp.pl/pl/jak-dzialamy/transformacja-energetyczna-/doliny-wodorowe/ (accessed on 24 October 2024).
- Available online: https://wodorowe.info/ (accessed on 21 October 2024).
- Available online: https://polishscience.pl/en/first-hydrogen-academy-is-over/ (accessed on 4 April 2024).
- Gnanapragasam, N.V.; Reddy, B.V.; Rosen, M.A. Hydrogen production from coal using coal direct chemical looping and syngas chemical looping combustion systems: Assessment of system operation and resource requirements. Int. J. Hydrogen Energy 2009, 34, 2606–2615. [Google Scholar] [CrossRef]
- Mechleri, E.; Brown, S.; Fennell, P.S.; Mac Dowell, N. CO2 capture and storage (CCS) cost reduction via infrastructure right-sizing. Chem. Eng. Res. Des. 2017, 119, 130–139. [Google Scholar] [CrossRef]
- Kaplan, R.; Kopacz, M. Economic Conditions for Developing Hydrogen Production Based on Coal Gasification with Carbon Capture and Storage in Poland. Energies 2020, 13, 5074. [Google Scholar] [CrossRef]
- Available online: https://klasterwodorowy.pl/images/zdjecia/9_Analiza_potencjalu_technologii_wodorowych_opracowanie.pdf (accessed on 2 April 2024).
- Budzianowski, W. Can ‘negative net CO2 emissions’ from decarbonised biogas-to-electricity contribute to solving Poland’s carbon capture and sequestration dilemmas? Energy 2011, 36, 6318–6325. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_14_1131 (accessed on 3 April 2024).
- Zaik, K.; Werle, S. Solar and wind energy in Poland as power sources for electrolysis process—A review of studies and experimental methodology. Int. J. Hydrogen Energy 2023, 48, 11628–11639. [Google Scholar] [CrossRef]
- McKenna, R.; Hollnaicher, S.; Ostman, P.; Leye, V.D.; Fichtner, W. Cost-potentials for large onshore wind turbines in Europe. Energy 2015, 83, 217–229. [Google Scholar] [CrossRef]
- Świsłowski, K. Setki milionów złotych pomocy publicznej na produkcję zielonego wodoru w Gdańsku. Przy rafinerii stanie elektrolizer. 13 kwietnia 2023 r. Available online: https://www.green-news.pl (accessed on 22 April 2024).
- Benalcazar, P.; Komorowska, A. Prospects of green hydrogen in Poland: A techno-economic analysis using a Monte Carlo approach. Int. J. Hydrogen Energy 2022, 47, 5779–5796. [Google Scholar] [CrossRef]
- Available online: https://biznes.pap.pl/en/news/all/info/3501469,polsat-plus-and-ze-pak-open-second-hydrogen-refueling-station-under-neso-brand (accessed on 10 April 2024).
- Available online: https://ceenergynews.com/hydrogen/orlen-signs-eu-deal-for-new-hydrogen-stations-in-poland/ (accessed on 10 April 2024).
- Dell’Isola, M.; Ficco, G.; Moretti, L.; Jaworski, J.; Kułaga, P.; Kukulska-Zając, E. Impact of Hydrogen Injection on Natural Gas Measurement. Energies 2021, 14, 8461. [Google Scholar] [CrossRef]
- Eikeng, E.; Makhsoos, A.; Pollet, B.G. Critical and strategic raw materials for electrolysers, fuel cells, metal hydrides and hydrogen separation technologies. Int. J. Hydrogen Energy 2024, 71, 433–464. [Google Scholar] [CrossRef]
- Available online: https://www.gov.pl/web/ia/polityka-energetyczna-polski-do-2040-r-pep2040 (accessed on 24 April 2024).
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Hemme, C.; Van Berk, W. Hydrogeochemical Modeling to Identify Potential Risks of Underground Hydrogen Storage in Depleted Gas Fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Tarkowski, R.; Czapowski, G. Salt domes in Poland—Potential sites for hydrogen storage in caverns. Int. J. Hydrogen Energy 2018, 43, 21414–21427. [Google Scholar] [CrossRef]
- Tarkowski, R. Perspectives of using the geological subsurface for hydrogen storage in Poland. Int. J. Hydrogen Energy 2017, 42, 347–355. [Google Scholar] [CrossRef]
- Available online: https://forumakademickie.pl/konkursy/wynalazki-z-pan-i-politechniki-gdanskiej-produktami-przyszlosci/ (accessed on 12 April 2024).
- Available online: https://www.gov.pl/web/klimat/ministerstwo-klimatu-i-srodowiska-rozpoczyna-prace-nad-instrumentami-wsparcia-dla-wykorzystania-wodoru-niskoemisyjnego-w-gospodarce (accessed on 10 April 2024).
- Available online: https://grupa.energa.pl/en/publications/524598/energa-to-implement-an-innovative-hydrogen-project-2020-06-10 (accessed on 21 April 2024).
- Available online: https://ftims.pg.edu.pl/en/news/2022-12/our-employees-create-technology-provides-ecological-transport-and-heating-waste (accessed on 12 April 2024).
- Available online: https://www.lotos.pl/322/n,5311/projekt_vetni_wystartowal (accessed on 5 April 2024).
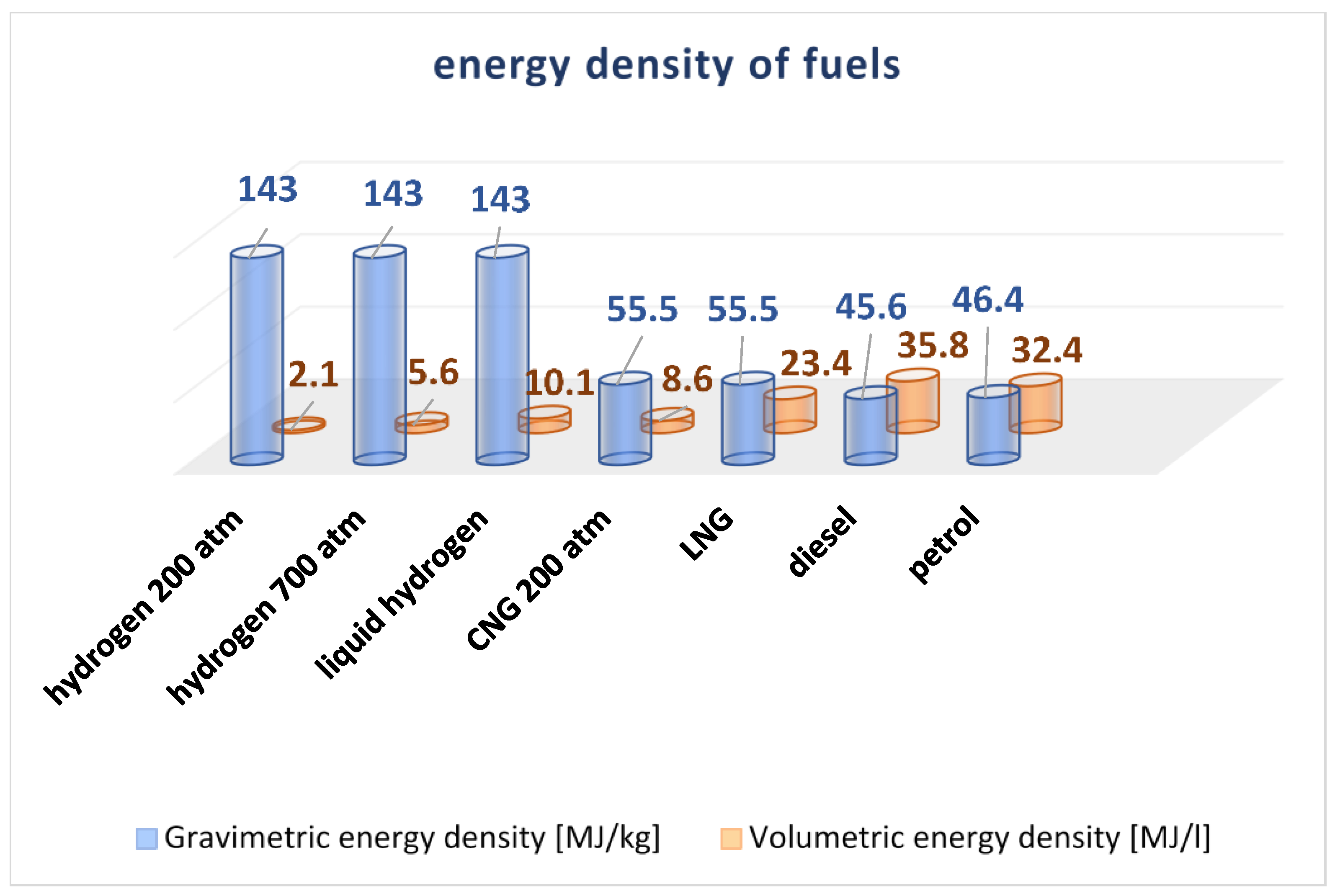
| Fuel | Flammability Limits [vol. %] | Octane Number | Temperature of Self- Ignition [°C] | Combustion Speed [m s−1] |
|---|---|---|---|---|
| Hydrogen | 4–75 | 130 | 585 | 2.65–3.25 |
| Methane | 5–16 | 125 | 540 | 0.37–0.45 |
| Methane + 20% hydrogen fraction | 4.6–19.9 | |||
| Methane + 60% hydrogen fraction | 4.4–26 | |||
| Methane + 80% hydrogen fraction | 4.6–47.6 | |||
| Propane | 2.2–9.6 | 105 | 490 | |
| Petrol | 1–7.6 | 87–98 | 230–480 | 0.37–0.43 |
| Diesel fuel | 0.6–5.5 | 30 | 254–285 |
| Hydrogen Production Technology | Estimated Proces Efficiency [%] | Emission of CO2[kgCO2/ kg H2] | Hydrogen Colour | Advantages | Disadvantages | Estimated Hydrogen Production Cost [EUR/kg H2] |
|---|---|---|---|---|---|---|
| Hard coal gasification process | 30–40 | 15–20 | black | well-known and available technology, low efficiency | high-emission technology, impurities in the raw material, low efficiency, fluctuations in the raw material prices, costs depending on the price of CO2 emission allowances | 1.3–2.8 |
| Methane steam reforming (SMR) | 74–85 | 8–12.9 | grey | proven technology/infrastructure, high efficiency, low cost | high-emission technology, variable natural gas prices, costs dependent on the price of CO2 emission allowances | 1.3–2.2 |
| Hydrogen produced from fossil fuels combined with CO2 capture process (CCS) | 2–4 | blue | low-emission technology | high cost of CCS installation, dependence on fossil fuels | 1.2–3.6 | |
| Water electrolysis using nuclear energy | 60–80 | 0–0.2 | pink | emission-free technology | high energy and water consumption, high prices and poor availability of electrolyzers, high cost of installing a nuclear power plant | 3.5 do 8.5 |
| Water electrolysis using renewable energy sources | 60–80 | 0 | green | emission-free technology | high energy and water consumption, high prices and poor availability of electrolyzers, dependence on weather conditions | 3.5 do 8.5 |
| Polish Hydrogen Valley (Year of Establishment) | Scope of Activity/Achievements |
|---|---|
| Pomeranian (2019) | the application of zero-emission hydrogen in public transport, production of green hydrogen from offshore areas, and production of electrolysers; Lotos Group implemented the Pure H2 project until 2023 |
| Subcarpathian (2021) | hydrogen buses, hydrogen in aviation, hydrogen in energy, green heat, and water pipelines; Polenergia is implementing the H2HUB Nowa Sarzyna project “Green Hydrogen Storage” with an application for project development assistance |
| Silesian–Lesser (2022) | the production of 350 tH2/y low-emission hydrogen, green steel, and the decarbonisation of public transport; Orlen Południe launched a green glycol production plant; the creation of the hydrogen portal H2Poland.eu, supporting the development of the hydrogen economy in Poland and the Silesian–Lesser Poland Hydrogen Valley |
| Lower Silesian (2022) | the production of green ammonia, green heat, and the use of green hydrogen in metallurgical processes, namely the production of green copper, hydrogen in river transport (hydrogen barges), hydrogen in public transport, and hydrogen storage; in 2022, two applications were submitted to an international consortium for the Horizon Europe programme for the development of a regional hydrogen economy; in 2023, the first commercial installation for the production of green hydrogen in Poland (electrolyser with a capacity of 5 MW, 720 tH2/y) powered by renewable energy (wind, PV) was launched; the produced hydrogen will be converted in a trigeneration installation (into heat, cold, electricity) and used as a green fuel |
| Mazovian (2022) | the production of green hydrogen, synthetic fuels, biogas, the petrochemical industry, hydrogen in river transport, and public transport, namely the production of hydrogen buses and hydrogen refuelling stations; PKN Orlen is implementing the Hydrogen Eagle project and IPCEI called Hy2USE; the Hydrogen Academy project was also launched |
| Central (2023) | hydrogen production from renewable energy, hydrogen haulers, hydrogen storage, renewable energy, green public transport, and hydrogen production from nuclear energy |
| West Pomeranian (2022) | green ammonia production, low-emission maritime transport, low-emission river transport, ammonia collection infrastructure, integration around a large chemical company, and the Shore H2 Valley concept |
| Greater (2021) | the production of clean hydrogen and hydrogen in housing, which is a project of the first housing estate in Poland heated with a zero-emission hydrogen boiler; hydrogen refuelling stations, the production of hydrogen buses, hydrogen in air transport; the “Economical 20250-H2 Greater Poland” project is being implemented |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giza, K.; Owczarek, E.; Piotrowska-Woroniak, J.; Woroniak, G. Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector. Appl. Sci. 2024, 14, 10024. https://doi.org/10.3390/app142110024
Giza K, Owczarek E, Piotrowska-Woroniak J, Woroniak G. Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector. Applied Sciences. 2024; 14(21):10024. https://doi.org/10.3390/app142110024
Chicago/Turabian StyleGiza, Krystyna, Edyta Owczarek, Joanna Piotrowska-Woroniak, and Grzegorz Woroniak. 2024. "Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector" Applied Sciences 14, no. 21: 10024. https://doi.org/10.3390/app142110024
APA StyleGiza, K., Owczarek, E., Piotrowska-Woroniak, J., & Woroniak, G. (2024). Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector. Applied Sciences, 14(21), 10024. https://doi.org/10.3390/app142110024